Abstract
Silver nanoparticles were prepared by chemical reduction of acetaldehyde gas in the absence of protective gas, and Ag/FePO4 nanocomposites were synthesised by modified silver mirror reaction at a gas-liquid interface. A hydrogen peroxide (H2O2) electrochemical sensor was constructed through immobilizing Ag/FePO4 nanocomposites on gold (Au) electrode. The morphology and composition of the nanocomposites were characterized by transmission electron microscopy (TEM) and energy-dispersive X-ray spectroscopy (EDS). The electrochemical investigations of the sensor indicated that it exhibited excellent analytical performance with a wide linear range from 3.0×10−5 to 1.1×10−2 mol⋅L−1 and a low detection limit of 4.7 μmol⋅L −1 at a signal-to-noise ratio of 3. Meanwhile, it also showed acceptable reproducibility and anti-interference ability. This study may provide a new method for the synthesis of highly dispersed metal nanoparticles which might be used in other related fields.

The process for preparing FePO4 nanocomposites decorated with Ag NPs is described. An H2O2 electrochemical sensor was fabricated by immobilizing Ag/FePO4 nanocomposites on gold electrode. The electrochemical investigations for this sensor exhibited excellent H2O2 sensing performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Hydrogen peroxide (H2O2) plays vital roles in industry, biomedicine, pharmaceutical chemistry and many other fields.[1–3] Therefore, it is of great importance to develop an efficient method for selective and sensitive determination of H2O2. Many methods have been reported for detecting H2O2, such as titrimetry,[4] fluorometry,[5] chromatography,[6] spectrophotometry,[7] chemiluminescence[8] and electrochemistry.[9] Among these methods, the electrochemical detection methods have attracted more and more attention because of low cost, simplicity and high efficiency.[10–12] The electrochemical detection of H2O2 could be classified into two main categories: enzymatic detection and non-enzymatic detection. However, enzyme-based sensors suffer from the expensive enzyme material, poor stability and complex fabrication procedure for sensor.[13,14] Thus, many people are committed to the study of non-enzymatic sensors. Moreover, with the emergence and development of nanotechnology, nanoparticles (NPs) play an important role in the non-enzymatic electrochemical sensors due to their good catalytic performance and biocompatibility.[15,16] Especially, metal nanoparticles with good catalytic properties and conductivity can be modified on the electrode. Among these nanoparticles, NPs of Pd,[17] Cu,[18] Au[19] and Ag[20] have been used to construct electrochemical sensors for detecting H2O2..
Comparing with other metal nanoparticles, excellent catalytic properties of Ag NPs are getting greater attention of the researchers. Ag NPs have attracted much interest in the sensor applications due to their low cost, high surface-to-volume ratio and excellent conductibility.[21,22] In addition, in our group, the dispersed AgNPs were synthesized by using methyl aldehyde as reducing agent at a gas-liquid interface, which could slow down the rate of reduction reaction, and thus the aggregation of AgNPs was avoided.[23]
In recent years, phosphate nanomaterials have been reported, such as LiFePO4,[24] AlPO4,[25] and FePO4.[26] Among them, FePO4 is a particularly promising material because of good electrochemical activity and has aroused wide attention.[27,28] Besides, FePO4 nanosphere has many advantages, including environment-friendliness, low-cost, large surface area, good electron transfer capability and good thermal stability.[29–31] It has been reported that the amorphous FePO4 nanosphere can be a catalyst supporter for loading Au NPs, and the obtained Au/FePO4 catalyst can catalyze oxidation of CO.[32] Therefore, FePO4 nanosphere could also be used as a catalyst support with a great potential. The high surface-to-volume ratio and negative surface charge of FePO4 nanosphere could provide a large surface to load more Ag NPs, and thereby improve the catalytic performance toward the reduction of H2O2. To the best of our knowledge, Ag NPs decorated FePO4 nanosphere has never been reported so far. Besides, the synthetic method of FePO4 nanosphere is much simpler than other catalyst supports such as graphene.[33]
In this study, Ag/FePO4 nanocomposites were prepared at a gas-liquid interface. Based on this, we developed a new type of non-enzymatic sensor for electrochemical detection of H2O2. This paper may provide a good catalyst support and a simple method for the development of H2O2 electrochemical sensors.
2 Experimental
2.1 Materials
Hydrogen peroxide (30%, v/v aqueous solution), acetaldehyde (C2H4O, 40%) and ammonium ferrous sulfate ((NH4)2Fe(SO4)2⋅6H2O) were purchased from Tianjin Tianli Chemistry Reagent Co., Ltd (Tianjin, China); Chitosan (CS, MW 5–6×105, >90% deacetylation) was purchased from Shanghai Yuanju Biotechnology Co, Ltd (Shanghai, China); 0.1 M phosphate buffered saline (PBS, pH 7.2) as the supporting electrolyte in electrochemical experiments. In addition, other reagents and chemicals were analytical reagent grade. Doubly distilled water was used in all the experiments.
2.2 Apparatus and Electrochemical Measurements
The transmission electron microscopic (TEM) measurements were executed with Tecnai G 2 F20 S-TWIN (FEI, USA). The energy-dispersive Xray spectroscopy (EDS) was performed during the scanning electron microscopic JSM-6700F (JEOL, Japan) measurements.
Electrochemical measurements were executed with a classical three-electrode electroanalysis system of CHI 660 electrochemical workstation (Shanghai CH Instrument Co. Ltd., China). The bare Au electrodes (diameter: 2.0 mm), FePO4 modified Au electrode and Ag/FePO4 modified Au electrode were used as working electrodes in respective experiments. The saturated calomel electrode (SCE) and platinum wire electrode were used as the reference electrode and counter electrode, respectively. The whole experiment was conducted at room temperature (25±2∘C).
2.3 Fabrication of the Sensor
2.3.1 Synthesis of FePO4 nanospheres
(NH4)2 Fe(SO4)2⋅6H2O (0.4 g), urea (1.0 g) H 3 PO 4 (14 μL) and sodium dodecylsulfate (SDS, 0.1 g) were dissolved in 20 mL doubly distilled water. The mixture was stirred vigorously for about ten minutes, and then decanted into a 25 mL-Teflon-lined autoclave, heated and kept at 80∘C for 12 h. The autoclave was naturally cooled to room temperature after completion of the reaction. The final sediment was collected by centrifugation at 7000 rpm for 8 min, washed twice with doubly distilled water and then dried at 6∘C for 12 h.
2.3.2 Synthesis of Ag/FePO4
10 mL of AgNO 3 (10 mM) was added to a 25 mL beaker containing 10 mL of doubly distilled water. Then, 200 μL of NaOH (1 mol L −1) solution was added to this beaker under stirring condition until a fine brown precipitate of Ag2O was formed. The brown precipitate of Ag2O gradually disappeared until clarification when 25% NH 3 ⋅H 2O (300 μL) was added drop by drop under stable stirring condition. Ag[(NH 3)2] + solution (4.88 mM) was prepared and left for future use.
FePO4 powder (15 mg) was completely dispersed to 45 mL of ethanol-water (1:1, v/v) solution by ultrasound for 30 min, and then ultrasonically mixed with 5 mL of abothe ve as-prepared Ag[(NH 3)2] + solution. After a while, the mixture and another beaker which with 10 mL of CH 3CHO solution were placed in a sealed beaker. This modified silver mirror reaction was performed for 1 h under stable stirring at room temperature. The composite products was collected by centrifugation at 7000 rpm for 8 min, washed thrice with doubly distilled water, and then dried at 5∘C for 10 h.
2.3.3 Modification of electrode
The Au electrode was prepared by a simple method. Before use, the Au electrode was polished with 1.0 and 0.3 μm alumina powder to obtain a mirror-like surface. Then washed with doubly distilled water, completely cleaned in ethanol solution and doubly distilled water in proper order under ultrasonic condition. The Au electrode was dried in a stream of nitrogen. 1 mg of Ag/FePO4 nanocomposite was dispersed in 1 mL of chitosan (wt%, 0.5%) solution under ultrasonic condition for 30 min. The obtained suspension (5 μL) was dropped to the surface of Au electrode and dried in air at room temperature. The modified electrode can be labeled as Ag/FePO4/Au electrode. The process of the synthesis of the material and the modification of the electrode are shown in scheme 1.
3 Results and Discussion
3.1 Characterization of Ag/FePO4 Nanocomposites
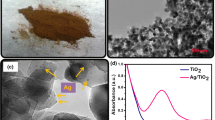
The transmission electron microscopic (TEM) measurements are an efficient tool to characterize the morphology of nanocomposites. The morphology of FePO4 (A, B) and Ag/FePO4 (C, D) nanocomposites are shown in figure 1. From figure 1A and B we can know that the FePO4 composite was more likely a spherical nanomaterial with an average diameter of 650 ± 50 nm. Compared with figure 1D, it is clearly seen that FePO4 nanospheres were successfully decorated with well-dispersed Ag nanoparticles according to the synthesis method. The average diameter of Ag NPs is ranging from 8 to 10 nm. Moreover, a large amount of Ag NPs with small spherical particles were observed uniformly on the surface of the FePO4 nanosphere. Furthermore, the EDS analysis had also been used to characterize the elements in Ag/FePO4 nanocomposites. The EDS spectrum is shown in figure S1 in Supplementary Information.
3.2 Electrochemical behavior of Ag/FePO4
Electrochemical impedance spectroscopy (EIS) is a powerful tool to investigate the interfacial properties of chemically modified electrodes. Generally, the semicircle diameter of the nyquist plot represents the electron transfer resistance (Rct).[34] The suitable equivalent circuit obtained by CHI 660 software is shown in figure 2. Here, R s is the ohmic resistance of the electrolyte solution; Cdl indicates an ideal capacitor; Q is used to represent a constant phase element which is an accumulation of all capacitive effects in the measurement; R et is the interfacial electron-transfer resistance and W is a Warburg impedance which models the diffusion of ions from the bulk electrolyte to the electrode. As shown in figure 2, the diameter of semicircle of the Ag/FePO4/Au electrode (curve a) is smaller than those of the bare Au electrode (curve b) and FePO4/Au electrode (curve c). Moreover, the semicircle diameter has increased from 50 Ω to 300 Ω when the FePO4 is fixed to the surface of Au electrode. However, the R et decreased extremely after addition of the Ag NPs to the surface of FePO4 composite. It should be noted that the curve for the Ag/FePO4/Au electrode looks approximately like a straight line in that frequency range, indicating that the R et of the Ag/FePO4 is pretty small. Therefore, the Ag/FePO4 could efficiently enhance the electron transfer efficiency.
Nyquist plots of (a) Ag/FePO4/Au electrode, (b) bare Au electrode and (c) FePO4/Au electrode in KCl solution (0.10 M) containing 5.0 mM [Fe(CN) 6] 4−/3− at open-circuit potential conditions. Frequency range: 0.01 Hz to 10 kHz; AC amplitude: 5.0 mV. Inset: Equivalent electrical circuit obtained by CHI 660 software.
Figure 3 shows the electrochemical responses of the bare Au electrode, FePO4 modified Au electrode and Ag/FePO4 modified Au electrode as working electrode, respectively. The red arrow indicates the direction of sweep (as in other CVs). From the figure 3, we can know that the bare Au electrode (curve b), FePO4 modified Au electrode (curve c) and Ag/FePO4 modified Au electrode (curve a) show almost no electrochemical response in the absence of H2O2. However, the electrochemical responses have been changed significantly when 5 mM H2O2 was added (figure 3B). Comparing with Ag/FePO4 modified Au electrode (curve d), the bare Au electrode (curve e) and FePO4 modified Au electrode (curve f) exhibited low electrochemical responses. The CV curves of Ag/FePO4/Au electrode (curve g) in O2-saturated 0.1 M PBS (pH 7.2) in the absence of H2O2 are shown in figure 3A. The reduction current of oxygen was small enough to be negligible. It shows that oxygen had a negligible effect in the electrochemical experiments. The Ag/FePO4 modified Au electrode showed an apparent catalytic current peak of approximately −65 μA at nearly −072 V. Thus, the Ag/FePO4 nanocomposites show good catalytic performance towards the electro-reduction of H2O2.
CV curves (b, e) of bare Au electrode, (c, f) FePO4 modified Au electrode and (a, d) Ag/FePO4 modified Au electrode in N2-saturated 0.1 M PBS (pH 7.2) in the absence (b, c and a) and presence (e, f and d) of 5 mM H2O2 at a scan rate of 0.1 V/s. (curve g) Ag/FePO4/Au electrode in O2-saturated 0.1 mol ⋅L −1 PBS (pH 7.2) in the absence of H2O2.
The mechanism of H2O2 electro-reduction can be expressed as follows:[35]



When the surface of the electrode is modified by the Ag NPs, the cathodic reaction is the reduction of H2O2 as the first electron transfer reaction and the anodic reaction is the dissolution of Ag NPs. Actually, the Ag NPs disproportionate the balance of the reaction and speed up the reaction rate in the buffer solution, and the reaction becomes more irreversible:[35]
However, the presence of oxygen in the above action could become the detection lsignal on electrode. The electroreduction of O2 on electrode occurs by the mechanism shown below.[36]


Then,

Because of the excellent catalytic properties of Ag NPs for H2O2, the response signal of H2O2 was detected after Ag NPs were fixed on the surface of FePO4.
The amount of as-prepared [Ag(NH 3)2] + solution is critical to electrochemical experiments because Ag NPs could enhance the electron transfer efficiency for the determination of H2O2.[23] The different amounts of as-prepared [Ag(NH 3)2] + solution can be used to synthesize Ag/FePO4 nanocomposites decorated with different amounts of Ag NPs. Therefore, the Ag/FePO4 nanocomposites with different amounts of Ag NPs were prepared to construct the modified electrode to detect H2O2 by CVs. The experimental results are shown in figure 4. As seen in figure 4, the peak current increased at the beginning and then decreased with the raise of [Ag(NH 3)2] + solution and the maximum peak current was observed with 6 mL as-prepared [Ag(NH 3)2] + solution. However, more Ag NPs were easily aggregated and the catalytic properties were then reduced. Therefore, we chose 5.0 mL of as-prepared [Ag(NH 3)2] + solution as appropriate.
The reduction peak current increased with the increasing concentration of H2O2 from 0.5 to 5.0 mM on the Ag/FePO4 modified Au electrode as shown in figure 5. Figure 5 inset shows that there is a good linear relationship (R = 0.9986) between reduction peak current and concentration of H2O2. These results strongly demonstrate that the Ag/FePO4 nanocomposites had remarkable electrocatalytic performance towards H2O2.
The reduction current at different scan rates with 4.0 mM H2O2 is shown in figure 6. The reduction peak current changed linearly with the square root of the scan rate when the scan rate gradually increased from 0.02 to 0.14 V s −1. Therefore, the electrochemical process of H2O2 reduction on the Ag/FePO4 modified Au electrode is diffusion-controlled.
Figure 7A shows the amperometric current −time curve of the Ag/FePO4 modified Au electrode in N2-saturated 0.1 M PBS (pH 7.2) under stirring condition. The calibration curve for the Ag/FePO4 modified Au electrode is shown in figure 7B. It can be observed that the Ag/FePO4 modified Au electrode exhibits quick response within 4 s. The linear regression equation is I p (μA) =−2.6 C (mM) - 1.24 with a correlation coefficient of 0.9995. Moreover, the Ag/FePO4 modified Au electrode has linear amperometric response to concentration of H2O2 in the range of 3 × 10−5−1.1 × 10−2 M is shown in figure 7B. The sensitivity and detection limit were calculated to be 82.8 μA cm 2 mM −1, 4.7 μM (Signal-to-noise ratio: 3), respectively. The comparison of the performance of our sensor with other non-enzymatic H2O2 sensors are listed in table 1. It is noticeable that our sensor had higher sensitivity than other reported sensors. In addition, our sensor has an acceptable linear range and detection limit. The good performance may be because excellent electrocatalytic properties of AgNPs for electro-reduction of H2O2
(A) Amperometric response of the Ag/FePO4 modified Au electrode upon successive injection of H2O2 in N2-saturated 0.1 M PBS (pH 7.2) under stirring condition Applied potential: −0.45 V. Inset: Amplification of the plot for low concentrations. (B) Calibration curve of current versus its concentration.
3.3 Interference Study
The amperometric response of the Ag/FePO4 modified Au electrode upon the injection of H2O2 (0.5 mM) and other electroactive species (0.5 mM) in N2-saturated 0.1 M PBS (pH 7.2) with stirring condition at −0.45 V are shown in figure 8A The addition of 0.5 mM H2O2 exhibits an obvious amperometric response, while the amperometric current was not changed obviously when glucose, ethanol, ascorbic acid (AA) and uric acid (UA) were added. The amperometric response of the Ag/FePO4 modified Au electrode in O2-saturated (curve a) and N2-saturated (curve b) 0.1 M PBS are shown in figure 8B. From the figure 8B, we can clearly see the stability of the current responses and a good signal-to-noise ratio in N2-saturated 0.1 M PBS at −0.45 V (curve b). The background noise increased because of the electro-reduction of O2 when it was detected in O2-saturated 0.1 M PBS (curve a). But the response current almost remained unchanged. So the Ag/FePO4 modified Au electrode exhibited excellent performance of anti-interference to O2. Hence we conclude that the H2O2 sensor has a favorable ability of anti-interference to other electroactive species and O2.
(A) Amperometric response of the Ag/FePO4 modified Au electrode to successive addition of H2O2, glucose, ethanol, ascorbic acid (AA), and uric acid (UA) (0.5 mM, respectively) in N2-saturated 0.1 M PBS (pH 7.2) under stirring condition. (B) Successive addition of H2O2 (0.15 mM) in (a) O2-saturated and (b) N2-saturated 0.1 M PBS (pH 7.2). Applied potential: −0.45 V.
3.4 Repeatability and stability study
The repeatability and stability of Ag/FePO4 modified Au electrode is extremely important for the entire experiment. The amperometric current responses of five the Ag/FePO4 modified Au electrodes at −0.45 V are compared. The relative standard deviation (RSD) was about 3%, so the modified electrode has good repeatability. The Ag/FePO4 modified electrode remained at 90% of its initial current response after four weeks. To sum up, the Ag/FePO4 modified electrode has acceptable reproducibility and stability.
3.5 Real sample analysis
The real sample analysis of our H2O2 sensor was tested for detecting a detergent by the standard addition method. Briefly, the detergent sample (1.0 mL) was added to 9.0 mL of 0.1 M PBS (pH 7.2) and recorded the amperometric responses of H2O2 reduction in N2-saturated 0.1 PBS. The experimental results are listed in table 2. It is noticed that our H2O2 sensor could be used for the detection of H2O2 in the real samples.
4 Conclusions
In summary, the Ag/FePO4 nanocomposites were synthesized by a modified silver mirror reaction at gas-liquid interface. Then the non-enzymatic H2O2 sensor was fabricated using this nanocomposites modifying the surface of the gold electrode. The non-enzymatic H2O2 electrochemical sensor presents excellent catalytic performance for the detection of H2O2 with a wide linear range and anti-interference ability. This study may be propitious to develop new methods for H2O2 electrochemical sensors.
References
Sanderson W R 2000 Pure. Appl. Chem. 72 1289
Barnard J P and Stinson M W 1999 Infect. Immun. 67 6558
Karthega M, Nagarajan S and Rajendran N 2010 Electrochim. Acta 55 2201
Klassen N V, Marchington D and McGowan H C E 1994 Anal. Chem. 66 2921
Gao Y, Wang G N, Huang H, Hu J J, Shah S M and Su X G 2011 Talanta 85 1075
Steinberg S M 2013 Environ. Monit. Assess. 185 3749
Hoshino M, Kamino S, Doi M, Takada S, Mitani S, Yanagihara R and Fujita Y 2014 Spectrochim. Acta A 117 814
Shi W B, Zhang X D, He S H and Huang Y M 2011 Chem. Commun. 47 10785
Liu M M, Liu R and Chen W 2013 Biosens. Bioelectron. 45 206
Abdulrahman O 2013 Nanoscale 5 8921
Zhang J and Zheng J B 2015 Anal. Methods 7 1788
Mase K, Ohkubo K and Fukuzumi S 2013 J. Am. Chem. Soc. 135 2800
Welch C M, Banks C E, Simm A O and Compton R G 2005 Anal. Bioanal. Chem. 382 12
Long L H, Hoi A and Halliwell B 2010 Arch. Biochem. Biophys. 501 162
Landon P, Collier P J, Papworth A J, Kiely C J and Hutchings G J 2002 Chem. Commun. 18 2058
Zhang J D and Oyama M 2005 J. Electroanal. Chem. 577 273
Jiang F X, Yue R R, Du Y K, Xu J K and Yang P 2013 Biosens. Bioelectron. 44 127
Luo B B, Li X M, Yang J C, Li X L, Xue L P, Li X L, Gu J K, Wang M Z and Jiang L 2014 Anal. Methods 6 1114
Jia F F, Zhong H, Zhu F X, Li X H, Wang Y Z, Cheng Z P, Zhang L L, Sheng Z H and Guo L P 2014 Electroanal. 26 2244
Yadav D K, Gupta R, Ganesan V, Sonkar P K and Rastogi P K 2016 J. Appl. Electrochem. 46 103
Michaels A M, Jiang J and Brus L 2000 J. Phys. Chem. B 104 11965
Iga M, Seki A and Watanabe K 2004 Sens. Actuators, B 101 368
Bai W S, Nie F, Zheng J B and Sheng Q L 2014 ACS Appl. Mater. Inter. 6 5439
Huang H, Yin S C and Nazar L F 2001 Electrochem. Solid-State Lett. 4 A170
Lee J G, Kim B, Cho J, Kin Y W and Park B 2004 J. Electrochem. Soc. 151 A801
Prosini P P, Lisi M, Scaccia S, Carewska M, Cardellini F and Pasquali M 2002 J. Electrochem. Soc. 149 A297
Qing C B, Bai Y, Yang J M and Zhang W F 2011 Electrochim. Acta 56 6612
Laffont L, Delacourt C, Gibot P, Wu M Y, Kooyman P, Masquelier C and Tarascon J M 2006 Chem. Mater. 18 5520
Song Y N, Yang S F, Zavalij P Y and Whittingham M S 2002 Mater. Res. Bull. 37 1249
Okada S, Yamamoto T, Okazaki Y, Yamaki J I, Tokunaga M and Nishida T 2005 J. Power Sources 146 570
Shi Z C, Li Y X, Ye W L and Yang Y 2005 Electrochem. Solid-State Lett. 8 A396
Li M J, Wu Z L, Ma Z, Schwartz V, Mullins D R, Dai S and Overbury S H 2009 J. Catal. 266 98
Yin Y J, Wu P, Zhang H and Cai C X 2012 Electrochem. Commun. 18 1
Chen D, Wang G, Lu W, Zhang H and Li J H 2007 Electrochem. Commun. 9 2151
Masataka H, Takuro K and Hideaki K 1986 Electrochim. Acta 31 377
Xu J, Huang W H and McCreery R L 1996 Electroanal. Chem. 410 235
Lu W B, Liao F, Luo Y L, Chang G H and Sun X P 2011 Electrochim. Acta 56 2295
Zhao W, Wang H C, Qin X, Wang X S, Zhao Z X, Miao Z Y, Chen L L, Shan M M, Fang Y X and Chen Q 2009 Talanta 80 1029
Hocevar S B, Ogorevc B, Schachl K and Kalcher K 2004 Electroanalysis 16 1711
Luo Y L, Lu W B, Chang G H, Liao F and Sun X P 2011 Electrochim. Acta 56 8371
Liu S, Tian J Q, Wang L, Li H Y, Zhang Y W and Sun X P 2010 Macromolecus 43 10078
Acknowledgements
The authors gratefully acknowledge the financial support of this project by the National Science Fund of China (No. 21275116), Specialized Research Fund for the Doctoral Program of Higher Education (No. 20126101120023), the Natural Science Fund of Shaanxi Province in China (No. 2012JM2013, 2013KJXX-25), the Fund of Shaanxi Province Educational Committee of China (No. 12JK0576), the Scientific Research Foundation of Shaanxi Provincial Key Laboratory (2010JS088, 11JS080, 12JS087, 12JS088, 13JS097, 13JS098) and the Graduate Innovation Fund of Northwest University (No. YZZ12019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information (SI)
The EDS spectrum of Ag/FePO4 nanocomposites (figure S1) is given in the supporting information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
RAO, D., ZHANG, J. & ZHENG, J. Synthesis of silver nanoparticles-decorated FePO4 nanosphere at a gas-liquid interface for the electrochemical detection of Hydrogen peroxide. J Chem Sci 128, 839–847 (2016). https://doi.org/10.1007/s12039-016-1062-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-016-1062-8