Abstract
Solid electrolytes-based lithium phosphates have achieved a great evolution due to the safety problem in the lithium-ion battery. This work targets to investigate the prepared glasses within the 20Li2O–(50 − x)Li2WO4–xTiO2–30P2O5 system, with 0 ≤ x ≤ 15 mol%. The bonds constituting the framework of these glasses were studied by Raman spectroscopy. The data analysis of the chemical durability showed that the dissolution rates depend on the composition of each glass. Thermal analysis by using the DSC technique was used to determine the activation energy of crystallization, it is found in the glassy composition (x = 5) that Ec= 184.482 kJ/mol. The determinate Avrami parameter is around 1.7, which allows suggesting the mechanism is surface crystallization. The crystallization process of the prepared glasses is carried out by heating samples at a suggested value of temperature around 550 °C for 4 and 12 h, with heating rate of 10 °C/min. The crystallized phases are identified by XRD. The results of the X-ray diffraction analysis confirm that TiO2 acts mainly as network forming units. The crystalline phases Li2WO4 and Li4P2O7 are formed during the crystallization process. The formation of these crystalline phases into the glasses depends on the time of heating at a fixed crystallization temperature. FTIR spectra of the glass–ceramics show nearly the same IR vibrational modes likewise their parent glasses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Glasses and glass–ceramics-based phosphates have attracted the attention of many researchers due to their potential applications in technology, like solid electrolyte [1]. All-solid-state batteries using solid electrolytes are expected to have long battery life [2]. In general, chemical side reactions are inhibited in inorganic solid electrolytes as the lithium ions move through these typical electrolytes. As a result of this, all-solid-state batteries are potentially safer than conventional lithium-ion batteries [3]. Also, they are considered to be ideal batteries for electric vehicles and plug-in hybrid electric vehicles [4]. The solid electrolytes with lithium-ion conductivity are potentially investigated considering their use in power sources [5, 6]. Solid electrolytes-based phosphate materials are good candidates owing to their easy preparation, low melting point, wide glass-forming region, and simple composition [7, 8]. Consequently, in the last decades, scientists paid considerable attention to Li+ ion glassy electrolytes [9, 10].
As well known, the ionic glasses have a wide thermal stability window, but they tend to crystallize at certain temperatures (Tc). For this reason, their direct uses affect the performance of the ionic devices during high-temperature applications [11, 12]. The glass–ceramics derived from glasses present lower conductivity than the parent glasses. This depends on the low ionic conductivity of the precipitated crystallites. Nevertheless, other glass–ceramics exhibit higher conductivity than the pristine glasses of the same compositions, for example, in Ag+ ion-conducting AgI–Ag2O–MoO3 glasses. Also, glass–ceramics obtained from the glass Li2O–Al2O3–TiO2–P2O5 system exhibited higher conductivity than the host glass matrix. Indeed, the high conductivity is attributed to the formation of the LiTi2(PO4)3 phase during crystallization [13]. There are many attempts to optimize ionic conductivity by stabilization the superionic α-phase of ionic compounds in the glass matrix. The first successful attempt has been realized with the stabilization of α-AgI within the glass AgI–Ag2O–B2O3 system by rapid quenching. Exceptionally, at room temperature, α-AgI has been established in glass–ceramics of the AgI–Ag2O–B2O3 system, which resulted in high Ag+ ion conductivity of 10−1 Ω−1 cm−1 [14].
Solid electrolytes-based crystalline phosphate materials are widely investigated in all-solid-state batteries, for example, Li4P2O7. The XRD data analysis showed that Li4P2O7 crystallized in triclinic structure with space group P-1. The linking LiO4 with P2O7 groups allows the formation of a framework with large voids [5]. In this framework, Li+ ions move easily; thus, high conductivity is obtained. In the literature, it reported that Li4P2O7 has a rather high conductivity (~ 10−1 s/m at 925 k) [15]. Kartini et al. studied Li4P2O7 in the Li4P2O7–Li3PO4 composite with the highest content of 93.56 % Li4P2O7. This composite achieved high ionic conductivity of 3.85 10−5 s/m at room temperature. This ionic conductivity is higher in comparison to the single phase of LiPO3, Li3PO4, and Li4P2O7. As a result of this, the composite Li4P2O7–Li3PO4 is a good solid electrolyte for all-solid-state batteries [16].
Another example, Li2WO4 compound crystallized in the monoclinic structure with space group (P2/m) and the lattice parameters: a = 9.753 A, b = 5.954 A, c = 4.994 A, α = γ = 90°, and β = 96.81°. A structural study showed that the presence of some porosity is observed [17]. The presence of porosity is very important for Li+ ions to move into the LiWO4 structure. The electrical conductivity was carried out in the [200–500 °C] temperature range. The electrical measurements showed the Li+ ion conductivity is about 2.18 × 10−7 s/cm at 300 °C, and the activation energy is 0.94 eV [18]. The Li2WO4 and Li4P2O7 ionic conductors have also been reported to exhibit electrical conductivity. Nevertheless, according to our best knowledge, there is no work dealing with the Li2WO4–Li4P2O7 compound. Our attempts focused on precipitating Li2WO4 and Li4P2O7 crystallites in Li2O–Li2WO4–TiO2–P2O5 glasses [18]. The precipitation of these crystallites would greatly enhance the ionic conductivity of glass–ceramics. This study was performed to stabilize single or more crystalline phases already known for their high ionic conductivity.
The aim of the work is to investigate the prepared glasses within the Li2O–Li2WO4–TiO2–P2O5 system. Their characterization was carried out by Raman spectroscopy and chemical durability tests. The crystallization kinetic study was performed by using thermal analysis. The glass–ceramics are characterized by X-ray diffraction and Infrared spectroscopy (IR).
2 Experimental procedure
2.1 Samples preparation
The phosphate glasses of the 20Li2O–(50 − x)Li2WO4–xTiO2–30P2O5 system (x = 0, 5, 8, 10, and 15 mol%) were prepared from the raw materials (purchased from Sigma–Aldrich, USA): lithium carbonate (Li2CO3), titanium trioxide (TiO2), lithium tungstate Li2WO4, and hydrogen ammonium phosphate (NH4H2PO4). The detailed experimental protocol of preparing glasses was described in our previous work [18]. The amorphous state of these colorless glasses was verified by XRD and confirmed by Raman spectroscopic analysis. Glassy samples were subjected to controlled thermal heat treatment through two-step regime. The glasses were heat treated at a rate of 10 °C/min to reach at temperature around 550 °C for 4 h and 12 h, sufficient to provide sufficient nucleation sites. The experimental processes to obtain glass–ceramics have summarized according to the schematic diagram in Fig. 1. The Muffle Furnace (Model LT 9/12, Nabertherm, Italy) with the samples inside was switched off and then left to cool to room temperature at a rate of 1 °C/min. These specific temperatures were collected from DSC measurements. The obtained glass–ceramics were analyzed by an X-ray Diffractometer (Model D5000, Bruker, USA) in the range of 2θ from 10° to 80°. The X-ray data were analyzed by X’Pert High Score Plus software to identify the crystalline phases.
2.2 Raman spectroscopy
The structural properties of the prepared glasses were studied by Raman spectroscopy. The Raman data are obtained at room temperature by using spectrometer (Model T64000, Horiba, Germany). The spectra were measured in backscattering geometry, under excitation with He–Ne laser radiation (632.8 nm) at a power of 12 mW. The exposure time was 3 s, the accumulated number was 10, and the spectral slit width was 1 mm.
2.3 Durability testing
Chemical durability test of the glasses is carried out to determine the dissolution rate in distilled water at 25 °C. The prepared blocks of the glasses were placed into a bottle full of distilled water with pH 6.8. The bottles are suspended in an Electrical Thermostatic Water Bath (Model HH-420, BILON, China) kept at 30 °C for 250 h. The dissolution rate, DR, is calculated from the equation DR = Dx/(S.t), where Dx is the mass loss (g), S is the glass surface (cm2) before the dissolution test, and ‘t’ is time of immersion (min). The pH value of leaching for each glass is measured every 24 h by a pH meter (Model Consort™ C3010, Thermo Fisher Scientific, USA).
2.4 Crystallization kinetics
The crystallization kinetics of the glasses is studied by differential scanning calorimetry (DSC). The fine grain powdered sample (< 50 μm) of about 40 mg was placed in an aluminum crucible and heated at a rate of 5 °C/min, 8 °C/min, 10 °C/min, and 12 °C/min using a DSC (Model 131 Evo analyzer, Thermo Fisher Scientific, UK) from ambient temperature to 550 °C. The obtained data are investigated to determine the peak crystallization temperature (Tp).
2.5 Infrared spectroscopy
The structural units formed the glass–ceramics were analyzed by a Fourier Transform Infrared (FTIR) spectrometer (Model Tensor 27, Bruker, UK). The IR spectra were recorded at room temperature in the frequency range (400–1500) cm−1. The samples were ground to a fine powder and then mixed with KBr powder to prepare pellets for this study.
3 Results and discussion
3.1 Raman spectroscopy
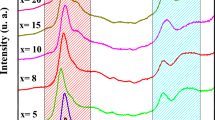
Figure 2 presents the Raman spectra of the phosphate 20Li2O–(50 − x)Li2WO4–xTiO2–30P2O5 glasses recorded in the wavenumber region 1200–150 cm−1. The band positions and their assignments are gathered in Table 1. The Raman spectra show different bands situated at different positions: 1055 cm−1, 930 cm−1, 870 cm−1, 740 cm−1, 635–510 cm−1, 372 cm−1, and 250 cm−1. The assignments of these bands were made by comparing with the literature data [19,20,21,22,23,24]. From the band positions, we divided the Raman spectra into the three wavenumber regions:
-
i)
In the high wavenumber region, there is a band of low intensity at the position 1055 cm−1. This band is attributed to the asymmetric vibration νas(P–O−), Q1 units. The band at 930 cm−1 of high intensity is assigned to the asymmetric vibration of the bond P–O− into Q0 units or to the symmetric vibration M–O− in a polyhedron with (M = W, Mo).
-
ii)
For the intermediate region, a band the low intensity at 870 cm−1 it observed associated with the symmetric vibration mode of the bond W–O bond of the WO6 polyhedron and/or W–O–P bond. Also, a band of low intensity is situated at the position 740 cm−1 attributed to the symmetric vibration of the P–O–P bond of the Q1 unit.
-
iii)
In the low wavenumber region, the bands situated between 635 and 250 cm−1 could be attributed to the stretching mode of the [PO4] units.
After the substitution by TiO2 in the glassy composition (x = 5), two supplementary bands are observed. These bands are situated around the positions 914 cm−1 and 742 cm−1 attributed to the TiO4 and TiO6, respectively [25]. At TiO2 content increased, Raman spectra change, especially in the region of low frequencies. This shows that several structural units are present in the various networks. Besides, there is a considerable change in the intensity of the structural units of TiO4. Such variations suggest that titanium ions occupy distorted octahedral positions and modify the vitreous matrix increasing the number of non-bridging oxygens (NBO). When TiO2 is present in larger quantities (x > 8), the P=O bond can be broken by structural units of TiO4, which can cause the creation of new NBO ions facilitating the formation of structural units [TiO6/2]2− [26]. The analysis of Raman spectra shows that the degree of depolymerization of the glass lattice and the quality of TiO6 structural units increases with the increasing TiO2 content.
3.2 Chemical durability
3.2.1 Weight loss
Chemical durability is expressed by the resistance of the glasses to attack chemical species found in a solution. This attack takes place through the process of ion exchange in the hydrated layer. When the chains are completely surrounded by water, they can separate from the partially hydrated chains and dissolve in solution. Once the hydrated layer is constant, the dissolution is linear over time [27].
Figure 3 represents the evolution of the weight loss at the initial surface (g cm−2) of the glass as a function of time (h). From Fig. 3, it is noted that the glasses present similar dissolution behavior. We can clearly differentiate two stages or two phases: (i) t < 100 h, the dissolution is almost linear. (ii) 100 h < t < h250h, where the dissolution rate slows down. The substitution of Li2WO4 by TiO2 has an observable effect on the dissolution behavior of the synthesized glasses. The dissolution of the glass (x = 0 mol%) is characterized by an initial dissolution, faster than that of the other glass. The solubility of the studied glasses depends on the degree of substitution of Li2WO4 by TiO2. Indeed, the introduction of TiO2 in the glass network leads to improved chemical durability of the network. In our similar study, we reported that incorporating Fe2O3 into the vitreous network leads to improve the chemical durability, which was explained by the formation of a new bond P–O–Fe in place of P–O–P [28]. By analogy, TiO2 also leads to the enhancement of the chemical durability of the network by the formation of bonds of the type P–O–Ti. These bonds are identified by Raman spectroscopy. These results agree with the thermal analysis, which showed that the introduction of TiO2 into the phosphate glass network makes the glass matrix harder.
The correlation between chemical durability and thermal analysis confirms that the introduction of TiO2 into the glass network leads to the strengthening of the chains, constituting the glass matrix of the Li2O–Li2WO4–TiO2–P2O5 systems [29].
3.2.2 pH variation
Figure 4 illustrates the evolution of the pH as a function of the immersion time in distilled water (pH ~ 6.8) at a temperature of 30 °C. The curves showed two zones: (i) before 48 h, there is a sudden decrease in pH curves; (ii) after 48 h, the pH value is almost constant. This variation in pH depends on the chemical composition of the glass. We can say that the alkali ions and the phosphate ions that constitute the glass surface have been released into the solution. This ion exchange plays a major role which that defines the acid-base character of the solution. In fact, decreasing pH curves can be attributed to the disintegration of the phosphate entities. The variation of pH depends on the formation of phosphoric acid controlled by the entities H2PO4− in water [30,31,32,33].
At TiO2 content increases, it is observed that the pH of the solution becomes smaller than the initial solution. These results indicate that the introduction of TiO2 into the glass network greatly improves chemical durability. In terms of this chemical study, it is believed that the corrosion mechanism of glasses occurs in two phases: (i) a rapid alteration phase where the thermodynamic imbalance between the glass and the altering solution is great, which creates a sudden decrease in pH; (ii) a corrosion slowing phase, which is associated with the saturation of the altering solution. This leads to the formation of a protective layer against diffusion through the surface of glass.
3.3 Crystallization kinetics by DSC
The crystallization kinetics have suggested calculating parameters of activation energy (Ec) and Avrami exponent (n). The activation energy (Ec) of crystallization was calculated by using the following modified form of Kissinger Eq. (1), established by Matusita and Saka [34]:
where β, R and Tp are the heating rate, the universal gas constant (8.314 J/mol k), and the crystallization peak, respectively. Different heating rates β (5 °C/min, 8 °C/min, 10 °C/min, 12 °C/min) are deliberated to study the crystallization kinetics of the prepared glasses. The crystallization activation energy Ec is determinate to review the crystallization kinetics mechanism of glasses.
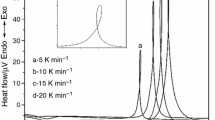
Figure 5 plots the DSC curves for the glassy composition x = 5 mol% obtained at different heating rates β. The DSC curves present a broad crystallization peak shifted to high temperature, with an increase in the heating rate. The obtained crystallization temperature (Tp) for the glass (x = 5 mol%) is listed in Table 2. The plot of ln(Tp2/β) versus 1/Tp is shown, a straight line is obtained (see Fig. 6), and from its slope, the value of Ec can be determined. The obtained value of the activation energy (Ec) is 184.482 kJ/mol. This determinate activation energy relies on the bond energy of the glassy framework. In submitting a material to heat treatment, its weak bonds destroy. Remarkably, stable crystalline phases can be precipitated into heated glasses. The studied glassy network consists of the bonds Li–O, P–O, Ti–O, and W–O, respectively, their bond energies are 333.5 kJ/mol, 599 kJ/mol, 666.5 kJ/mol, and 720 kJ/mol [35]. At first glance, we bet on destroying the Li–O bonds, because it needs low energy in comparison with other bonds. Therefore, the crystallization of crystalline phases in the glass–ceramics is expected to the break of the Li–O bonds (see the section of the X-ray diffraction of glass–ceramics). Additionally, the crystallization kinetics mechanism could be clear by using the Avrami parameter n calculated by applying the Augis–Bennett Eq. (2) [36]:
where ΔT is the full width of the exothermic DSC peak at the half-maximum intensity, and (n) is the Avrami exponent or crystallization index. The Avrami exponent (n) had indicated the nucleation and growth mechanism. According to the Johnson–Mehl–Avrami (JMA) theory, (n) was also related to crystallization pattern, n = 2 means that the surface crystallization dominants the overall crystallization, n = 3 means two-dimensional crystallization, n = 4 means that three-dimensional crystallization for bulk materials [37,38,39]. The value calculated for (n) is very close to 1.7, suggesting that surface crystallization is dominant during the crystallization of the glasses [40, 41].
3.4 X-ray diffraction of glass–ceramics
From the DSC analysis, it has been suggested the crystallization temperature around 550 °C, as an average value of the analyzed glasses (x = 0, 5, 8, 10, 15 mol%), where the crystallization temperature is picked at the maximum of the crystallization peak of each DSC curve. This temperature is determinate for the compositions x = 0 mol% (about 503 °C) and x = 5 mol% (around 532 °C). But, for the compositions x = 8, 10, and 15 mol%, the crystallization temperature is not determinate in the 500–575 °C region [18]. As a result of the treatment temperature, which fixed at 550 °C (suggested crystallization temperature), it has obtained glass–ceramics. The crystalline phases formed into heated glasses are identified by using X-ray diffraction. Figures 7 and 8 correspond to the X-ray patterns of glass–ceramics derived from the glasses of the 20Li2O–(50 − x)Li2WO4–xTiO2–30P2O5 system, with 0 ≤ x ≤ 15 mol%. The X-ray patterns gathered in Fig. 7 showed the crystallization of crystalline phases into the compounds (x = 0 and 5) Li2WO4 (JCPDS# 01-072-0086) and Li4P2O7 (JCPDS# 01-087-0409) during 4 h. These two formed crystalline phases are characterized by low intensity of the peaks. At high TiO2 content (x = 8, 10, and 15 mol%), it observed no crystalline phase formed. Nevertheless, at increasing the heating duration of 12 h, it observed that the intensity of the peaks is slightly increased in crystalline phases Li2WO4 (JCPDS# 01-072-0086) and Li4P2O7 (JCPDS# 01-087-0409) for the compositions (x = 0 and 5). But, in the compositions (x = 8, 10, and 15), there are peaks appeared which corresponding to the crystalline phases Li4P2O7 (JCPDS# 01-087-0409). According to the literature, the crystallographic data reported that the compound Li2WO4crystallized with monoclinic symmetry, C2c, a = 9.753(1) Å, b = 5.954(1) Å, c = 4.994(1) Å, β = 90.58(2)°. In addition, the lithium and the tungsten atoms are located in octahedral sites. Furthermore, the structure is found in four octahedral layers parallel to (100). The first and the third layers are filled with lithium atoms, resulting in LiO6 octahedral sheets, while the second and the fourth contain zigzag chains of WO6 octahedral reported in the Wolframite structure [42]. Also, the compound of the crystal structure of Li4P2O7 was interpreted as triclinic, space group P-1, as confirmed by Voronin et al. [5]. As well known, the existing of titanium or tungsten ions in glass could play a double role: former in the glass of covalent [MO4] groups (M = Ti, W) and modifier in the ionic glass of the [MO6] polyhedrons. WO3 itself brings three oxygen atoms in [WO6/2] structural units. On the other hand, TiO2 itself takes stoichiometrically two oxygen atoms in [TiO4/2] and passing to six coordination. Therefore, the titanium atoms obtain oxygen from the glassy network. The determination of layer size is an important test for the characterization of heat-treated glasses.
The crystallite size values are estimated from X-ray patterns by using the Scherrer Eq. (3) [43]:
where D is the average crystallite size, λ is the X-ray wavelength, β is the width of the X-ray peak on the 2θ axis, normally measured as full width at half maximum (FWHM), θ is the Bragg angle, and K is the Scherrer constant with K = 0.9. K depends on the crystallite shape and the size distribution, indices of the diffraction line, and the actual definition used for β whether FWHM or integral breadth [44]. Since the exact value of K for the present materials system is not known, crystallite size calculations are only estimates. The calculated crystal sizes of the crystalline phases (Li2WO4 and Li4P2O7) are given in Table 3, where the crystal size of the Li2WO4 is determinated from the peak of the h, k, l value (1 0 1) and for Li4P2O7 was determinated from the peak of the h, k, l value (2 1 0). From the measurements of the crystal size, it observed that the crystal size average is constant for each crystalline phase, where D(Li2WO4)= 18 nm and D(Li4P2O7)= 26 nm. As the crystal size distribution and evolution during growth are well known, quantitative studies on the nucleation and growth of a fragment reveal that, with increasing growth time, both the crystal size and the size spectrum increase. During the early stages of growth, crystals are small and relatively uniform sized. As films grow, the average crystal size increases, and at the same time, the size distribution broadens. But the crystal size is independent of the diffraction peaks [45].
It seems that the vitreous network of glasses contains structural units of the pyrophosphate type. The crystallization times of the glasses for 4 and 12 h, insufficient for the formation of crystallized phases, contain the titanium, within the pieces of the heat-treated glass.
3.5 Infrared spectra of glass–ceramics
Figure 9 shows the IR spectra of the prepared glass–ceramics heated at 550 °C for 12 h. These spectra contain the same principal bands of the phosphate glasses with sharp differences between the glasses (previous work [18]) and their derived glass–ceramics. The spectra of the heat-treated glasses (glass–ceramics) show different absorption bands localized around 1275 cm−1, 1120 cm−1, 1085 cm−1, 1050 cm−1, 940 cm−1, 860 cm−1, 745 cm−1, 605 cm−1, 510 cm−1, and 450 cm−1. The assignment of the absorption bands of these glass–ceramics of the Li2O–Li2MoO4–TiO2–P2O5 system was done according to our previous work, interested in studying the glassy structure of this system [18]. The band positions and their assignments are listed in Table 4. The broad weak band at position 1275 cm−1 is described to the asymmetric vibration of the P=O bond [νas(P=O)] and/or νas(PO2−), in the metaphosphate Q2 units, while the band at 1120 cm−1 is attributed to symmetric stretching vibration in the νs (PO2)− bond in Q2 chains. The strong band situated at 1085 cm−1 is associated with asymmetric stretching modes of the νas(PO3)2−, in pyrophosphate Q1 units, whereas the weak band at 1025 cm−1 is identified with the symmetric stretching modes of the pyrophosphate (PO3)2− units. The low shoulder band situated at the position 9040 cm−1 is ascribed with the orthophosphate PO43− units. The bands at 860 cm−1 are attributed to the asymmetric vibration modes of the P–O–P and/or P–O–M (M = W, Ti) bonds. The band at 745 cm−1 is attributed to the symmetric stretching vibrations of the P–O–P νs(P–O–P) bond, of bridging oxygen atoms. The band about 605 cm−1 is associated with the symmetric vibration of the P–O–P bond in the Q2 units. The broad bands in the 510–450 cm−1 range belong to the deformation mode of the PO4 units of phosphate glasses. In comparison, between IR band assignments of the glasses and their glass–ceramics, we conclude that the structure of these materials (glasses and glass–ceramics) is mainly formed from the bonds of the phosphate units: metaphosphate PO2− units, pyrophosphate PO32− units, and orthophosphate PO43− units.
At the substitution of the Li2WO4 by TiO2 in the glasses and their glass–ceramics, the band positions and their intensities have changed. We attributed these changes to the interaction between (50 − x)Li2O with xTiO2 or/and (50 − x)WO3 with xTiO2. But, for the glass–ceramics, the changes are due to the bonds breaking as a result of heat treatment to format crystalline phases: Li2WO4 and Li4P2O7 (see the section of the X-ray diffraction of glass–ceramics). Existing the tungsten into structural phosphates, well the IR spectra show bands attributed to WO4 (725 cm−1) and WO6 (920 cm−1) units [46]. At high TiO2 content, the intensities of the bands at 1000 cm−1 are associated with PO43− units and TiO4 units, decreasing with slightly shifting towards higher wavenumber. Additionally, an increase of TiO2 content in the glasses leads to increasing the intensity of bands associated with the metaphosphate (PO2)− units. But the intensity of bands attributed to the pyrophosphate (PO3)2− groups decreased [18]. In contrary, at low TiO2 content in glass–ceramics (from x = 8 to x = 0 mol%), it observed changes in form and intensity of bands, especially the bands range in 1247–1000 cm−1. These changes are associated with the precipitation of the crystalline phases (Li2WO4 and Li4P2O7) in the heat-treated glasses. But, for the composition x = 10 mol% and x = 15 mol% of heat-treated glasses, it observed no change in form or intensity. Therefore, it assumed that the P–O–M (M = P, W) bonds are interrupted by the presence of the LiO2, then formation of new P–O–Ti bonds [47]. As a result of increasing, the P–O–Ti bonds in glasses lead to increasing of hardness of the glassy structure. Therefore, the formation of new crystalline phases contain titanium that needs more temperature and/or time for heat treatment of the glasses.
In conclusion, the IR spectra of the glass–ceramics show nearly the same IR vibrational modes as their parent glasses. The IR spectra of the glass–ceramics reveal a few sharp peaks in the far IR region and the mid-region spectrum. Due to the stretching of main phosphate network groups, it appears lower in the intensity of the vibrational bands. These results confirm that the crystalline phases separated, while the X-ray data indicated that the formation of these new crystalline phases (Li2WO4 and Li4P2O7), which are suggested to be somewhat compacted or limited to two crystalline phases.
4 Conclusions
The Raman spectra of the prepared glasses show a conversion of metaphosphate units into pyrophosphate and orthophosphate units during the substitution of Li2WO4 by TiO2. Crystallization kinetics of glasses is reported by DSC. Therefore, the activation energy of crystallization (Ec ) depends on the chemical composition of the glass (x = 5 mol%), where its value is around ≈ 184.482 kJ/mol, while the obtained value of the parameter Avrami (n) is about ≈ 1.7, where it allowed us to predict that the crystallization of the glasses takes place according to a surface mechanism. In addition, the formation of the crystalline phases depends on the temperature and heat treatment time. Glass–ceramics obtained from the studied glasses submitted to heating at two different durations were exploited by XRD and FTIR, whereas the crystallized phases (Li2WO4 and Li4P2O7) into prepared glasses depend on the composition and the conditions of treatment. Also, X-ray patterns of crystalline phases are investigated to determine the crystal size for each crystallized phase into heat-treated glasses, where it found that the D(Li2WO4)= 18 nm and D(Li4P2O7)= 26 nm. Furthermore, the crystal size of these crystalline phases was fixed with the variation of TiO2 mol%. FTIR analysis has shown that the absorption bands of both spectra (glasses and glass–ceramics) were presented the same bands with quite different. Also, the results of the IR analysis confirmed the formation of the crystalline phases have been identified by X-ray diffraction. In the perspective work, we will focus on the study the electrical conduction mechanism into these glass–ceramics.
References
T. Ishiyama, S. Suzuki, J. Nishii, T. Yamashita, H. Kawazoe, T. Omata, Solid State Ionics 262, 856–859 (2014). https://doi.org/10.1016/j.ssi.2013.10.055
S. Hussain, X. Yang, M.K. Aslam, A. Shaheen, M.S. Javed, N. Aslam et al., Chem. Eng. J. 391, 123595 (2020). https://doi.org/10.1016/j.cej.2019.123595
F. Zheng, M. Kotobuki, S. Song, M.O. Lai, L. Lu, J. Power Sources 389, 198–213 (2018). https://doi.org/10.1016/j.jpowsour.2018.04.022
T. Minami, A. Hayashi, M. Tatsumisago, Solid State Ionics 177, 2715–2720 (2006). https://doi.org/10.1016/j.ssi.2006.07.017
V.I. Voronin, E.a. Sherstobitova, V.a. Blatov, G.S. Shekhtman, J. Solid State Chem. 211, 170–175 (2014). https://doi.org/10.1016/j.jssc.2013.12.015
E. Kartini, M. Nakamura, M. Arai, Y. Inamura, K. Nakajima, T. Maksum et al., Solid State Ionics 262, 833–836 (2014). https://doi.org/10.1016/j.ssi.2013.12.041
T. Minami, J. Non-Cryst. Solids 73(1–3), 273–284 (1985). https://doi.org/10.1016/0022-3093(85)90353-9
P. Knauth, Solid State Ionics 180(14–16), 911–916 (2009). https://doi.org/10.1016/j.ssi.2009.03.022
M. Tatsumisago, Solid State Ionics 175(1–4), 13–18 (2004). https://doi.org/10.1016/j.ssi.2004.09.012
K. Minami, F. Mizuno, A. Hayashi, M. Tatsumisago, Solid State Ionics 178(11–12), 837–841 (2007). https://doi.org/10.1016/j.ssi.2007.03.001
A.D. Robertson, A.R. West, A.G. Ritchie, Solid State Ionics 104(1–2), 1–11 (1997). https://doi.org/10.1016/S0167-2738(97)00429-3
G.L. Henriksen, D.R. Vissers, Journal of power sources 51(1–2), 115–128 (1994). https://doi.org/10.1016/0378-7753(94)01965-7
S. Soman, Y. Iwai, J. Kawamura, A. Kulkarni, J. Solid State Electrochem. 16(5), 1761–1766 (2012). https://doi.org/10.1007/s10008-011-1592-4
M. Tatsumisago, Y. Shinkuma, T. Minami, Nature 354(6350), 217–218 (1991). https://doi.org/10.1038/354217a0
E. Kartini, V. Yapriadi, H. Jodi, M. Manawan, C. Panghegar, Prog. Nat. Sci. Mater. Int. (2020). https://doi.org/10.1016/j.pnsc.2020.01.020
H. Horiuchi, N. Morimoto, S. Yamaoka, J. Solid State Chem. 33(1), 115–119 (1980). https://doi.org/10.1016/0022-4596(80)90554-X
D.M.M. Krishantha, R.M.G. Rajapakse, D.T.B. Tennakoon, H.V.R. Dias, J. Compos. Mater. 40(11), 1009–1021 (2006). https://doi.org/10.1177/0021998305056388
H. Es-Soufi, L. Bih, B. Manoun, P. Lazor, J. Non-Cryst. Solids 463, 12–18 (2017). https://doi.org/10.1016/j.jnoncrysol.2017.02.013
M.A. Sayed, A.M. Ali, A. El-Rehim, A.F. Wahab, E. A., & K.S. Shaaban, J. Electron. Mater. (2021). https://doi.org/10.1007/s11664-021-08921-9
S.F. Ismail, M.R. Sahar, S.K. Ghoshal, Mater. Res. Bull. 74, 502–506 (2016). https://doi.org/10.1016/j.materresbull.2015.11.022
K.S. Rao, M.S. Reddy, V.R. Kumar, N. Veeraiah, Mater. Chem. Phys. 111(2–3), 283–292 (2008). https://doi.org/10.1016/j.matchemphys.2008.04.012
G.K. Marasinghe, M. Karabulut, C.S. Ray, D.E. Day, P.G. Allen, J.J. Bucher et al., Ceram. Trans. 93, 195–202 (2003)
X. Fang, C.S. Ray, A. Moguš-Milanković, D.E. Day, J. Non-cryst. Solids 283(1–3), 162–172 (2001). https://doi.org/10.1016/S0022-3093(01)00416-1
G.M. Krishna, B.A. Kumari, M.S. Reddy, N. Veeraiah, J. Solid State Chem. 180(10), 2747–2755 (2007). https://doi.org/10.1016/j.jssc.2007.07.025
M. Nagarjuna, T. Satyanarayana, Y. Gandhi, N. Veeraiah, J. Alloys Compd. 479(1–2), 549–556 (2009). https://doi.org/10.1016/j.jallcom.2008.12.132
F. Delahaye-Carrière (1997). (Doctoral dissertation, Compiègne)
H. Es-Soufi, L. Bih, J. Non-Cryst. Solids 558, 120655 (2021). https://doi.org/10.1016/j.jnoncrysol.2021.120655
A. El Hadrami, M. Mesnaoui, M. Maazaz, J.J. Videau, J. Non-cryst. Solids 331(1–3), 228–239 (2003). https://doi.org/10.1016/j.jnoncrysol.2003.08.066
L. Ma, R.K. Brow, M.E. Schlesinger, J. Non-Cryst. Solids 463, 90–101 (2017). https://doi.org/10.1016/j.jnoncrysol.2017.02.022
X. Li, Z. Xiao, M. Luo, X. Dong, T. Du, Y. Wang, J. Non-Cryst. Solids 469, 62–69 (2017). https://doi.org/10.1016/j.jnoncrysol.2017.04.023
L. Ma, R.K. Brow, L. Ghussn, M.E. Schlesinger, J. Non-Cryst. Solids 409, 131–138 (2015). https://doi.org/10.1016/j.jnoncrysol.2014.11.019
P.P. Poluektov, O.V. Schmidt, V.A. Kascheev, M.I. Ojovan, J. Nucl. Mater. 484, 357–366 (2017). https://doi.org/10.1016/j.jnucmat.2016.10.033
H.E. Kissinger, J. Res. Nat. Bur. Stand. 57, 217 (1956)
J.A. Augis, J.E. Bennett, J. Therm. Anal. 13(2), 283–292 (1978). https://doi.org/10.1007/BF01912301
D.R. Lide (ed.), CRC Handbook of Chemistry and Physics (CRC Press, Boca Raton, 2001)
M. Avrami, J. Chem. Phys. 7(12), 1103–1112 (1939). https://doi.org/10.1063/1.1750380
M. Avrami, J. Chem. Phys. 8(2), 212–224 (1940). https://doi.org/10.1063/1.1750631
M. Avrami, J. Chem. Phys. 9, 177–184 (1941). https://doi.org/10.1063/1.1750872
E.C. Cardillo, S. Terny, C. Lopez, G. Narda, M.A. Frechero, Ceram. Int. (2020). https://doi.org/10.1016/j.ceramint.2020.05.168
E. Haily, L. Bih, A. El Bouari, A. Lahmar, M. El Marssi, B. Manoun, Phase Transit. (2020). https://doi.org/10.1080/01411594.2020.1837369
F.H. ElBatal, M.A. Marzouk, H.A. ElBatal, J. Mol. Struct. 1121, 54–59 (2016). https://doi.org/10.1016/j.molstruc.2016.05.052
H. Horiuchi, N. Morimoto, S. Yamaoka, J. Solid State Chem. 33(1), 115–119 (1980)
P. Scherrer, Nachr. Ges. Wiss. Göttingen 2, 98–100 (1918)
J.I. Langford, A.J.C. Wilson, J. Appl. Cryst. 11, 102–113 (1978)
D.J. Lim, N.A. Marks, M.R. Rowles, Carbon 162, 475–480 (2020). https://doi.org/10.1016/j.carbon.2020.02.064
L. Koudelka, O. Kupetska, P. Kalenda, P. Mošner, L. Montagne, B. Revel, J. Non-Cryst. Solids 500, 42–48 (2018). https://doi.org/10.1016/j.jnoncrysol.2018.05.028
M. Azmoonfar, M.H. Hekmat-Shoar, M. Mirzayi, Ionics 15(4), 513–518 (2009). https://doi.org/10.1007/s11581-008-0299-8
Acknowledgements
The authors are grateful to G. Bánhegyi (Medicontur Medical Engineering Ltd., Herceghalmi út 1, Zsámbék 2072, Hungary) for his comments and valuable suggestions.
Author information
Authors and Affiliations
Contributions
HE-S: Conceptualization, Writing—review & editing. LB: Supervision. ARFL: Visualization. AEB: Visualization. BM: Investigation. SH: Visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Es-Soufi, H., Bih, L., Lima, A.R.F. et al. Investigation DSC and XRD on the crystallization kinetics in the phosphate Li2O–Li2WO4–TiO2–P2O5 glassy ionic system. J Mater Sci: Mater Electron 33, 8747–8758 (2022). https://doi.org/10.1007/s10854-021-06804-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06804-7