Abstract
Four types of doxorubicin (DOX)-loaded polymeric micelles based on hydrophobically-modified sulfated chitosan (SCTS) were prepared. The hydrophobic group was composed of glycyrrhetinic acid (GA), cholic acid, stearic acid (SA) or lauric aldehyde. DOX encapsulation depended on several parameters, including the degree of substitution of the sulfate group and the hydrophobic group, and the type of hydrophobic group. Of these micelles, GA–SCTS micelles had the best capability to solubilize DOX. In addition, GA–SCTS micelles had the ability to target HepG2 cells, and the IC50 for DOX-loaded GA–SCTS micelles was 54.7 ng/mL, which was much lower than that of the other micelles. Further studies on the DOX-loaded GA–SCTS micelles showed that they were stable in salt and protein solutions, in cell culture media, and during long-term storage (6 months). Based on these results, these micelles may be a promising DOX-encapsulated formulation, particularly, GA–SCTS as a potential vehicle for liver-targeted delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Doxorubicin (DOX), a well known anticancer drug, is a member of anthracycline ring antibiotics. It is commonly used in the treatment of a wide range of cancers, including hematological malignancies, many types of carcinoma, and soft tissue sarcomas [1, 2]. However, severe side effects, such as myelosuppression and cardiotoxicity [1, 3], limit the application of DOX in cancer therapy, and this is primarily ascribed to the non-specific, indiscriminate distribution of the drug in various tissues [1]. Therefore, significant research has been devoted to improvements in the therapeutic index of DOX. Doxil®, a novel formulation of DOX in long-circulating (Stealth) liposomes, dramatically changed the pharmacokinetics and biodistribution of DOX [4], resulting in a significant decrease in DOX-associated toxicity and an increase in the effectiveness of chemotherapy [5, 6]. Thus, it can be concluded that an effective method of improving the therapeutic index of a drug is drug reformulation. Many attempts have been made to identify carriers to deliver DOX to the desired tissue while reducing adverse reactions in other tissues. In addition, liposomes and numerous drug delivery systems including microspheres [7, 8], polymer–drug conjugates [9, 10], nanoparticles [11, 12], solid lipid nanoparticles (SLNs) [13, 14], and polymeric micelles [15, 16], have been extensively studied and evaluated as carriers of DOX.
In particular, polymeric micelles, have received significant attention due to their remarkable advantages as drug carriers. These advantages include the low critical micelle concentration (CMC) value of the polymeric micelles which promotes drug stability in aqueous environments; the hydrophobic segment in the core–shell architecture which solubilizes lipophilic drugs, and prolonged blood circulation. The emerging polymeric micellar drug carriers include amphiphilic block copolymers [17–19], hydrophobically modified water-soluble polymers [20, 21], and natural polysaccharides bearing hydrophobic groups [22–24]. In recent years, sulfated chitosan (SCTS), a water soluble chitosan (CTS) derivative with sulfate substituents on some or both of the hydroxyl and amino groups of CTS, and the close structural analogs of the natural blood anticoagulant heparin, has attracted increasing attention as a drug carrier because of its good biocompatibility and biological activities, such as blood anticoagulant, hemagglutination inhibition and antimicrobial activities [25]. A series of polymeric micelles based on SCTS has been synthesized. Ping et al. [26, 27] reported on PEG-conjugated N-octyl-O-sulfate chitosan (mPEGOSC) micelles for the delivery of paclitaxel (PTX). The solubility of PTX in micellar solution was significantly improved (by 4000-fold), compared with that of free PTX in water. Tissue distribution studies indicated that micelles greatly decreased the elimination of PTX by the reticuloendothelial system (RES) and its accumulation in liver and spleen. The targeting efficiency of PTX-mPEGOSC to uterus was 2.27 times higher than that of Taxol®. These results indicate that micelles based on SCTS are excellent carriers for drug delivery.
In this study, polymeric micelles based on hydrophobically modified SCTS were designed for the encapsulation of DOX, in which the hydrophobic moiety was composed of glycyrrhetinic acid (GA), cholic acid (CA), stearic acid (SA), or lauric aldehyde (LA). CA, SA and LA are commonly used as hydrophobic segments in the preparation of polymeric micelles [28–30]. GA was selected due to the presence of receptors on hepatocytes [31], and GA–SCTS was expected to have the ability both to solubilize DOX and to target liver cancer cells. The micelle-forming properties of these amphiphilic polymers had been discussed previously. Generally, drug encapsulation is tightly associated with the properties of polymeric micelles [27, 32, 33], such as the composition of the micelle, the degree of substitution (DS) of each segment (hydrophilic group and hydrophobic moiety), the molecular weight of the micelle, as well as other factors. Therefore, the influence of the composition of the micelle on DOX encapsulation and in vitro release were evaluated in some detail, to evaluate the capacity of these micelles as carriers of DOX. The biocompatibility, the cellular uptake, and the cytotoxicity of the DOX-loaded micelles were also investigated.
2 Materials and methods
2.1 Materials
Chitosan (Mw = 50,000, DD > 95 %) was supplied by Ao’xing Biotechnology Co., Ltd. (Zhejiang, China). GA (purity >98 % by HPLC) was purchased from Fujie Pharmaceutical Co., Ltd. (Xi’an, China). SA, LA and chlorosulfonic acid (HClSO3) were supplied by Tianjin Chemical Company (Tianjin, China). CA (purity >98 % by HPLC) was supplied by Guangfu Co., Ltd (Tianjin, China). DOX·HCl was obtained from Huafeng United Technology Co., Ltd. (Beijing, China). 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxy-succinimide (NHS) were obtained from GL Biochem., Ltd. (Shanghai, China). Bovine Serum Albumin (BSA, purity >98 % by HPLC) was supplied by Tianjin Dingguo Bio. Co., Ltd. (Tianjin, China). All other agents were of analytical grade.
2.2 Synthesis of the amphiphilic polymers
SCTS was prepared according to the Ref. [34]. CTS (1.0 g) was suspended in a mixture of 60 mL of H2SO4 (98 %) and 20 mL of HSO3Cl (98 %), previously cooled at 0–4 °C. Then the solution was stirred for 50 min at room temperature and precipitated with cold diethyl ether. The precipitate was filtered and repeatedly washed with diethyl ether, then solubilized in water, neutralized with 0.5 M NaOH, dialyzed against water for 3 days and lyophilized.
GA–SCTS was prepared by two steps. Firstly, GA (5.0 mmol) and succinic anhydride (20.0 mmol) were dissolved in 50 mL anhydrous pyridine. The mixture was refluxed for 14–15 h, poured into water and acidified to pH 3–4. The precipitate was filtered and purified by silica gel (eluted in an ethanol-ethyl acetate solvent system, 11:3, v/v) and the 3-O-hemisuccinate glycyrrhetinic acid (suc-GA) was obtained. Then, SCTS was dissolved in DMF/H2O (3:1, v:v) followed by adding different amounts of suc-GA with EDC/NHS in the solution. After stirring at room temperature for 24 h, the solution was precipitated in acetone, and the precipitate was filtered and dried.
SA–SCTS and CA–SCTS were also prepared in the same way as GA–SCTS, in which SA or CA took place of suc-GA.
LA–SCTS was prepared according to Ref. [27]. SCTS (1.0 g) was dissolved in methanol/water (3:1, v:v) with stirring at room temperature. LA (1.0 g) was added to the reaction mixture and the solution was stirred for 24 h. Then NaBH4 (0.5 g) was slowly added into the solution. After a further 24 h of continuous stirring, the solution was neutralized with HCl. Subsequently, methanol was added to obtain precipitate. The precipitate was filtered and dried in vacuum at 40 °C.
2.3 Characterization of the amphiphilic polymers
1H NMR spectrum was used to analyze the synthesized product and recorded on an NMR spectrometer (Varian Unity-Plus 400) in D2O.
FT-IR spectrum was recorded on a FT-IR spectrometer (Bio-Rad FTS-6000) by mixing the sample with Potassium bromide (KBr).
The molecular weight of SCTS was determined by gel permeation chromatography (GPC) (apparatus: Waters 1515 HPLC pump, Waters 2410 refractive index detector, mobile phase: 0.2 M NaNO3, flow rate: 1.0 mL/min, poly(ethylene glycol) standard).
S content in SCTS was investigated by inductively coupled plasma-atomic emission spectrometer (ICP-AES) (ICP 9000, Jarrell-Ash Division).
The content of GA/SA/CA/LA was determined by elemental analysis (Vanio-EL, Heraeus, Germany). The DS was defined as the number of GA/SA/CA/LA groups per 100 glucosamine units of CTS.
2.4 Critical micelle concentration of the amphiphilic polymers
The CMC was estimated by fluorescence measurement (F-7000 fluorescence spectrophotometer, Hitachi, Japan) using pyrene as a probe [35]. Samples were prepared by adding a certain amount of pyrene in acetone to a series of 20 mL vials, and then removing the acetone by evaporation. Various amounts of the amphiphilic polymer were then added to each vial, and the final pyrene concentration was 6.0 × 10−7 M. The solution was left overnight to equilibrate the pyrene and the micelles. The excitation wavelength was 336 nm, and the emission spectra were recorded ranging from 360 to 460 nm. The intensity ratio of I373/I383 from the emission spectrum was analyzed as a function of the concentration of amphiphilic polymer to calculate the CMC.
2.5 Preparation of doxorubicin-loaded micelles
DOX-loaded micelles were prepared by dialysis. Briefly, 20.0 mg of the amphiphilic polymer was dissolved in 20 mL PBS (pH = 7.4), followed by the addition of different amounts of DOX in 1 mL DMF. The solution was stirred at room temperature overnight, and subsequently dialyzed against distilled water for 48 h using a dialysis membrane (MWCO 7,000). Finally, the DOX-loaded micelles (DOX/GA–SCTS, DOX/SA–SCTS, DOX/CA–SCTS and DOX/LA–SCTS) were collected by lyophilization.
2.6 The size and morphology of micelles
The size and zeta potential of the blank and DOX-loaded micelles were measured by dynamic light scattering (DLS) and laser doppler anemometry using a Zetasizer 3000 (Malvern Instruments, UK) with the concentration of micelles keeping at 1.0 mg/mL.
The morphology of different micelles was observed by transmission electron microscopy (TEM, Philips T20ST).
2.7 Determination of drug encapsulation efficiency and drug loading
DOX-loaded micelles (1.0 mg) were dissolved in 10.0 mL of DMF/H2O (9:1, v:v) and the absorbance of DOX was analyzed by UV–Vis spectrophotometry (UV-4802H, Unic) at λ = 480 nm. A calibration curve was pre-established under the same condition. The loading content (LC) and the encapsulation efficiency (EE) of the micelles for DOX were calculated by Eqs. (1) and (2), respectively.
2.8 In vitro DOX release from micelles
DOX-loaded micelles (5.0 mg) were dissolved in 5.0 mL PBS (pH = 7.4, 5.8). The solution was transferred into dialysis tubing and dialyzed against 30.0 mL PBS at 37 °C under shaking at 90 ± 5 rpm. At predetermined time intervals, 5.0 mL medium out of the dialysis membrane was taken out and replaced with 5.0 mL fresh medium. The drug concentration in the medium was determined by HPLC equipped with a C18 volume (L6-P6, Purkinje General). The mobile phase was a mixture of CH3OH:CH3CN:H2O:H3PO4 = 12:100:112:0.15 (volume ratio), the flow rate was 1.0 mL/min at 25 °C, and the wavelength was set at 232.8 nm. All the tests were performed thrice.
2.9 Cytotoxicity assay
The cytotoxicities of the DOX-loaded micelles against HepG2 cells were evaluated using MTT method [11]. Cells (8.0 × 103) were incubated in each well of a 96-well plate. After incubation for 24 h, the culture medium was replaced by 150 μL of DMEM containing different concentrations of DOX-loaded micelles, and the cells were further incubated for 48 h. The micelle concentration was adjusted to maintain the DOX concentration in the range of 3.9 × 10−3–4.0 μg/mL. Following incubation, cells were washed thrice with the culture medium. Then, 20 μL of MTT solution (5.0 mg/mL) was added. After additional 4 h incubation, the MTT medium was removed from each well, and 150 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals. Then, 50 μL of the solution was taken out, and the optical density (OD) was measured at 570 nm with a microplate reader (Model 550, Bio-Rad, USA). The untreated cells were used as control. The cell viability was calculated as follows:
where A is the absorbance of the cells incubated with the culture medium and B is the absorbance of the cells incubated with the drug-loaded micelles or free drug.
2.10 In vitro cellular uptake
HepG2 cells were cultured in DMEM medium in a 96-well plate at a density of 8 × 103 cells/well. After a 24 h incubation, 150 μL DOX-loaded micelles solution containing 1.5 μg DOX (10 μg/mL) was added. After additional 4 h incubation, the culture medium was removed, and the cells were rinsed three times with PBS prior to fluorescence observation (Olympus, Tokyo, Japan).
To quantitatively study the cellular uptake of the micelles, HepG2 cells were incubated with the DOX-loaded micelles at a DOX concentration of 10 μg/mL in a 6-well plate for 4 h. The cells were washed thrice with PBS, and harvested by trypsinization. The intracellular fluorescence intensity was measured with a flow cytofluorometer (BD FACSCalibur, USA). Approximately 1.0 × 104 cells were counted to determine the trend of micelle uptake by the HepG2 cells.
2.11 Stability assays
To study colloidal stability, DOX-loaded micelles (1.0 mg/mL) were incubated in solutions containing different amounts of Na2SO4 (0–0.6 M) and BSA (0–1.0 mg/mL). The effect of incubation time on the micelle stability in DMEM media was also studied. The size of the micelles after these operations was measured to evaluate the colloidal stability.
The quality of DOX-loaded micelles after long-term storage was investigated by measuring their morphology and size. Briefly, DOX-loaded micelles were stored in a brown bottle at room temperature. At predetermined time intervals, micelles were suspended in PBS (1.0 mg/mL). The morphology and the size of the micelles were investigated. In addition, the morphology of the micelles before and after lyophilization was also observed.
2.12 Statistical analysis
All data expressed as means ± standard deviation (SD) were representative of at least three different experiments. Statistical analysis was performed in Origin 7.0. A two-tailed paired Student’s t-test was used to compare the differences. A value of P < 0.05 was considered statistically significant.
3 Results
3.1 Synthesis and self-aggregation of sulfated chitosan derivatives
The routes of synthesis of the amphiphilic polymers are shown in Scheme 1. The process of sulfation was carried out according to a previously published study using a mixture of H2SO4 and HSO3Cl [34]. It is well known that CTS is vulnerable to acidic solution due to the presence of glycosidic bonds in the backbone. Therefore, it is reasonable to expect that SCTS will have a reduced molecular weight compared to CTS itself. Table 1 shows the molecular weight and the DS of the –SO3 − groups of SCTS prepared at different experimental time.
The structures of the final products were also characterized by IR and NMR. From the IR spectra (Fig. 1), SCTS showed new peaks at 1230 cm−1, 1002 cm−1 and 806 cm−1 compared with CTS, which were attributed to the O=S=O bonds. The peak at 1,076 cm−1 for –OH of CTS disappeared, and the peak at 1,597 cm−1 for –NH2 was not significantly changed in the spectrum of SCTS (1,604 cm−1). These findings indicated that the sulfate groups were introduced mainly at the hydroxyl groups of CTS. After conjugation of SCTS with each hydrophobic molecule, taking GA for example, a peak at 1,728 cm−1, belonging to the carbonyl of the ester bond of suc-GA, appeared in the spectrum of GA–SCTS. In addition, the absorption band at 1,647 cm−1 (amide I) increased, and the peak at 1,604 cm−1 (–NH2) decreased, indicating the formation of new amide bonds. From the NMR spectra (Fig. 2), the conjugations, taking GA for example, showed new peaks at 0.6–1.6 ppm compared with SCTS, which attributed to the protons of CH3, CH2, and CH of GA, indicating that GA molecules were successfully conjugated.
By varying the reaction conditions, several types of amphiphilic polymers were synthesized (Table 2). The number after the sample code X-SCTS is the DS value, for example, GA5–SCTS52 indicates that the DS value of GA is 5 (5 GA groups per 100 glucosamine units) and the DS value of –SO3 − is 52 (52 –SO3 − groups per 100 glucosamine units).
The CMC value is also an important factor for the usage of polymeric micelles as drug carriers. The CMC of each polymer was investigated by fluorescence spectrometry using pyrene as a fluorescent probe. As shown in Table 2, the CMC values were found to be in the range 29–90 μg/mL for each polymer. This low CMC value is beneficial for drug delivery purpose, because it would prevent the micelles from disassociation while diluted in the body fluid, and thus avoid leakage of the encapsulated drug.
3.2 Characterization of doxorubicin-loaded micelles
3.2.1 Size and zeta potential
Doxorubicin-loaded micelles were prepared using a dialysis technique. GA, CA, SA and LA were used as hydrophobic groups to facilitate the encapsulation of DOX. The characteristics of DOX-loaded micelles are listed in Table 3. The size and the zeta potential of micelles are very important properties and play a key role in the fate of micelles in vivo. For cancer treatment, the ideal particle size is between 70 and 200 nm [36]. Except for LA5–SCTS52, the sizes of all other micelles were below 200 nm, which is suitable for cancer treatment. It was also shown that the micelle sizes of GA5–SCTS52 and CA5–SCTS52 (about 150 nm) were smaller than that of LA5–SCTS52 and SA5–SCTS52 (232.3 and 179.6 nm, respectively). It is very interesting that the micelle size decreased from 196.4 to 153.3 nm when the drug feed ratio increased from 0 to 20 %. This phenomenon may due to two reasons. One is the electrostatic interaction between the sulfate group of GA–SCTS and the amino group of DOX. With the addition of DOX, more and more –SO3 − groups are combined with DOX, which decreases the repulsion between the sulfate groups and results in the shrinkage of the micelles. The other reason is the hydrophobicity of DOX. The hydrophobic interaction between DOX and the hydrophobic segments could make the micelle core denser, leading to a decrease in micelle size.
Zeta potential gives an indication of the potential stability of the colloidal system. A large negative or positive zeta potential in the suspension diminishes the aggregation behavior of the particles. It was reported that a value below −30 mV (or above +30 mV) indicated a stable colloidal dispersion [37, 38]. The zeta potentials of all the drug-loaded micelles were around −30 mV. This was beneficial for the colloidal stability of the micelles in aqueous solution.
3.2.2 Drug encapsulation and in vitro release
From Table 3 it can be seen that the LC and EE were significantly affected by the feed ratio, the DS values of the hydrophobic and hydrophilic groups, and the category of the hydrophobic group used. Firstly, LC increased as the feed ratio increased from 5 to 20 %, LC increased from 3.56 to 12.04 % for the GA5–SCTS52 micelles, while EE decreased from 74.76 to 62.13 %. Secondly, increase in hydrophobicity resulted in higher LC and EE. LC values were 10.63 and 12.04 %, and EE values were 54.21 and 62.13 % for the GA2–SCTS52 and GA5–SCTS52 micelles, respectively. Thirdly, it appeared that the introduction of more –SO3 − groups also improved the LC and EE. The GA5–SCTS80 micelles resulted in the highest LC (13.99 %) and EE (83.10 %) among the drug-loaded micelles prepared under identical conditions. Fourthly, the type of hydrophobic segment slightly altered the LC and the EE. The multi-ring structure of the hydrophobic groups supported relatively higher LC and EE: e.g., GA5–SCTS52, CA5–SCTS52 and SA5–SCTS52 micelles. Finally, the longer hydrocarbon chains in the hydrophobic groups greatly increased the LC and EE. The LC and EE were about 5.47 and 32.82 % for the LA5–SCTS52 micelles, respectively. These values increased to 10.61 and 42.65 %, respectively, when the hydrophobic group LA was replaced with SA.
The release profiles of DOX from micelles are shown in Fig. 3. DOX/GA5–SCTS52 and DOX/CA5–SCTS52 micelles released about 40 % of the drug in 24 h at pH 7.4, and DOX/SA5–SCTS52 micelles released 58 % under the same condition. The DOX release rates for DOX/GA5–SCTS52 and DOX/CA5–SCTS52 micelles were slower than that for DOX/SA5–SCTS52 micelles, and DOX/LA5–SCTS52 had the lowest release rate of all the micelles. It was also noted that the rate of DOX release was faster at pH 5.8. Sixty percent of the total drug diffused into the medium from GA5–SCTS52 micelles in 24 h at pH 5.8; however, only 40 % of the drug was detected in the medium at pH 7.4 in 24 h.
In any given condition, the release process of a drug from each micelle can be divided into two stages, with a rapid release over the first 12 h, followed by a slow release. DOX can be associated with micelles in three different states [12]: (1) adsorbed on or near to the surface of the micelles; (2) in the core as a reversible complex with the polymer matrix; or (3) in the core as an irreversible complex with the polymer matrix. Fast release is attributed to DOX located at or close to the surface of the micelles, whereas the subsequent slow release is probably the result of the release of drug molecules entrapped in the core of the micelles. From Fig. 3, it is obvious that a considerable amount of the drug was not released. These unreleased DOX molecules were assumed to be well entrapped within the micelles, and tightly associated with the micelles by hydrophobic and electrostatic interactions. Therefore, degradation of the micelles might be required to accomplish the complete release. Unfortunately, we have no way of confirming this hypothesis since DOX degradation has only been confirmed after long-term incubation in the medium and by chitosanase treatment [39].
3.3 In vitro cell assays
Biocompatibility is one of the most important prerequisites of biomaterials. The toxicity of the polymers against HepG2 cells was evaluated using the MTT assay. As shown in Fig. 4a, cell viabilities in the presence of these polymers were more than 90 %, even at high concentration (250 μg/mL). This indicated that these polymers have low toxicity and are potential drug carriers.
In this work, an in vitro cytotoxicity assay was used to demonstrate the retention of drug bioactivity after encapsulation. The cytotoxicity of these micelles against HepG2 cells is shown in Fig. 4b. The cells were incubated with the DOX-loaded micelles at a dose equivalent to free DOX. The IC50 (50 % inhibitory concentration) values of free DOX, DOX/GA5–SCTS52, DOX/CA5–SCTS52, SA5–SCTS52 and DOX/LA5-SCTS52 micelles were 57.9, 54.7, 87.0, 96.0 and 114.0 ng/mL, respectively. These findings indicated that DOX maintained its bioactivity after encapsulation.
3.4 Stability assay
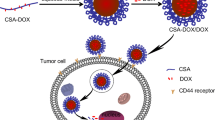
The development of a drug delivery system usually faces several challenges, and one of these challenges is the stability in blood circulation. The aggregation and dissociation of micelles by adsorbing proteins or being diluted after administration would induce a rapid clearance from plasma and leakage of the encapsulated drug, both of which are disadvantageous for drug delivery. Thus, particle sizes in various solutions containing high salt or protein concentrations were investigated to assess the stability of micelles. Of the four types of micelles prepared in this work, GA–SCTS micelles had the best capability to solubilize DOX. In addition, due to the special interaction between GA and its receptors on hepatocytes, GA-modified micelles or nanoparticles also have the ability to target liver cells [31, 40, 41]. Thus, the stability of DOX/GA–SCTS was investigated further. Figure 5a shows the stability of DOX/GA5–SCTS52 micelles in Na2SO4 solutions. The micelles were very stable in solutions with a low Na2SO4 concentration. Negligible changes in size were found at Na2SO4 concentrations below 0.2 M. Although aggregation occurred in solutions with a higher Na2SO4 concentration (≥0.4 M), this does not limit their application in vivo, as the Na+ concentration in blood is about 0.14 M (NaCl is the main component of the electrolytes in blood). Particle size was studied as a function of the amount of BSA in PBS and as a function of the incubation time in RPMI media. As shown in Fig. 5b, no aggregation was observed even at high BSA concentrations. These findings indicated that these micelles were less susceptible to aggregation and non-specific interaction with BSA, as a result of their negative surface charge acting as a protective layer to prevent any non-specific adsorption of protein [32]. Conversely, particle size was gradually reduced as BSA concentration increased. Similar results have been reported in previous studies [42]. This may due to the small size of BSA (about 15 nm) which contributed to the calculation of particle size, leading to a decreased trend in the size of the micelles as the BSA concentration increased. It was noted that the size remained almost constant up to 180 min (Fig. 5c) and tended to increase slightly after 24 h incubation in RPMI media.
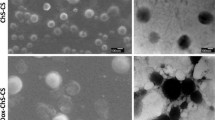
Lyophilization is an effective method of collecting the drug-loaded micelles. However, during lyophilization the structure of the micelles may be destroyed, and therefore excipients such as saccharide/polyol, polymers (PVP, PEG), surfactants or amino acids are used to maintain the structure of the particles. In this work, the DOX-loaded micelles were collected by lyophilization without using any cryoprotectants. The morphology of the DOX/GA5–SCTS52 micelles before and after lyophilization was shown in Fig. 6a, b. Micelles maintained their regular and spherical shape after lyophilization, and the diameter changed negligibly. These results also indicated that the DOX/GA5–SCTS52 micelles had high stability which is beneficial during long-term storage. As shown in Fig. 5d, small fluctuations in size and zeta potential were observed during shortage. The sizes were 153.3, 159.2, 159.4, 169.2, 169.6 173.9 and 180.0 nm at 0, 1, 2, 3, 4, 5 and 6 months of storage respectively. The micelles also had a regular spherical shape (Fig. 6c) after 6 months of storage.
These colloidal stability results revealed that DOX/GA5–SCTS52 micelles had high stability and would be an excellent drug carrier.
4 Discussions
4.1 Synthesis and self-aggregation of sulfated chitosan derivatives
In this work, the amphiphilic polymers were obtained by coupling sulfate to the hydroxyl groups of CTS to prepare SCTS. The final products were then acquired following modification with several hydrophobic molecules. According to previous studies, sulfation occurred mainly at the hydroxyl groups of CTS [34], thus, the unreacted amino groups in the backbone of CTS made it possible and easy to chemically conjugate various groups to confer multifunctionality to the material. In this study, GA, CA, SA and LA were chosen to modify SCTS to confer micellar properties to the polymer.
From Table 2, it can be seen that the hydrophobic and hydrophilic segments significantly affected micelle behaviors. The increase in hydrophobicity following the introduction of more identical hydrophobic groups reduced the CMC value. The CMC values were 90.8 and 22.3 μg/mL for GA2–SCTS52 and GA5–SCTS52 micelles, respectively, and the DS values ranged from 2.12 to 4.73. An increase in the number of hydrophilic groups, however, produced the opposite results. The greater the number of hydrophilic groups, the higher were the CMC values. For instance, the CMC values were 22.33 and 78.46 μg/mL for GA5–SCTS52 and GA5–SCTS80 micelles. In addition, the CMC value was dependent on the type of hydrophobic group. The addition of GA and CA, with multiple ring structures, resulted in lower CMC values (22.33 and 29.87 μg/mL, respectively). However, the linear compounds SA and LA resulted in higher CMC values (77.39 and 79.18 μg/mL, respectively). This may be the result of the more hydrophobic and rigid nature of the compounds with the multi-ring structure compared with the linear compounds.
4.2 Characterization of doxorubicin-loaded micelles
It can be seen that the hydrophilic segments significantly affected the drug encapsulation and release profiles. The reason for the increased LC and EE and decreased drug release from the micelles with more hydrophilic groups may be the molecular structures of DOX and SCTS. The electrostatic interactions between the –SO3 − groups of SCTS and the amino group of DOX play a role in the association between drug molecules and drug carriers. In a previous report [43], Kim et al. showed that electrostatic interactions caused DOX binding to the ionic core of micelles, and an increase in low molecular mass electrolyte concentration weakened DOX binding with anionic groups, which resulted in a faster release of DOX. DOX encapsulation and release also depended on the number of hydrophobic groups. The more hydrophobic groups in the polymer, the more drug molecules could be entrapped by hydrophobic interactions, leading to a lower release rate and higher encapsulation. The category of the hydrophobic group can also affect drug loading and release profile. This may be attributed to the rigid structure of GA and CA which offered more hydrophobic force than that of the linear structure (SA and LA). In our previous work, we used a molecular docking method (AutoDock 4.0) to simulate the interactions between DOX and polymers. The results of this study proved the existence of electrostatic interactions between the amino group of DOX and the sulfonate group of SCTS, and hydrophobic interactions between the multi-ring structure of DOX and hydrophobic segment of the polymers (data not shown here).
It is apparent from Fig. 3 that DOX/LA5–SCTS52 had the lowest release rate among the micelles studied. This result contradicted the rationales given above. It is possible, however, that DOX/LA5–SCTS52 micelles had the lowest LC (5.47 %) of the micelles prepared under identical conditions, and there were more negatively charged groups (–SO3 −) available in the micelles to retain DOX through electrostatic interactions [43].
The accelerated DOX release from these micelles in an acidic environment was probably because of the lower pH which weakened the drug-polymer interaction by causing partial protonation of the sulfate groups in the micelles, and increased the solubility of DOX, both of which were favorable for the faster release of the drug.
4.3 Cell toxicity of the drug-loaded micelles
It is very interesting that DOX/GA5–SCTS52 micelles had the best anti-cancer cell proliferation activity of the micelles studied. Although drug release from the micelles was incomplete, DOX/GA5–SCTS52 micelles were able to slow HepG2 cell proliferation relative to the free DOX solution. The IC50 for free DOX and DOX/GA5–SCTS52 micelles was 57.9 and 54.7 ng/mL, respectively, which was significantly lower than that for other micelles. Considering the similar size and zeta potentials of these micelles, we assumed that the main reason for the difference in cytotoxicity was that the DOX/GA5–SCTS52 micelles had a high affinity for hepatocytes, due to the abundant GA receptors on hepatocyte membranes [31]. Figure 7a shows the fluorescence microscopy images of HepG2 cells following incubation with the DOX-loaded micelles for 4 h. A strong DOX fluorescence was observed in the cells after incubation with the DOX/GA5–SCTS52 micelles. In contrast, a relatively weak fluorescence was observed with HepG2 cells incubated with the other micelles. The mean fluorescence intensity of the cells treated with the DOX/GA5–SCTS52 micelles was approximately 1.8–2.4-fold higher than that obtained with the other micelles (Fig. 7b). This is in agreement with the earlier results indicating that GA-modified carriers have the ability to target the liver or hepatocytes [40, 44–46].
5 Conclusions
In this study, four types of DOX-loaded polymeric micelles based on hydrophobically-modified SCTS were prepared using a simply dialysis process. Of these micelles, the GA–SCTS micelles had the highest encapsulation capability for DOX and greater storage stability. The cellular uptake of DOX/GA–SCTS micelles by HepG2 cells was approximately 1.8–2.4-fold higher than that of the other micelles. These advantages were attributed to the hydrophobicity of the multiple ring structures and liver-target ability of GA.
References
Blum RH, Carter SK. Adriamycin. Ann Intern Med. 1974;80(2):249–59.
Carter SK. Adriamycin—thoughts for the future. Cancer Chemother Rep. 1975;63:1833–77.
Bally MB, Nayar R, Masin D, Cullis PR, Mayer LD. Studies on the myelosuppressive activity of doxorubicin entrapped in liposomes. Cancer Chemother Pharm. 1990;27(1):13–9.
Bao A, Goins B, Klipper R, Negrete G, Phillips WT. Direct 99mTc labeling of PEGylated liposomal doxorubicin (Doxil) for pharmacokinetic and non-invasive imaging studies. J Pharmacol Exp Ther. 2004;308(2):419–25.
Lasic DD. Doxorubicin in sterically stabilized liposomes. Nature. 1996;380(6574):561–2.
Safra T, Muggia F, Jeffers S, Tsao-Wei DD, Groshen S, Lyass O, Henderson R, Berry G, Gabizon A. Pegylated liposomal doxorubicin (doxil): reduced clinical cardiotoxicity in patients reaching or exceeding cumulative doses of 500 mg/m(2). Ann Oncol. 2000;11(8):1029–33.
Sun Y, Chen L, Yu J, Zhi X, Tang S, Zhou P, Wang C. Folate-bearing doxorubicin-loaded magnetic poly(N-isopropylacrylamide) microspheres as a new strategy for cancer therapy. Anti-Cancer Drug. 2009;20(7):607–15.
Yang X, Chen L, Huang B, Bai F, Yang X. Synthesis of pH-sensitive hollow polymer microspheres and their application as drug carriers. Polymer. 2009;50(15):3556–63.
Lim S-H, Jeong Y-I, Moon K-S, Ryu H–H, Jin Y-H, Jin S-G, Jung T-Y, Kim I-Y, Kang S–S, Jung S. Anticancer activity of PEGylated matrix metalloproteinase cleavable peptide-conjugated adriamycin against malignant glioma cells. Int J Pharm. 2010;387(1–2):209–14.
Seymour LW, Ferry DR, Anderson D, Hesslewood S, Julyan PJ, Poyner R, Doran J, Young AM, Burtles S, Kerr DJ. Hepatic drug targeting: phase I evaluation of polymer-bound doxorubicin. J Clin Oncol. 2002;20(6):1668–76.
Janes KA, Fresneau MP, Marazuela A, Fabra A, Alonso MJ. Chitosan nanoparticles as delivery systems for doxorubicin. J Control Release. 2001;73(2–3):255–67.
Lin A, Liu Y, Huang Y, Sun J, Wu Z, Zhang X, Ping Q. Glycyrrhizin surface-modified chitosan nanoparticles for hepatocyte-targeted delivery. Int J Pharm. 2008;359(1–2):247–53.
Jain A, Agarwal A, Majumder S, Lariya N, Khaya A, Agrawal H, Majumdar S, Agrawal GP. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Control Release. 2010;148(3):359–67.
Kang KW, Chun M-K, Kim O, Subedi RK, Ahn S-G, Yoon J-H, Choi H-K. Doxorubicin-loaded solid lipid nanoparticles to overcome multidrug resistance in cancer therapy. Nanotechnol Biol Med. 2010;6(2):210–3.
Kim JO, Sahay G, Kabanov AV, Bronich TK. Polymeric micelles with ionic cores containing biodegradable cross-links for delivery of chemotherapeutic agents. Biomacromolecules. 2010;11(4):919–26.
Liu J, Huang W, Pang Y, Zhu X, Zhou Y, Yan D. Self-assembled micelles from an amphiphilic hyperbranched copolymer with polyphosphate arms for drug delivery. Langmuir. 2010;26(13):10585–92.
Arimura H, Ohya Y, Ouchi T. Formation of core-shell type biodegradable polymeric micelles from amphiphilic poly(aspartic acid)-block-polylactide diblock copolymer. Biomacromolecules. 2005;6(2):720–5.
Sun H, Guo B, Cheng R, Meng F, Liu H, Zhong Z. Biodegradable micelles with sheddable poly(ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials. 2009;30(31):6358–66.
Yoo HS, Park TG. Folate receptor targeted biodegradable polymeric doxorubicin micelles. J Control Release. 2004;96(2):273–83.
Despa F, Luo JT, Li J, Duan Y, Lam KS. Cholic acid micellesa—controlling the size of the aqueous cavity by PEGylation. Phys Chem Chem Phys. 2010;12(7):1589–94.
Kim C, Lee SC, Kang SW, Kwon IC, Kim Y-H, Jeong SY. Synthesis and the micellar characteristics of poly(ethylene oxide)–deoxycholic acid conjugates. Langmuir. 2000;16(11):4792–7.
Cho YW, Park SA, Han TH, Son DH, Park JS, Oh SJ, Moon DH, Cho K-J, Ahn C-H, Byun Y, Kim I-S, Kwon IC, Kim SY. In vivo tumor targeting and radionuclide imaging with self-assembled nanoparticles: mechanisms, key factors, and their implications. Biomaterials. 2007;28(6):1236–47.
Choi KY, Chung H, Min KH, Yoon HY, Kim K, Park JH, Kwon IC, Jeong SY. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials. 2010;31(1):106–14.
Nichifor M, Lopes A, Carpov A, Melo E. Aggregation in water of dextran hydrophobically modified with bile acids. Macromolecules. 1999;32(21):7078–85.
Jayakumar R, Nwe N, Tokura S, Tamura H. Sulfated chitin and chitosan as novel biomaterials. Int J Biol Macromol. 2007;40(3):175–81.
Qu G, Yao Z, Zhang C, Wu X, Ping Q. PEG conjugated N-octyl-O-sulfate chitosan micelles for delivery of paclitaxel: in vitro characterization and in vivo evaluation. Eur J Pharm Sci. 2009;37(2):98–105.
Yao Z, Zhang C, Ping Q, Yu L. A series of novel chitosan derivatives: synthesis, characterization and micellar solubilization of paclitaxel. Carbohydr Polym. 2007;68(4):781–92.
Besheer A, Hause G, Kressler J, Mäder K. Hydrophobically modified hydroxyethyl starch: synthesis, characterization, and aqueous self-assembly into nano-sized polymeric micelles and vesicles. Biomacromolecules. 2007;8(2):359–67.
Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, Cheng RH, Wang L, Lam KS. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–16.
Ye Y-Q, Yang F-L, Hu F-Q, Du Y-Z, Yuan H, Yu H-Y. Core-modified chitosan-based polymeric micelles for controlled release of doxorubicin. Int J Pharm. 2008;352(1–2):294–301.
Negishi M, Irie A, Nagata N, Ichikawa A. Specific binding of glycyrrhetinic acid to the rat liver membrane. Biochim Biophys Acta Biomembr. 1991;1066(1):77–82.
Du Y-Z, Wang L, Yuan H, Wei X-H, Hu F-Q. Preparation and characteristics of linoleic acid-grafted chitosan oligosaccharide micelles as a carrier for doxorubicin. Colloid Surf B. 2009;69(2):257–63.
Hu F-Q, Ren G-F, Yuan H, Du Y-Z, Zeng S. Shell cross-linked stearic acid grafted chitosan oligosaccharide self-aggregated micelles for controlled release of paclitaxel. Colloid Surf B. 2006;50(2):97–103.
Terbojevich M, Carraro C, Cosani A, Focher B, Naggi AM, Torri G. Solution studies of chitosan 6-O-sulfate. Die Angew Makromol Chem. 1989;190(11):2847–55.
Yuan X-B, Li H, Yuan Y-B. Preparation of cholesterol-modified chitosan self-aggregated nanoparticles for delivery of drugs to ocular surface. Carbohydr Polym. 2006;65(3):337–45.
Gaumet M, Vargas A, Gurny R, Delie F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69(1):1–9.
Cunningham D, Littleford RE, Smith WE, Lundahl PJ, Khan I, McComb DW, Graham D, Laforest N. Practical control of SERRS enhancement. Faraday Discuss. 2006;132:135–45.
Dilnawaz F, Singh A, Mohanty C, Sahoo SK. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials. 2010;31(13):3694–706.
Missirlis D, Kawamura R, Tirelli N, Hubbell JA. Doxorubicin encapsulation and diffusional release from stable, polymeric, hydrogel nanoparticles. Eur J Pharm Sci. 2006;29(2):120–9.
Huang W, Wang W, Wang P, Tian Q, Zhang C, Wang C, Yuan Z, Liu M, Wan H, Tang H. Glycyrrhetinic acid-modified poly(ethylene glycol)-b-poly([gamma]-benzyl l-glutamate) micelles for liver targeting therapy. Acta Biomater. 2010;6(10):3927–35.
Zhang C-N, Wang W, Liu T, Wu Y-K, Guo H, Wang P, Tian Q, Wang Y-M, Yuan Z. Doxorubicin-loaded glycyrrhetinic acid-modified alginate nanoparticles for liver tumor chemotherapy. Biomaterials. 2012;33(7):2187–96.
Son S, Kim WJ. Biodegradable nanoparticles modified by branched polyethylenimine for plasmid DNA delivery. Biomaterials. 2010;31(1):133–43.
Kim JO, Kabanov AV, Bronich TK. Polymer micelles with cross-linked polyanion core for delivery of a cationic drug doxorubicin. J Control Release. 2009;138(3):197–204.
Mao SJ, Bi YQ, Jin H, Wei DP, He R, Hou SX. Preparation, characterization and uptake by primary cultured rat hepatocytes of liposomes surface-modified with glycyrrhetinic acid. Pharmazie. 2007;62(8):614–9.
Tian Q, Zhang C-N, Wang X-H, Wang W, Huang W, Cha R-T, Wang C-H, Yuan Z, Liu M, Wan H-Y, Tang H. Glycyrrhetinic acid-modified chitosan/poly(ethylene glycol) nanoparticles for liver-targeted delivery. Biomaterials. 2010;31(17):4748–56.
Tian Q, Wang X-H, Wang W, Zhang C-N, Wang P, Yuan Z. Self-assembly and liver targeting of sulfated chitosan nanoparticles functionalized with glycyrrhetinic acid. Nanomed Nanotechnol Biol Med. doi:10.1016/j.nano.2011.11.002.
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (No. 50873048, No. 51073080), Key Project of Scientific and Technical Supporting Programs of Tianjin (No. 10ZCKFSY07500), and State Key Fundamental R&D Project (No. 2011CB606202) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiu-Hua Wang and Qin Tian contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Wang, XH., Tian, Q., Wang, W. et al. In vitro evaluation of polymeric micelles based on hydrophobically-modified sulfated chitosan as a carrier of doxorubicin. J Mater Sci: Mater Med 23, 1663–1674 (2012). https://doi.org/10.1007/s10856-012-4627-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-012-4627-1