Abstract
To improve the hydrogen detection performance, a flexible palladium-based hydrogen sensor was designed and fabricated on normal photocopy paper. The paper substrate offers advantages such as light weight, low cost, flexibility and unique surface texture. A conventional vacuum evaporation technique was utilized for 60 nm palladium deposition on the paper and glass substrates. The unique surface texture of the paper effectively increased the surface area to volume ratio for the sensing element, which achieved a higher gas response with faster speed than the glass-based sensor. In addition, we investigated the temperature impacts on sensing performance of the paper-based hydrogen sensor at room temperature and 50 °C. Furthermore, the flexibility test results of the paper-based hydrogen sensor showed that the sensing performances were impervious to mechanical bending of 5.7°.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The inescapable exhaustion of fossil fuel reserves has led to a deep interest in alternative and renewable energy resources [1]. Hydrogen (H2), as a clean, affordable and environmentally friendly energy carrier, is a potential replacement for fossil fuels. Consequently, the problem of depleting fossil fuels can be addressed with H2 wide-ranging applications, such as H2 fuel cells [2], propulsion systems, and H2-fueled cars [3]. However, H2 is a highly flammable gas and detonates when its concentration is over 4% in air; and it is also colourless, odourless and tasteless. These properties of H2 make the safety of hydrogen applications to be a serious consideration. Therefore, a fast, inexpensive, and reliable detection device of H2 gas is required to avoid the hazard of H2-based applications [3].
Conventionally, the most economically available H2 detection devices are metal-oxide based, such as tungsten oxide sensors [4, 5], and tin oxide sensors [6]. While they have an acceptable lifetime, they have poor H2 selectivity, and require a high operational temperature during the gas detection process. In the recent decade, palladium (Pd) and its alloy [7,8,9] have been presented as a superior sensing material compared with metal oxide, which lead to high sensitivity and selectivity of H2 at room temperature. Although the detection performance of Pd alloy is better than pure Pd, the fabrication process is complex and expensive [10,11,12]. In order to achieve a low-cost, fast response, high-sensitivity H2 sensing, pure Pd is the practicable sensing material for H2 leakage detection and concentration measurement.
Although pure Pd has high sensitivity and selectivity to H2, the pure Pd thin film H2 sensor has two major problems: surface peeling off and slow response. These problems are significant matters for practical applications. To address these issues, the low-dimensional nanostructured Pd-based H2 sensor has been a promising research topic in the last few decades [1, 3, 12, 13]. The nanosized Pd sensing element can inhibit its crystal defects, and improve the mechanical strength of the film. Therefore, the Pd thin film H2 sensor is able to avoid the peeling off phenomenon. In addition, the nanosized Pd-based H2 sensor also provides a faster response and higher resolution, owing to the high surface area to volume ratio [14,15,16].
Recently, researchers have presented various Pd nanostructures for H2 gas detection devices [1, 3, 13, 17]. Kim et al. [1] reported that a nanostructured Pd-based H2 sensor using anodic aluminium oxides (AAOs). The AAOs template was applied to form nanoporous Pd film, which led to enhancing the surface area of the sensing layer and improving the performance of the H2 sensor. However, the sensor was not impressive in its sensitivity. A Pd wire network-based hydrogen sensor on glass was presented by Walia et al. [17]. The research indicated that the Pd wire network was implemented with the crackle lithography technique, which was able to fabricate the microsized crackles on the glass. Because this novel fabrication technique was utilized, the reaction interface of Pd and H2 molecules was increased, and thus achieved high sensitivity and fast response. Lim et al. [3] investigated a flexible Pd-based H2 sensor with high sensitivity and short response time. The Pd nanowires were fabricated by using the nanoimprint lithography technique, and then the nanowires were transferred to a polycarbonate substrate. Overall, the aforesaid nanostructured Pd-based H2 sensors were fabricated by using advanced fabrication techniques to achieve the large surface area to volume ratio of Pd, however the processes were complex and/or costly [13, 18, 19].
In the present work, a low-cost and flexible Pd-based H2 sensor is designed, fabricated and characterized. In order to enhance the H2 detection performance of the sensor, the surface texture of the normal photocopy paper (NPP) is effectively utilized to achieve low-dimensional nanostructures in this investigation. The 60 nm Pd thin film is coated on the NPP by using the vacuum evaporation system, and the characterization of the microfibre networks sensing layer is analysed by a scanning electron microscope (SEM). The combination of the 2-dimensional (2D) nanosized sensing layer and microfibre networks can form a nanoshell sensing structure, thereby significantly increasing the surface area to volume ratio. Thanks to the conventional fabrication process and the ubiquitous substrate, the sensor offers advantages such as simple fabrication, low cost, flexibility, light weight, and mass production. The presented H2 sensor is capable of detecting the low concentration of 100 ppm H2, and has a short response time of 12 s when exposed to 10,000 ppm H2 at room temperature. In addition, the temperature effect and bending effect of the sensor were investigated during the experimental testing process.
2 Design and experiment
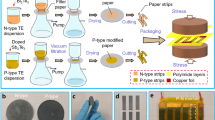
The process flow for the fabrication of the Pd microfibre networks H2 gas sensor is illustrated in Fig. 1. The vacuum evaporation technique is utilized to deposit pure Pd thin film on the surface of the NPP (A4/80gsm, Paper Australian Pty Ltd.) substrate. Because of the surface texture of the NPP substrate, the Pd microfibre networks are formed on the NPP substrate. Thus, the large surface area to volume ratio of the sensing element is achieved according to the microfibre morphology of the NPP. Moreover, the NPP is a low-cost, flexible, light, and ubiquitous material for the substrate of the H2 sensor.
The facile fabrication process flow of the H2 sensor is schematically described in Fig. 1. First, dust on the surface of the NPP was removed by using a high-pressure nitrogen gun. Then, Pd with a purity of 99.95% was deposited on the NPP surface by employing a vacuum evaporation system (VPC-260, ULVAC Technologies, Inc.) to form the microfibre networks structure with high density (superficial density) Pd layer. High film density is a critical parameter to improve the mechanical properties of the Pd film, and the adhesion to the substrate. To fabricate the superior mechanical property and smooth surface of the Pd thin film, the film deposition system was operated under a vacuum of 2.0 × 10−5. Torr, and a current of 90 A. For sensing performance comparison and thickness reference purpose, a microscope cover glass slip was chosen to be placed adjacent to the NPP substrate. The glass was cut into the size of 20 mm by 5 mm, cleaned in ethanol for 10 min in an ultrasonic bath, and then dried using a high-pressure nitrogen gun. With the same evaporation conditions, 60 nm Pd thin films on the NPP and glass substrates were achieved after 3 min of deposition time. The Pd thin film on NPP substrate was cut into pieces of 20 mm by 5 mm after the whole NPP substrate was transferred out of the vacuum evaporation system. Finally, the electrodes were made on the edges of sensors with silver epoxy.
The H2 sensor testing was implemented by placing the sensors in a sealed quartz tube (gas chamber) and connecting the sensors with measurement circuitry using soft gold wires, as illustrated in Fig. 2. Pure nitrogen and 1% H2 gas were connected to the mass flow controller (MFC), and the H2 concentration was controlled by the MFC. The gas flow rates of MFCs were controlled to obtain various H2 concentrations of 100 ppm, 2000 ppm, 4000 ppm, 6000 ppm, 8000 ppm and 10,000 ppm for the sensor characterization.
Before the sensing experiments, pure nitrogen was fed into the chamber for 10 min to remove all the residual gases. During H2 concentration measurement, the mixed gas flow rate at the inlet of the gas chamber was set at 100 standard cubic centimetres per minutes (SCCM). Each concentration of H2 was flowed into the gas chamber for 300 s and was cut off for 300 s for recovery while nitrogen gas was flowing. To convert the resistance change of the sensor to voltage readings, a voltage divider was employed in the measurement setup. The applied DC voltage (E) was 1 V and the standard resistor (R) was set to be \(2\ \text{k}\Omega\) and 220 \(\Omega\) to match the resistances of NPP (\(1.79\ \text{k}\Omega\)) and glass-based (\(207.85 \ \Omega\)) sensors, respectively. In real-time, the voltage change of R was recorded by a digital multimeter once per second and logged into a computer while the H2 sensor was exposed to different concentrations of H2. All the tests of the NPP-based hydrogen sensor were carried out under three different conditions, including room temperature, 50 °C, and bending at room temperature. The gas response (G) of the H2 sensor was described as shown in Eq. (1):
where \(R_{N}\) and \(R_{H}\) are the sensor resistance in nitrogen and after the mixed H2 gas injection, respectively. The range of the H2 concentration was from 100 ppm to 10,000 ppm.
3 Results and discussion
A scanning electron microscope (SEM) was used to image the surface morphology of the NPP-based H2 sensor. To avoid charging effects during SEM imaging, it was necessary to connect the surface of the Pd thin film to the testing platform with double-sided carbon tape. The surface morphology images of the NPP-based H2 sensor are presented in Fig. 3 with two different magnification. The network structure of multilayer microfibre is clearly shown in Fig. 3a; and up to approximate 30 μm in diameter of the Pd microfibre can be observed in Fig. 3b. The combination of the nanosized sensing layer and microfibre networks can improve the surface area to volume ratio owing to the transformation from 2D to nanoshell networks structure. The detailed analysis will be discussed later in this section. Additionally, the surface elements of the sensor were plotted according to the energy dispersive X-ray analysis, as shown in Fig. 4, which demonstrated that carbon, oxygen, palladium, and calcium were obviously contained on the surface of the NPP-based H2 sensor.
The response time, recovery time and gas response of the Pd-based H2 sensor when exposed to various H2 gas concentrations are illustrated in Fig. 5. The response time is defined as the time needed to change the resistance of the sensor from the initial value to 90% of the final stable value, whereas the recovery time is defined as the time needed to change the resistance from the stable value to 10% of it.
As well known, 4% H2 concentration is a critical value for H2 gas to be explosive. Due to the safety consideration, the maximum H2 concentration was set at well below 4% all the time. Figure 5a represents the response times of the H2 sensor with the glass and NPP substrates when exposed to specific concentrations from 100 to 10,000 ppm. For instance, the resistance of the glass-based H2 sensor rose quickly when exposed to 10,000 ppm H2, and its resistance reached 90% of the final stable value within 47 s. The large surface area to volume ratio of the H2 sensor was attained because the nanosized Pd thin film was coated on a flat glass substrate. Therefore, the glass-based H2 sensor was able to provide the fast response speed. On the other hand, as shown in Fig. 5a, NPP-based H2 sensor achieved much shorter response time of 12 s at 10,000 ppm H2. The surface morphology of the NPP is an effective means to further improve the surface area to volume ratio of the Pd thin film, as shown in Fig. 6.
The principles of absorption and desorption are same for glass-based and NPP-based H2 sensors. In terms of the distinctive interaction between Pd and H2 gas, the H2 partial pressure changes along with the absorption and desorption processes of the Pd-based H2 sensor when exposed to H2 gas [20]. During the absorption process, the H2 partial pressure of the outside of the Pd thin film is higher than the inside of the sensing element, leading to hydrogen atoms diffusion into the Pd thin film. Based on this phenomenon, the Pd–H hydride is formed and results in the Pd physical properties changes, involving mass, volume and electrical resistance. When the Pd-based H2 sensor is exposed to air or nitrogen, the H2 partial pressure in the Pd thin film is higher than that on the surface, which causes the desorption occurrence [21].
As presented in Fig. 6, the NPP-based H2 sensor is capable of achieving higher detection performance compared with the glass-based sensor, and the reason can be explained by the following equations. The surface area to volume ratio of the Pd thin film on the glass substrate is inversely proportional to its thickness, as shown in Eq. (2).
where \((A/V)_{{{\text{Glass}}}}\) is the surface area (A) to volume (V) ratio, and HPd is the thickness of the Pd film on the glass substrate.
During the Pd deposition process, the flat Pd thin film with the average thickness of HPd was coated on the glass substrate, as shown in Fig. 6a. However, the thickness of the Pd thin film on NPP microfibre was not uniform during the same deposition conditions: the Pd film on the side wall of the fibre was thinner than that on the top and bottom, as presented in Fig. 6b. In that case, we presumed the thickness of the Pd film on the top and bottom of the fibre was HPd, the side part was HS. Thus, the surface area to volume ratio of the Pd thin film on NPP substrate, \((A/V)_{NPP}\), can be defined as Eq. (3)
where \(A_{TB}\) is the total surface area of the top and bottom parts; and \(A_S\) is the total area of side wall. \(H_S\) is the film thickness of the side wall. Because (\(H_S\)-\(H_{Pd}\)) is less than zero, thus Eq. (3) can be simplified as presented as Eq. (4).
Combination of Eqs. (2) and (4) indicates that the NPP-based H2 sensor has a larger surface area to volume ratio than glass-based. Thus, the nanoshell network structure of Pd microfibres with larger surface area to volume ratio was formed after Pd coated the surface of the NPP, which led to increasing the reaction interface of the Pd and H2 molecules. Therefore more diffusion pathways of atomic hydrogen were provided on the surface of Pd thin film. As a result, the gas response speed can be improved.
The recovery times of the NPP-based and glass-based H2 sensors are presented in Fig. 5b, the recovery speed of both H2 sensors are also affected by the types of the substrates. The hydrogen atoms in NPP-based \({\text{H}}_{2}\) sensor were fully released to the chamber within 117 s for 10,000 ppm H2, which was about 60 s faster than the glass-based sensor. In addition, the larger surface area to volume ratio of the Pd thin film can have smaller transverse dimensions, therefore the more hydrogen atoms can be absorbed in the interstitial sites of the Pd lattice [22]. Thus, more electron scattering in Pd decreases the mobility of electrons, and the resistance of the Pd-based H2 sensor is increased. This indicates that the larger surface area to volume ratio of the Pd-based H2 sensor has a higher gas response, as proved in Fig. 5c. At H2 concentrations of 100, 2000, 4000, 6000, 8000 and 10,000 ppm, the gas responses of the NPP-based H2 sensor were 0.55, 1.37, 1.75, 1.94, 2.10 and 2.25%, respectively. By comparison, the gas response of the sensor based on the glass substrate was 1.25% for 10,000 ppm H2, which was 44.4% lower than that of the NPP-based H2 sensor at the same H2 concentration. This validated that the NPP-based sensor has a higher surface area to volume ratio as discussed in this section.
Apart from the microfibre texture, the NPP substrate has many other advantages over the glass substrate, for example its flexible property. To investigate the flexibility performance of the NPP-based H2 sensor, the bending test was implemented, as shown in Fig. 5d. For comparison purpose, the bending test of the H2 sensor was based on the same NPP-based hydrogen sensor. The NPP-based sensor was bended by 5.7o (1 mm above the platform) as shown in Fig. 5d. The response time, recovery time and gas response of the bended NPP-based H2 sensor with the H2 concentrations from 100 to 10,000 ppm are included in Fig. 5a–c, respectively. Evidently, there were not obvious discrepancies in the response time, recovery time, and gas response before and after the NPP-based H2 sensor was bended. Therefore, it implies that the NPP-based H2 sensor is suitable for flexible sensing applications without performance degradation.
The working temperature of sensors has been confirmed to be a significant factor effecting sensor performances. As shown in Fig. 7, the response time, recovery time, and gas response of the NPP-based H2 sensor were plotted during exposure to 100–10,000 ppm hydrogen at room and high temperature (25 °C and 50 °C). Theoretically, the movement of gas atoms increases with the increase of temperature, especially for hydrogen with small molecular mass [10]. Thus, the absorption and desorption rates increase at higher temperatures, resulting in shorter response time and recovery time, as proved in Fig. 7a and b. However, lower gas response was obtained when temperature was increased from room temperature to 50 °C, as presented in Fig. 7c. In addition, the standard deviations of the gas responses for the NPP-based H2 sensor at room temperature and 50 °C were less than ± 0.05% and ± 0.03%, respectively. Therefore, the high repeatability and stability of the sensor were proved at both detection temperatures. To investigate the gas response details at same H2 concentration and different temperatures, the gas response curves were redrawn with the same starting time, as plotted in Fig. 7d. The gas response of the NPP-based H2 sensor has decreased from 2.25 to 1.98%. The unique relationship can be explained by Sieverts’ Law, which states that the logarithm of H2 solubility increases linearly with the decreased temperature under the specified H2 partial pressure [1, 7, 8].
The detailed relationship between the gas response and H2 concentration can be also clarified by Sieverts’ Law in Eq. (5)
where (\(\text{ H/Pd}\)) is the concentration of the \({\text{H}}_{2}\) dissolved in the Pd thin film (equilibrium condition), \(K_{H}\) is the Sieverts’ constant, and \(P_{(H_{2} )}\) is the H2 partial pressure on the Pd thin film.
As depicted in Eq. (5), the ratio of the dissolved hydrogen to palladium atoms is dependent on the square root of the H2 partial pressure (H2 concentration in this experiment). The change of this ratio leads to a proportional gas response (G), as reported in [13, 20]. Using the experimental data in Fig. 7c, the H2 partial pressure (From H2 concentrations >100 ppm) versus the gas response relationships at different temperatures are plotted in linear curves, as shown in Fig. 8. Specifically, the calculated value of \(K_H\) is reduced from 0.1566 to 0.1255 when temperature is increased from 25 to 50 °C. According to Sieverts’ Law, the lower value of \(K_H\) can reduce the gas response at the same concentrations. Therefore, as shown in Fig. 7c, the gas response is reduced when temperature increases.
4 Conclusion
This paper presents the superior gas sensing performance of a Pd microfibre networks H2 sensor which was fabricated using a simple vacuum evaporation technique. The networks of the 60 nm Pd microfibres were formed on a NPP substrate, which was capable of improving the surface area to volume ratio of the Pd thin film. The experimental results indicated that the performance of the NPP-based H2 sensor was better than the glass-based sensor during the same detection conditions. This sensor presented a fast response of 12 s and high gas response of 2.25% at 10,000 ppm H2. Comparatively, our sensor has a higher gas response than Wang’s work [10], and more rapid response compared with Walia [17] and Lee [20]. The response time and recovery time was reduced at the high temperature, but the gas response was decreased. On the other hand, Its detection performances were unaffected by mechanical bending. Therefore, the NPP-based H2 sensor is flexible and suitable for room temperature H2 leakage detection. In addition, the advantages of the present sensor include simple fabrication, low cost, flexibility, light weight, and mass production.
References
B.J. Kim, J.S. Kim, Int. J. Hydrogen Energy 39, 16500–16505 (2014)
H. Görgün, M. Arcak, S. Varigonda, S.A. Bortoff, Int. J. Hydrogen Energy 30, 447–457 (2005)
S.H. Lim, B. Radha, J.Y. Chan, M.S.M. Saifullah, G.U. Kulkarni, G.W. Ho, A.C.S. Appl, Mater. Interfaces 5, 7274–7281 (2013)
J. Tamaki, T. Hashishin, Y. Uno, D.V. Dao, S. Sugiyama, Sensors Actuators B 132, 234–238 (2008)
D.V. Dao, K. Shibuya, T.T. Bui, S. Sugiyama, Procedia Eng. 25, 1149–1152 (2011)
B. Wang, L.F. Zhu, Y.H. Yang, N.S. Xu, G.W. Yang, J. Phys. Chem. C 112, 6643–6647 (2008)
J.H. Yoom, B.J. Kim, J.S. Kim, Mater. Chem. Phys. 133, 987–991 (2012)
H. Song, Y.P. Chen, G. Zhang, Y. Liu, P.C. Huang, H.W. Zhao, M.H. Yang, J.X. Dai, Z. Li, Sensors Actuators B 216, 11–16 (2015)
H. Song, Z.J. Luo, M.Y. Liu, G. Zhang, W. Peng, B. Wang, Y. Zhu, Sensors 18, 1448 (2018)
M. Wang, Y. Feng, Sensors Actuators B 123, 101–106 (2007)
N. Tasaltin, S. Öztürk, N. Kilinc, Z.Z. Öztürk, J. Alloy. Compd. 509, 4701–4706 (2011)
B. Wang, Y. Zhu, Y.P. Chen, H. Song, P.C. Huang, D.V. Dao, Mater. Chem. Phys. 194, 231–235 (2017)
X.Q. Zeng, Y.L. Wang, H. Deng, M.L. Latimer, Z.L. Xiao, J. Pearson, T. Xu, H.H. Wang, U. Welp, G.W. Crabtree, W.K. Kwok, ACS Nano 5, 7443–7452 (2011)
P. Offermans, H.D. Tong, C.J.M. van Rijn, P. Merken, S.H. Brongersma, M. Crego-Calama, Appl. Phys. Lett. 94, 223110 (2009)
Y. Lim, Y. Lee, J. Heo, H. Shin, Sensors Actuators B 210, 218–224 (2015)
B. Wang, Y. Zhu, V. Huynh, M.A. Rahman, B. Hawkett, S. Sriram, D.V. Dao. (2019) https://doi.org/10.1007/978-3-030-04290-5_12
S. Walia, R. Gupta, K.D. Rao, G.U. Kulkarni, A.C.S. Appl, Mater. Interfaces 8, 23419–23424 (2016)
F. Yang, S.C. Kung, M. Cheng, J.C. Hemminger, R.M. Penner, ACS Nano 4, 5233–5244 (2010)
K.J. Jeon, J.M. Lee, E. Lee, W. Lee, Nanotechnology 20, 135502 (2009)
E. Lee, J.M. Lee, J.H. Koo, W. Lee, T. Lee, Int. J. Hydrogen Energy 35, 6984–6991 (2010)
B. Wang, M.A. Rahman, Y. Nara, T. Hashishin, D.V. Dao, Y. Zhu, J. Phys. Chem. Solids 131, 50–54 (2019)
X.Q. Zeng, M.L. Latimer, Z.L. Xiao, S. Panuganti, U. Welp, W.K. Kwok, T. Xu, Nano Lett. 11, 262–268 (2011)
Acknowledgements
The authors would like to thank Professor Hiroshi Kubota, faculty of engineering, Kumamoto University, Japan for providing micro-fabrication and characterization facilities, and the technical support from Mr. Abe.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, B., Nara, Y., Hashishin, T. et al. Palladium on paper as a low-cost and flexible material for fast hydrogen sensing. J Mater Sci: Mater Electron 31, 5298–5304 (2020). https://doi.org/10.1007/s10854-020-03090-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03090-7