Abstract
Silver/titania (Ag/TiO2) nanocomposites, synthesized through a simple one-step chemical reduction method, are used for electrochemical sensing of hydrogen peroxide (H2O2). The images of transmission electron microscopic (TEM) demonstrate a well dispersion of Ag nanoparticles (NPs) with a particle size range of approximately 3 nm on the TiO2 surface, which makes the sensor based on Ag/TiO2 exhibit an excellent performance toward H2O2. The AgNPs presented in the nanocomposite exhibit electrocatalytic reduction of H2O2 and the limit of detection (LOD) is found to be 1.23 µM. Density functional theory (DFT) calculation studies reveal that H2O2 could be easily adsorbed onto Ag rather than TiO2 surface of Ag/TiO2 via a partial electron transfer from Ag to H2O2. The nanocomposite-modified electrode has also excellent selectivity toward the detection of H2O2 over the interferents even when the interferents have a 100 times higher concentration than H2O2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The sensitivity and accurate determination of small quantities of H2O2 is utmost important because of its wide use in food industry, pharmaceutical, environmental analysis, biomedical fields, and enzymatic reactions [1]. Accidental ingestion of H2O2 will lead to diseases such as asthma, cancer, and diabetes and even will cause death for high consumption of H2O2. With the increasing number of important applications of H2O2, different methods have been utilized toward the detection of H2O2, such as chemiluminescence [2], fluorescence [3], and spectrophotometry [4]. Yet, these techniques are usually not easy to use, time consuming, and sometimes costly. Since H2O2 itself is an electroactive material, constructing a competent electrochemical sensor has gained much interest among researchers due to its ease of the operation, low cost, and high selectivity [5, 6], certainly depending on the material used for the sensor.
In recent year, metal oxide materials emerged as promising materials for sensing applications. A large variety of oxide compounds can be formed from metal elements that can adopt a vast number of structural geometries with an electronic structure that can exhibit metallic, semiconductor, or insulator character. Due to the widespread structural, physical properties, chemical properties, and functionalities, metal oxides had stand out as one of the most common, diverse, and richest classes of materials [7]. Metal oxides proved to be very promising for a variety of technological applications due to their unique and tunable properties such as optical, magnetic, electrical, catalytic, photochemical properties [8,9,10]. The metal oxides, such as modified zinc oxide (ZnO) [11], titanium oxide, (TiO2) [12, 13] tin oxide (SnO2) [14], zirconium oxide (ZrO2) [15], manganese dioxide (MnO2) [16], copper(II) oxide (CuO) [17], and cerium oxide (CeO2) [18, 19], have been widely investigated as sensing material. So far, TiO2 has attracted much interest owing to its low cost, abundance, non-toxicity, safety, high specific surface area, and biocompatibility [20, 21].
Nowadays, non-enzymatic electrochemical sensors have attracted much attention from the researchers to avoid the disadvantage of the enzyme-based biosensors [22]. Therefore, a plenty of studies have been carried out to improve the non-enzymatic electrochemical sensors with low detection limit. With the development of nanotechnology, many nanomaterials have been applied in the field of enzyme-free sensors due to their excellent conductivity, extraordinary electrocatalytic, large specific surface areas, and biocompatibilities. Among these, metal nanoparticles, such as gold (Au) [23, 24], copper (Cu) [25, 26] platinum (Pt) [27, 28], and palladium (Pd) [29, 30] were employed to construct H2O2 sensor. Silver nanoparticles (AgNPs) have aroused growing interest in recent years, because they not only have common characteristics of noble metals nanoparticles but also have unique properties of outstanding conductivity, electroactivity, low toxicity, and low cost [12,13,14, 31, 32]. However, the aggregation of AgNPs prohibits extensive applications because of the poor stability and reproducibility, and thus highly dispersed AgNPs are very important in fabricating a sensor [33]. In this regard, the supported system had a major influence on fabricating highly dispersed AgNPs for efficient sensor application. Recently, TiO2 has gained increasing attraction as an ideal material for the supported system to fabricate sensors owing to its special characteristics, especially the property of the strong metal-support interactions (SMSI). The TiO2 particles will provide large surface area and strong interaction between Ti and Ag for the stable deposition of AgNPs and the AgNPs facilitate an efficient electron transfer process during the electrocatalytic reduction of H2O2, thus improving the sensing performance.
In this study, we successfully developed a facile, one-step chemical reduction method to prepare homogeneously distributed Ag nanoparticles deposited on TiO2 without addition of any stabilizer or surfactant. The Ag/TiO2 nanocomposite-modified electrode showed better catalytic performance compared to that of a bare TiO2. The effect of the Ag content on the catalytic performance was also investigated. The detection of H2O2 was studied using the amperometric i–t curve technique and limit of detection was found to be 1.23 µM with signal-to-noise (S/N) ratio around 3. Furthermore, the nanocomposite-modified electrode demonstrated excellent selectivity toward the detection of H2O2 in the presence of 100 times higher concentration of other important interferents.
2 Experimental methods
2.1 Materials
Titanium dioxide (P25), silver nitrate (AgNO3), and sodium borohydride (NaBH4) were purchased from Merck and used them as received. H2O2 was obtained from Sigma-Aldrich. All chemicals used were of analytical reagent grade and doubly distilled water was used for all the experiments.
2.2 Synthesis of Ag/TiO2 nanocomposite
Firstly, 500 mg of P25 was added to different amounts of aqueous AgNO3 (5, 10, 20, and 40 wt%). Each mixture was vigorously stirred for half hour at room temperature. The reduction of Ag+ was initiated when NaBH4 was added and the solution changed to greenish yellow. The mixture was then stirred for another half hour. The greenish yellow solution was centrifuged and washed repeatedly using distilled water and ethanol. Finally, the solution was then dried in an oven at 60 °C to obtain the Ag/TiO2 nanocomposite. The nanocomposite with 20 wt% of Ag was used for all the characterizations and detection of H2O2 unless otherwise stated.
2.3 Fabrication of Ag/TiO2-modified electrode and electrochemical measurements
The Ag/TiO2 nanocomposite was prepared following the previous method [34] with some modifications. The detailed method is described in supplementary information. Prior to modification, the glassy carbon electrode (GCE) was carefully polished with alumina slurry suspension (5 µM) on the micro-cloth polishing pad followed by ultrasonically cleaned with distilled water and ethanol for 2 min. Next, 5μL of the nanocomposite solution (1 mg/mL) was dropped onto a GCE surface, allowed to dry at room temperature (25 °C) for 1 h. The prepared Ag/TiO2-modified GCE was used as a working electrode for further investigation.
2.4 Characterization techniques
Transmission electron microscopy (TEM) images were captured using JEOL JEM-2100 F high-resolution transmission electron microscope (HRTEM) operated at 200 kV. The optical absorption properties in the spectral region of 200–800 nm were examined using a Thermo Scientific Evolution 300 UV–Vis absorption spectrophotometer. Raman spectra were recorded with Renishaw inVia 2000 system with an argon ion laser emitting at 532 nm. The crystalline phase of the samples was studied via X-ray diffraction (XRD; D5000, Siemens), using copper Kα radiation (λ = 1.5418 Å) at a scan rate of 0.02° s−1. All the electrochemical studies were carried out using a VersaSTAT-4 electrochemical analyzer (Princeton Applied Research, USA) with a conventional three-electrode system. The fabricated GCE was used as a working electrode, silver/silver chloride (Ag/AgCl) as reference electrodes, and Platinum (Pt) as counter electrode. X-ray photoelectron spectroscopy (XPS) measurements were taken by synchrotron radiation from beam line no. 3.2 at the Synchrotron Light Research Institute, Thailand.
2.5 Density functional theory (DFT) calculation
All DFT calculations, i.e., geometry optimization, were performed using the DMol3 program package [35] (Materials Studio 8.0, BIOVIA, San Diego, USA). The method proposed by Tkatchenko and Scheffler (TS) was employed to correct the calculations for missing van der Waals interaction [36]. Generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional was employed to account for the exchange–correlation interaction [37]. Spin-polarized calculations were carried out with double numerical plus polarization (DNP) basis set. All electrons were included in the calculations. The Brillouin zone was sampled only at Gamma-point. The orbital occupancy thermal smearing was set to 0.005 Ha. The convergence criteria for the maximum energy change, the maximum force, and the maximum displacement were set to 10–5 Ha, 0.002 Ha·Å−1, and 0.005 Å, respectively.
3 Results and discussion
3.1 Morphological studies and optical properties of Ag/TiO2 nanocomposite
The physical appearance of Ag/TiO2 with is shown in Fig. 1a. The morphology and structure of the Ag/TiO2 nanocomposite was characterized with TEM. The analysis of TEM images revealed that the TiO2 particles have a size range of 20–25 nm (Fig. 1b, c). AgNPs had a diameter of approximately 3 nm and are well dispersed and deposited on the surface of TiO2 (Fig. 1c). Figure 1d indicates the UV–Vis absorption spectra of the TiO2 particles and Ag/TiO2 nanocomposite. TiO2 did not have any absorbance in the visible region because of the wide band gap energy (~ 3.2 eV). The deposition of AgNPs on the TiO2 surface significantly influenced the absorption in the visible regions of 450–600 nm, which was due to the surface plasmon resonance (SPR) band of AgNPs. It can be seen that the absorption edge also shifts toward visible region for Ag/TiO2 sample due to the influences of AgNPs on the absorption properties of TiO2 based on the figure that compared to pure TiO2.
3.2 XPS and Raman analyses of Ag/TiO2 nanocomposite
In order to understand the chemical components of the samples, the Ag/TiO2 nanocomposite XPS spectra were analyzed and are demonstrated in Fig. 2. The scanned surface spectra illustrate the chemical composition of elemental Ti, O, and Ag in the Ag/TiO2 nanocomposite. The binding energies of Ag 3d5/2 and Ag 3d3/2 levels are found to be 368.5 and 374.5 eV in Fig. 2a, respectively, with a peak separation of 6 eV attributing to the formation of the metallic silver [38]. Ti 2p spectra for Ag/TiO2 are observed at 454.2 and 459.8 eV corresponding to the binding energies of the Ti 2p3/2 and Ti2 p1/2 core levels in the presence of the Ti(IV) state (Fig. 2b). The X-ray diffraction patterns indicated that TiO2 and Ag/TiO2 were composed of mixed anatase and rutile phases (Figure S1), which closely matches the reference patterns of JCPDS card No. 83-2243 and 21-127, respectively. The diffraction peaks observed at the 2θ values of 25.35°, 38.78°, 47.15°, and 75.4° corresponded to the anatase phase of TiO2 and were assigned to the (101), (004), (200), and (215) crystallographic planes, respectively. In contrast, the peaks at the 2θ values of 54.8° and 63.8° agreed well with the rutile phase of TiO2 and were are assigned to the (220) and (002) crystallographic planes, respectively. From the XRD analysis, the crystallographic peaks due to Ag overlapped with those for the rutile phase of TiO2. Hence, the peaks were indistinguishable in Ag@TiO2. Raman spectroscopy for TiO2 and Ag/TiO2 nanocomposite was also collected in order to evaluate the phase identification of the nanocomposites (Fig. 3). The bands at 153.35, 198.74, 396.32, 521.19, and 637.41 cm–1 are characteristic of anatase phase TiO2. However, no signal related to AgNPs was identified perhaps because of the relatively low concentration of Ag loaded onto TiO2 and its weak Raman scattering.
3.3 EIS study
The interfacial electron transfer properties and impedance changes of the surface-modified electrodes were studied by the electrochemical impedance spectroscopy (EIS) technique. The 1 mM [Fe(CN)6]3−/4− in 0.1 M KCl was applied as a redox analyte to study the conducting behavior of the Ag/TiO2 nanocomposite-modified electrode. Normally, the diameter of the semicircle at higher frequencies in the Nyquist diagram is related to the electron transfer resistance (Rct) [39]. As shown in Fig. 4a, the Ag/TiO2-modified electrode shows a smallest Rct value (~ 54,569 Ω) compared to bare GCE (~ 198,628 Ω) and TiO2 (~ 67,833 Ω)-modified electrode under given experimental conditions. This indicated that the presence of Ag at the modified electrode efficiently facilitated the electron transfer process. Furthermore, bode phase of the modified electrodes was collected in the frequency range of 0.01–1000 Hz that corresponds to the charge-transfer resistance of the modified electrodes. The shifting of peaks toward the low-frequency region for the TiO2 and Ag/TiO2 nanocomposite indicates the efficient electron transfer behavior of the nanocomposites as compared to the bare GCE (Fig. 4b).
3.4 Electrocatalytic reduction of H2O2
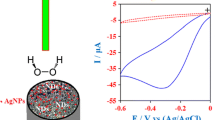
The electrocatalytic behavior of the Ag/TiO2 nanocomposite toward the reduction of a 1 mM H2O2 in 0.1 M PBS (pH 7.2) was investigated with cyclic voltammetry and the results are presented in Fig. 5a. No significant catalytic response peak was observed for the reduction of H2O2 at bare GCE and TiO2-modified electrode. However, a significant catalytic current response was observed at an over potential of − 0.57 V for Ag/TiO2 nanocomposite-modified electrode toward the reduction of 1 mM H2O2. This clearly reveals that excellent electrocatalytic properties of AgNPs for 1 mM H2O2 reduction. Hence, the current response corresponding to the reduction of H2O2 can be amplified by optimizing Ag content on TiO2. Optimization of the amount of Ag on TiO2 is essential for the economic and high-performance perspectives of a sensor. The current response increased with the increasing amount of Ag on TiO2 and the catalytic peak at − 0.57 V became significant for Ag/TiO2(20 wt% of Ag) nanocomposite-modified electrode. The TiO2 particles provide large surface area for the stable deposition of AgNPs and the AgNPs facilitate an efficient electron transfer process during the electrocatalytic reduction of H2O2. A further increase in the Ag content eventually led to a decrease in the catalytic current for the reduction of 1 mM H2O2.
a Cyclic voltammograms obtained for bare GCE, TiO2, and the Ag/TiO2 nanocomposite with different wt% of Ag in N2 saturated 0.1 M PBS (pH 7.2) containing 1 mM H2O2 at a scan rate of 50 mV/s. b CVs obtained for the Ag/TiO2 nanocomposite (20 wt% Ag) in N2 saturated 0.1 M PBS (pH 7.2) containing different concentrations of H2O2 at a scan rate of 50 mV/s. c Calibration curve of peak current versus concentration of H2O2
The cyclic voltammograms were recorded at the Ag/TiO2(20 wt% Ag) nanocomposite-modified electrode for the reduction of different concentrations of H2O2 in 0.1 M PBS (pH 7.2) and the voltammetric curves are displayed in Fig. 5b. It can be seen that no characteristic peak was observed in the absence of H2O2. When H2O2 was introduced into the PBS, the reduction peak appeared and it increased with respect to the concentration of the H2O2, indicating the good electrocatalytic activity of the nanocomposite toward H2O2 reduction. It was also observed that the plot of peak current versus concentration of H2O2 showed a linear relation (Fig. 5c). The plot of log(current) versus log[H2O2] (Figure S2) showed a linear relation with a slope ~ 1, which indicates that the electro-reduction of H2O2 at Ag/TiO2-modified electrode follows the first-order kinetics with respect to the H2O2 concentration. The cyclic voltammograms of Ag/TiO2 nanocomposite-modified electrode were recorded at different scan rates from 10–100 mV/s for the reduction of 1 mM H2O2 in 0.1 M PBS and are shown in Figure S3A. A linear relation between the peak currents and square root of scan rates was also obtained (Figure S3B) which indicates that the H2O2 reduction at the nanocomposite-modified electrode is controlled by the diffusion process.
3.5 Amperometric detection of H2O2
Selective detection of the particular analyte for the common interferent species can be obtained using amperometric (i–t) technique tool. The Ag/TiO2 nanocomposite with 20 wt% Ag was chosen for the sensitive and non-enzymatic determination of H2O2 because of the better catalytic performance in CV. Figure 6 displays the amperometric current–time (i–t) curve of the Ag/TiO2 (20 wt% Ag) nanocomposite-modified electrode in continuously stirred N2-saturated 0.1 M PBS (pH 7.2) for the injection of different concentrations of H2O2 at a regular interval of 60 s with applied potential of − 0.5 V. The lower applied potential was used to avoid or decrease the interferences caused by the electroactive species present in the solution [40]. It can be observed that a significant current response was observed and the sensor showed the current response in less than 3 s for each addition of H2O2 with a sample interval of 60 s. This indicates that the AgNPs present in the Ag/TiO2 nanocomposite can enhance the H2O2 accessibility and efficiently promote the electron transfer rate between the H2O2 and electrode surface in PBS. The current response increased for the successive addition of H2O2 and multi-linear lines was observed for different concentration ranges. The limit of detection of Ag/TiO2 (20 wt% of Ag) was found to be 1.23 µM toward the detection of H2O2. The comparison of the analytical performance of the present Ag/TiO2 nanocomposite-modified GCE with previous reports toward detection of H2O2 is summarized in Table 1. The present work stands for simple fabrication method for the catalyst and without employing any polymeric binder material or tedious electrode modification process. The proposed modified electrode displayed a satisfactory performance in terms of the detection limit and good selectivity.
a Amperometric i–t curve obtained at the Ag/TiO2(20 wt% of Ag) nanocomposite-modified electrode in N2 saturated 0.1 M PBS (pH 7.2) for the addition of various concentrations of H2O2 at a regular internal of 60 s. Applied potential was − 0.5 V. b Calibration curve of peak current versus concentration of H2O2
3.6 Interference study
Investigation on the selectivity of the Ag/TiO2 nanocomposite toward the detection of H2O2 was then carried out by injecting various possible physiological interferents in the same homogeneously stirred PBS containing H2O2. The current responses of the interferents such as ascorbic acid, dopamine, glucose, KCl, NaNO3, Na2SO4, and uric acid were studied by adding in sequence after the few consecutive addition of H2O2 (50 μM) in the stirred PBS. However, the added interferents did not indicate any current response even with a 100 times higher concentration than the concentration of H2O2 (Fig. 7). Addition of interferents creates disturbance in i–t curve signal but they did not lead to a stable and significant current response. After the sudden increase in the current response, a fast decay was detected before they reach the steady state. It was then followed by introduction of H2O2 to the same solution again which gave a quick response. Moreover, the presence of higher concentration of the interfering ions did not disturb the current signal of H2O2 and almost the same magnitudes of current response were reproduced. The present sensor displayed good selectivity and sensitivity when compared among the other common interferent species.
3.7 Density functional theory (DFT) calculation
In order to have a deep insight of the adsorption and reduction of H2O2 on Ag/TiO2 nanohybrid, DFT calculation is performed for the nanohybrid model in this work. The calculation followed the procedure reported by Tada et al. with slight modification [44]. As shown in Fig. 8, TiO2 surface is represented by a Ti4O10H4 cluster truncated from rutile (110) surface. Hydrogen atom is used to stabilize the dangling bond at peripheral O atom. Even though P25 is mainly composed of anatase (79%), stable and often exposed surface of anatase is not established when compared with the one of rutile. Band structure calculation shows that both surfaces have almost the same electronic property except that the surface of rutile has a slightly smaller band gap. Furthermore, H2O2 interacts with Ag nanoparticles rather than TiO2 surface, and thus the difference in local structure between anatase and rutile does not influence the reduction of H2O2 significantly.
As illustrated in Figure S4, Ag nanoparticle is represented by Ag3 and Ag4 clusters in this work. On optimizing the structure of Ag cluster on Ti4O10H4, Ag atoms and the O atom at the top position, which is close to Ag cluster, are optimized, while the other part of Ti4O10H4 cluster is fixed to the crystalline geometry and the O–H bond length is fixed to 1.0 Å. Figure S5 shows the optimized geometries of Ag clusters on Ti4O10H4 cluster. The optimized geometry of H2O2 is shown in Figure S6. In the case of H2O2 adsorption, H2O2 is placed initially right above the Ag cluster. H2O2 is optimized as well as the Ag atoms and the above-mentioned O atom. Similar to the result of Tada et al. [44], the Ag clusters deposited on Ti4O10H4 are positively charged. The charges are + 0.714 eV and + 0.767 eV estimated by Mulliken population for Ag3 and Ag4, respectively.
The optimized geometry of H2O2/Ag3/Ti4O10H4 is shown in Fig. 9a. Upon adsorption on Ag3/Ti4O10H4, H2O2 obtains extra electron from Ag3 cluster, resulting in the elongation of O–O bond. H2O2 is then dissociated into two hydroxyl groups, one of which is adsorbed on Ag3 cluster with a bond length of 2.246 Å. The hydroxyl groups formed will react further with H+ to form H2O. The adsorption energy of H2O2 (Eads = EH2O2/Ag4/Ti4O10H4 − EH2O2 − EAg4/Ti4O10H4) is calculated to be − 0.84 eV. The charges estimated by Mulliken population are − 0.161 e and − 0.156 e for the adsorbed hydroxyl group and free one, respectively. The charge of Ag3 cluster increases from + 0.714 eV to + 0.926 eV upon the adsorption of H2O2, i.e., the loss of electron is less than the one gained by H2O2. That means electron is also transferred from Ti4O10H4 cluster to H2O2 via Ag3 cluster. This is in good agreement with the mechanism presented by Khan et al. [8] Dissociative adsorption of H2O2 on Ag4/Ti4O10H4 (Fig. 9b) is similar to that on Ag3/Ti4O10H4. The adsorption energy of H2O2 is − 0.76 eV.
To investigate the role of charge in the catalytic activity, H2O2 adsorption on charged Ag4/ Ti4O10H4 cluster was calculated and the optimized geometries are shown in Figure S7. In the case of H2O2 adsorption on anionic Ag4/Ti4O10H4 cluster (− 1.0 eV), H2O2 obtains more electron, i.e., − 0.845 eV from Ag4/Ti4O10H4. H2O2 is dissociated into H2O and O bonded to Ag4 cluster. The length of Ag–O bond is 1.988 Å, which is in good agreement with experimental value of 2.0 Å [45]. The adsorption energy of H2O2 is calculated to be − 2.12 eV. While in the case of H2O2 adsorption on cationic Au4/Ti4O10H4 cluster (+ 1.0 eV), dissociation of H2O2 is not observed due to the lack of free electron. H2O2 adsorbed physically to cationic Au4/Ti4O10H4 cluster with an adsorption energy of − 0.66 eV.
For comparison, Cu4 and Au4 clusters are also calculated using the same procedure. Optimized geometries are shown in Figure S8. Upon adsorption on Cu4/Ti4O10H4 cluster, H2O2 is dissociated into H2O and O bonded to Cu4 cluster. The length of Cu–O bond is 1.714 Å, which compares well with the measured value of 1.72 Å for CuO [46]. The adsorption energy of H2O2 is calculated to be − 2.15 eV. Au4 cluster is not stable in the presence of H2O2. H2O2 adsorbed physically to deformed Au4 cluster with an adsorption energy of − 0.46 eV. DFT calculation supports the experimental results that TiO2 alone has nearly no activity toward H2O2 reduction and the active sites are actually Ag4/Ti4O10H4 although Ag plays the key role on the absorption of H2O2.
4 Conclusion
In summary, a facile route to prepare Ag nanoparticles deposited on TiO2 via a simple chemical reduction method was provided in this work. The Ag/TiO2 nanocomposite-modified glassy carbon electrode demonstrated a better catalytic performance toward H2O2 reduction compared to bare TiO2. The improvement was mainly attributed to the presence Ag nanoparticles which showed an excellent catalytic activity for the complete reduction of H2O2, while the existing TiO2 gives Ag/TiO2 to be a stable Ag4/Ti4O10H4. The optimum Ag content in Ag/TiO2 for efficient sensor was found to be 20 wt%. It showed better performance with a detection limit of 1.23 µM using amperometric i–t curve technique. Furthermore, the nanocomposite-modified electrode showed selectivity toward the detection of H2O2 in the presence of a 100 times higher concentration of other analytes. This sensor was stable and selective toward the detection of H2O2 and it could add further credit to the TiO2-based electrochemical sensor.
References
B.E. Watt, A.T. Proudfoot, J.A. Vale, Toxicol. Rev. 23, 51–57 (2004)
W. Lei, A. Dürkop, Z. Lin, M. Wu, O.S. Wolfbeis, Microchim. Acta 143, 269–274 (2003)
U. Pinkernell, S. Effkemann, U. Karst, Anal. Chem. 69, 3623–3627 (1997)
S. Guo, D. Wen, S. Dong, E. Wang, Talanta 77, 1510–1517 (2009)
N.I. Ikhsan, P. Rameshkumar, A. Pandikumar, M.M. Shahid, N.M. Huang, S.V. Kumar, H.N. Lim, Talanta 144, 908–914 (2015)
M.M. Shahid, P. Rameshkumar, A. Pandikumar, H.N. Lim, Y.H. Ng, N.M.J. Huang, Mater. Chem. A 3, 14458–14468 (2015)
J.M. George, A. Antony, B. Mathew, Microchim. Acta 185, 358 (2018)
C. Tortolini, P. Bollella, R. Zumpano, G. Favero, F. Mazzei, R. Antiochia, Biosensors 8, 108 (2018)
Y. Cho, N.S. Parmar, S. Nahm, J.-W.J. Choi, Alloys Compd. 694, 217–222 (2017)
A.L. Stroyuk, V.V. Shvalagin, S.Y. Kuchmii, J. Photochem. Photobiol. A 173, 185–194 (2005)
D. Sivalingam, J.B. Gopalakrishnan, U.M. Krishnan, S. Madanagurusamy, J.B.B. Rayappan, Phys. E 43, 1804–1808 (2011)
M.M. Khan, S.A. Ansari, J. Lee, M.H. Cho, Mater. Sci. Eng. C 33, 4692–4699 (2013)
W. Wang, Y. Xie, C. Xia, H. Du, F. Tian, Microchim. Acta 181, 1325–1331 (2014)
Y.-E. Miao, S. He, Y. Zhong, Z. Yang, W.W. Tjiu, T. Liu, Electrochim. Acta 99, 117–123 (2013)
G. Mohseni, M. Negahdary, R. Malekzadeh, J. Manoochehri, A. Hadaegh, A. Sayad, H. Akbari-dastjerdi, M. Fazilati, S. Rezaei-Zarchi, Int. J. Electrochem. Sci. 7, 7033–7044 (2012)
S. Yao, J. Xu, Y. Wang, X. Chen, Y. Xu, S. Hu, Anal. Chim. Acta 557, 78–84 (2006)
S. Weng, Y. Zheng, C. Zhao, J. Zhou, L. Lin, Z. Zheng, X. Lin, Microchim. Acta 180, 371–378 (2013)
W. Zhang, G. Xie, S. Li, L. Lu, B. Liu, Appl. Surf. Sci. 258, 8222–8227 (2012)
D. Wang, L. Pang, H. Mou, Y. Zhou, C. Song, RSC Adv. 5, 24101–24109 (2015)
S.P. Lim, A. Pandikumar, N.M. Huang, H.N. Lim, Int. J. Hydrog. Energy 39, 14720–14729 (2014)
S.P. Lim, A. Pandikumar, N.M. Huang, H.N. Lim, G. Gu, T. Ma, RSC Adv. 4, 48236–48244 (2014)
X. Chen, G. Wu, Z. Cai, M. Oyama, X. Chen, Microchim. Acta 181, 689–705 (2014)
Y.-H. Won, K. Huh, L.A. Stanciu, Biosens. Bioelectron. 26, 4514–4519 (2011)
M. Zheng, P. Li, C. Yang, H. Zhu, Y. Chen, Y. Tang, Y. Zhou, T. Lu, Analyst 137, 1182–1189 (2012)
R. Qiu, H.G. Cha, H.B. Noh, Y.B. Shim, X.L. Zhang, R. Qiao, D. Zhang, Y. Kim, U. Pal, Y.S. Kang, J. Phys. Chem. C 113, 15891–15896 (2009)
T.-H. Tsai, T.-W. Chen, S.-M. Chen, K.C. Lin, Int. J. Electrochem. Sci. 6, 4628–4637 (2011)
Y. Liu, D. Wang, L. Xu, H. Hou, T. You, Biosens. Bioelectron. 26, 4585–4590 (2011)
H. Zhong, R. Yuan, Y. Chai, Y. Zhang, C. Wang, F. Jia, Microchim. Acta 176, 389–395 (2012)
J.-M. You, Y.N. Jeong, M.S. Ahmed, S.K. Kim, H.C. Choi, S. Jeon, Biosens. Bioelectron. 26, 2287–2291 (2011)
F. Jiang, R. Yue, Y. Du, J. Xu, P. Yang, Biosens. Bioelectron. 44, 127–131 (2013)
W. Lu, F. Liao, Y. Luo, G. Chang, X. Sun, Electrochim. Acta 56, 2295–2298 (2011)
Q. Yi, F. Niu, L. Li, R. Du, Z. Zhou, X. Liu, J. Electroanal. Chem. 654, 60–65 (2011)
Y. Han, J. Zheng, S. Dong, Electrochim. Acta 90, 35–43 (2013)
S.P. Lim, A. Pandikumar, N.M. Huang, H.N. Lim, RSC Adv. 4, 38111–38118 (2014)
M. Yu, J. Zhang, S. Li, Y. Meng, J. Liu, RSC Adv. 5, 5604–5610 (2014)
A. Tkatchenko, M. Scheffler, Phys. Rev. Lett. 102, 73005 (2009)
J.P. Perdew, K. Burke, M. Ernzerhof, Phys. Rev. Lett. 78, 1396 (1997)
Z. Tian, L. Wang, L. Jia, Q. Li, Q. Song, S. Su, H. Yang, RSC Adv. 3, 6369–6376 (2013)
S.P. Lim, A. Pandikumar, H.N. Lim, R. Ramaraj, N.M. Huang, Sci. Rep. 5, 11922 (2015)
Y. Zhang, S. Liu, L. Wang, X. Qin, J. Tian, W. Lu, G. Chang, X. Sun, RSC Adv. 2, 538–545 (2012)
X. Qin, W. Lu, Y. Luo, G. Chang, A.M. Asiri, A.O. Al-Youbi, X. Sun, Electrochim. Acta 74, 275–279 (2012)
Y. Jiang, B. Zheng, J. Du, G. Liu, Y. Guo, D. Xiao, Talanta 112, 129–135 (2013)
B.-H. Jeon, D.-H. Yang, Y.-D. Kim, J.S. Shin, C.-S. Lee, Electrochim. Acta 292, 749–758 (2018)
H. Tada, F. Suzuki, S. Ito, T. Akita, K. Tanaka, T. Kawahara, H. Kobayashi, J. Phys. Chem. B 106, 8714–8720 (2002)
K.P. Huber, G. Herzberg, (Springer, Boston, 1979)
M.L. Polak, M.K. Gilles, J. Ho, W.C. Lineberger, J. Phys. Chem. 95, 3460–3463 (1991)
Acknowledgements
This work was funded by Xiamen University Malaysia Research Fund (Grant no. XMUMRF/2018-C1/IENG/0001.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lim, S.P., Shahid, M.M., Rameshkumar, P. et al. Amperometric detection of hydrogen peroxide and its density functional theory for adsorption on Ag/TiO2 nanohybrid. J Mater Sci: Mater Electron 31, 6017–6026 (2020). https://doi.org/10.1007/s10854-020-03153-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03153-9