Abstract
Chitosan (CS) has been widely studied as polycation to form the polyion complex (PIC) micelles, which have emerged as promising delivery systems for sustained release of hydrophilic drugs. However, poor solubility in aqueous solutions limits the application of CS. In this study, trimethyl chitosan (TMC), the partially quaternized derivative of CS, was synthesized and investigated the PIC formation coupled with poly(acrylic acid) (PAA) at different cationic: anionic ratios (named as TMC/PAA micelles). The encapsulation of hydrophilic anticancer drug, doxorubicin (DOX), release profile, and cytotoxicity against murine colon cancer cells were also evaluated. The results showed that the sustained release of DOX from TMC/PAA micelles was confirmed. We found that the release of DOX from TMC/PAA micelles in acidic pH buffer (pH 5.5) was significantly higher than in physiological pH (7.4). Finally, the anticancer activity of DOX-loaded TMC/PAA micelles was remarkably higher than that of DOX. The results in this study suggest the potential application of TMC/PAA micelles as controlled drug delivery for cancer therapy.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Chitosan (CS), a deacetylated derivative of chitin, is a copolymer composed of β-(1,4)-2-acetamido-D-glucose monomer and β-(1,4)-2-amino-D-glucose monomer. CS has attracted great attention in biomedical applications, including the development of a safe drug delivery tool since it exhibits high biocompatibility and biodegradability [1, 2]. However, the well-known limitation of CS is the poor water solubility at neutral and alkaline conditions, and it basically is soluble in only organic acids such as acetic acid [3]. Numerous studies have reported that the modification of chitosan to form new derivatives alters the physical, chemical, and biological properties of original CS. For example, N,N,N-trimethyl chitosan chloride (TMC), which is the partially quaternized derivative of CS, is highly soluble at neutral pH and improves biological activity and physicochemical stability of CS due to the high charge density [4, 5]. Since TMC possesses a highly positive recharge of quaternary amine groups, TMC achieves considerable attention as a drug and gene carrier. For example, TMC is used as polycation coupled with another polyanion to prepare the polyion complex (PIC) micelles improving stability and delivering the therapeutic agents including chemotherapy, protein, or nucleic acids [6, 7].
Doxorubicin (DOX) is well-known as an anticancer chemotherapy used in the treatment of a large variety of cancers. The anticancer mechanism of DOX is to interact with DNA to inhibit the DNA replication via the disruption of the topoisomerase II binding to DNA molecules during DNA synthesis. Besides, DOX releases free radicals that lead to damage to cellular membranes, DNA, and induces intracellular oxidative stress. However, since DOX is a low-molecular-weight agent, DOX distributes non-specially in the target tissues. Therefore, frequent administration of DOX causes multiple side effects consisting of myelotoxicity and haematological toxicity [8, 9], which limits its use in clinical practice.
In this study, TMC was synthesized, and a water-soluble PIC (named TMC/PAAc micelle) was prepared via the electrostatic interactions between TMC and poly(acrylic acid) (PAAc) to deliver DOX for the anticancer application. The DOX release profile from DOX-loaded TMC/PAAc micelle with different ratio of cation: anion polymer at varying physiological pH environments (pH 5.5 and pH 7.4) was investigated. In addition, the antitumor activity of DOX-loaded TMC/PAAc micelle was also evaluated.
2 Materials and Methods
2.1 Synthesis of TMC
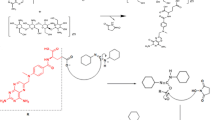
Chitosan (80% deacetylation), iodomethane (CH3I), sodium iodide (NaI), N-methyl-2-pyrrolidone (NMP), isopropanol (IPA), and acetic acid were purchased from Wako Pure Chemical Industries, Ltd., Japan. The TMC was synthesized via the methylation of CS by using CH3I (Fig. 1), as previously described with slight modification [10, 11]. Briefly, 5 g of was dissolved in 120 mL of NMP in an oil bath at 40 °C overnight with stirring. After, 4.8 g of NaI and 11 mL NaOH 15% was added to the reaction for 24 h at 60 °C. The TMC was collected by cold IPA reprecipitation following by dissolving in water and lyophilizing. The product was dissolved in D2O, and the chemical structure was confirmed by 1H NMR (JEOL ECS-400, JEOL Ltd., Tokyo, Japan).
2.2 Preparation of TMC/PAAc Micelle and DOX-Loaded TMC/PAAc Micelle
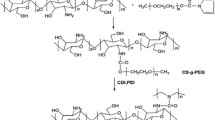
TMC/PAAc micelles were formed by ionic interactions between the positively charged TMC backbone and negatively charged PAAc (Fig. 2). The TMC/PAAc micelle formation were investigated by dissolving PAAc and TMC solution (10 mg/mL) in different ratio of cation and anion polymer (0.25, 0.5, 1, 2, 4 and 10). The average particle size, the size distribution of TMC/PAAc micelle was determined by dynamic lighting scattering (DLS, Malvern Zetasizer, UK) measurements. During the TMC/PAAc nanoparticles forming, DOX (Wako Pure Chemical Industries, Ltd., Japan) at a concentration of 1 mg/mL was slowly added to the micelle solution, followed by stirring to obtain the DOX-loaded TMC/PAAc micelles.
2.3 DOX Release Profile
The in vitro release of DOX from the DOX-loaded TMC/PAAc nanoparticles was investigated by a dialysis method using the membrane tube (Spectrum, molecular weight cut-off 3.5 kDa). In brief, the DOX-loaded TMC/PAAc micelles were added in the membrane tube and dialyzed against a PBS solution at pH 5.5 and 7.4. At predetermined time intervals, the amount of released DOX was measured with a UV–vis spectrophotometer at a wavelength of 490 nm.
2.4 Anticancer Activity In Vitro
The anticancer effect of nanoparticles on the murine colon cancer cell C-26 (RIKEN, Japan) was investigated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphehyltetrazolium bromide (MTT) assay (Roche Diagnostics, Tokyo, Japan), as previously reported [12]. Briefly, C-26 cells were added in 96-well plates with a density of \(1 \times 10^{4}\) cells/well. After 24 h incubation for cell attachment, C-26 cells were treated with DOX-loaded TMC/PAAc micelle or free DOX with different concentrations (0.5, 1, 5, 10 µg/mL) and blank TMC/PAAc micelle (5, 10, 50, 100 µg/mL) for 24 h. Then, treatment solutions were removed, and MTT (0.2 mg/mL) was added to each well for 4 h. Subsequently, MTT crystals were solubilized with solubilization buffer or dimethyl sulfoxide in each well. Thereafter, cell viability was measured using a microplate reader at a wavelength of 540 nm.
Percent cell viability curves using the following formula:
3 Results and Discussion
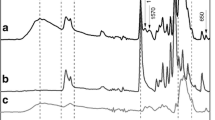
The synthesis of TMC was based on the methylation of CS with CH3I in a strong base solution. After the reaction, the obtained polymer was analyzed using 1H NMR measurement, as shown in Fig. 3. The degree of quaternization (DQ) of TMC was about 24% as compared to the 1H NMR spectrum of CS, and the obtained polymer was highly soluble in water, suggesting the successful synthesis of TMC from CS. The obtained TMC was further used for the preparation of PIC by coupling with polyanion PAAc at different ratios of TMC and PAAc. As shown in Fig. 4, the size of micelles was increased from about 68 nm to 170 nm with the increase in TMC:PAAc ratio. The data suggests that changing different amount of polymer, different sizes of the micelle can be obtained, those sizes of TMC/PAAc micelle provide the potential for accumulation at tumor sites via the enhanced permeability and retention effect [13, 14]. It should be noted that the polydispersity index of all formulations was lower than 0.2, suggesting the narrow size distribution, which is suitable for in vivo study [15].
Next, the release of DOX-loaded TMC/PAAc micelle in vitro was investigated through a dialysis method. As shown in Fig. 5a, the release of DOX was depended on the ratio of TMC and PAAc forming micelles. The micelle with TMC:PAAc ratio of 1:1 exhibited significantly slower release of DOX as compared to micelles with TMC:PAAc ratio of 4:1 and 10:1. This data suggests that cation:anion of 1:1 is the suitable ratio to form the stable micelle since the neutralization of the charged polymers generates the hydrophobic segments to stabilize the core–shell structure of PIC micelle [16, 17]. Over 80% of free DOX molecule released within 2 h while only less than 50% of DOX released from the DOX-loaded TMC/PAAc micelle, suggesting the sustained release of TMC/PAAc micelle system. Interestingly, we also obtained a pH-responsive release profile of DOX at pH 5.5 as compared to pH 7.4 (Fig. 5b). The release of DOX from DOX-loaded TMC/PAAc micelle was significantly higher at pH 7.4 as compared to pH 5.5. At the acidic pH, the electrostatic interaction between TMC and PAAc may be stronger due to the highly protonated quaternary amine of TMC leading to the higher stability of micelles, resulting in the lower release of DOX at acidic conditions. This nano-carrier can be considered to apply for oral drug delivery to suppress the release of drug in the gastric acid environment.
Finally, we investigated the anticancer potential of DOX-loaded TMC/PAAc micelle using a murine colon cancer cell line (C-26). In cytotoxicity assay, treating C-26 cells with blank TMC/PAAc micelle was over 95% cell viability for 24 h at all tested concentrations (Fig. 6), suggesting low toxicity of this nano-carrier. Compared with free DOX, the DOX-loaded TMC/PAAc micelle exhibited significantly higher cytotoxicity on C-26 colon carcinoma cells at a range of concentration from 0.5 to 1 μg/mL, indicating a high potential of DOX@TMC/PAAc micelle in the anticancer treatment. When higher DOX concentrations were used (5 and 10 µg/mL), the cell viability in DOX and DOX-loaded TMC/PAAc was not different due to high anticancer activity of DOX. This result suggested that a suitable dose of DOX should be considered to apply in vitro and in vivo to minimize the toxicity of DOX.
The anticancer activity of DOX-loaded TMC-PAA micelle and free DOX against colon cancer C-26 cell by MTT assay. a Quantitative cell viability (%) was evaluated by comparing cells treated with samples with control. Data was expressed as mean ± SD with n = 3 and *p < 0.05, **p < 0.01. b Phase contrast images of C-26 cells at 24 h after treated with samples
4 Conclusion
In this study, the water-soluble TMC was successfully synthesized by methylation of the amino groups in the water-insoluble CS. The PIC micelle with different sizes could be prepared by changing the ratio of positively recharged TMC and negatively recharge PAAc. The release of DOX was dependent on the charged ratio of TMC/PAAc micelles, and the pH-responsive release DOX from DOX-loaded TMC/PAAc micelle was obtained. The anticancer activity of DOX-loaded TMC/PAAc was significantly higher than that of free DOX treatment in a colon cancer cell. Further investigations, including cellular uptake, toxicity, or side effects, and the anticancer efficacy in the in vivo animal model should be investigated to clearly evaluate the potential of DOX-loaded TMC-PAAc micelle in the cancer therapy.
References
Bowman K, Leong KW (2006) Chitosan nanoparticles for oral drug and gene delivery. Int J Nanomedicine 1(2):117–128
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK (2017) An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 9(4):53
Sogias IA, Khutoryanskiy VV, Williams AC (2010) Exploring the factors affecting the solubility of chitosan in water. Macromol Chem Phys 211(4):426–433
Wu QX, Lin DQ, Yao SJ (2014) Design of chitosan and its water soluble derivatives-based drug carriers with polyelectrolyte complexes. Mar Drugs 12(12):6236–6253
Mourya VK, Inamdar NN (2009) Trimethyl chitosan and its applications in drug delivery. J Mater Sci Mater Med 20(5):1057–1079
Germershaus O, Mao S, Sitterberg J, Bakowsky U, Kissel T (2008) Gene delivery using chitosan, trimethyl chitosan or polyethylenglycol-graft-trimethyl chitosan block copolymers: establishment of structure-activity relationships in vitro. J Control Release 125(2):145–154
Zhou Z, Zhong C, Dongyuan S, Ting X, Xiang L, Fengbo W (2018) Synthesis, antitumor activity and molecular mechanism of doxorubicin conjugated trimethyl-chitosan polymeric micelle loading Beclin1 siRNA for drug-resisted bladder cancer therapy. RSC Adv 8(62):35395–35402
Thorn CF, Oshiro C, Marsh S, Hernandez-Boussard T, McLeod H, Klein TE, Altman RB (2011) Doxorubicin pathways: pharmacodynamics and adverse effects. Pharmacogenet Genomics 21(7):440–446
Patel AG, Kaufmann SH (2012) How does doxorubicin work? eLife 1:e00387
Sieval AB, Thanou MM, Kotzé AF, Verhoef JC, Brussee J, Junginger HE (1998) Preparation and NMR characterization of highly substituted N-trimethyl chitosan chloride. Carbohyd Polym 36:157–165
Ignacio I, Andrew W, Francisco FT (2016) Polyion complex (PIC) particles: preparation and biomedical applications. Eur Polymer J 81:198–215
Vong LB, Nagasaki Y (2016) Combination treatment of murine colon cancer with doxorubicin and redox nanoparticles. Mol Pharm 13(2):449–455
Matsumura Y, Maeda HA (1986) A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res 46(12):6387–6392
Fang J, Nakamura H, Maeda H (2011) The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63(3):136–151
Vong LB, Tomita T, Yoshitomi T, Matsui H, Nagasaki Y (2012) An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology 143(4):1027–1036
Vong LB, Ibayashi Y, Lee Y, Ngo DN, Nishikawa Y, Nagasaki Y (2019) Poly(ornithine)-based self-assembling drug for recovery of hyperammonemia and damage in acute liver injury. J Control Release 310:74–81
Kudo S, Nagasaki Y (2015) Facile and quantitative synthesis of a poly(ethylene glycol)-b-poly(L-arginine) block copolymer and its use for the preparation of polyion complex micelles with polyanions for biomedical applications. Macromol Rapid Commun 36(21):1916–1922
Acknowledgements
This work was supported by the Vietnam National Foundation of Science and Technology Development (NAFOSTED) under grant number 108.05-2017.327.
Conflicts of Interest
The authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Nguyen-Trinh, QN., Trinh, NT., Nguyen, H.T.N., Long, V.B. (2022). Preparation of Trimethyl Chitosan-Based Polyion Complex Micelle as Drug Delivery System for Cancer Therapy. In: Van Toi, V., Nguyen, TH., Long, V.B., Huong, H.T.T. (eds) 8th International Conference on the Development of Biomedical Engineering in Vietnam. BME 2020. IFMBE Proceedings, vol 85. Springer, Cham. https://doi.org/10.1007/978-3-030-75506-5_23
Download citation
DOI: https://doi.org/10.1007/978-3-030-75506-5_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75505-8
Online ISBN: 978-3-030-75506-5
eBook Packages: EngineeringEngineering (R0)