Abstract
Renewed interest for volatile organic compound (VOC) sensors using nanomaterials are increasing due to several limitations of existing commercially available sensors for healthcare, food quality, and environmental applications. A disposable, flexible, and room temperature, paper-based formaldehyde sensor has been developed using graphene quantum dot ink as the sensing material. The PEDOT:PSS conductive ink acts as an electrode on the porous paper substrate with graphene quantum dot ink as sensing material making the device novel, low cost, and potential candidate for large area roll to roll solution process-able formaldehyde sensors fabrication. The sensitivity is measured for a concentration varying from 7 to 20 ppm exposure of formaldehyde at ambient temperature at dry air condition. The sensor device shows a sensitivity of 0.26% at 15 ppm HCHO. The p-type GQDs reveal a resistance increment of the sensing film in presence of reducing gas HCHO, which is explained using charge transfer dynamics between sensing film and HCHO molecules. The finding may lead to new opportunities in flexible formaldehyde sensors operating at room temperature for healthcare applications.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Monitoring of volatile organic compounds (VOC) such as formaldehyde (HCHO) is crucial as HCHO has detrimental effects on human health, including irritation and dryness of the skin, nose, throat, and olfactory organs (Srivastava et al. 2000). It is a common mucous-membrane irritant that engenders conjunctivitis and lacrimation (Liu et al. 2018). This colorless, flammable chemical is one of the most common carbonyl compounds that can be found naturally in various raw foods like meat, fish, milk, or dairy products and packaged food by way of preservatives (Zhang et al. (2014)). Exposure to HCHO can cause eye irritation at 0.3–0.9 ppm concentration in industrial workers and might develop conjunctivitis and lacrimation for exposure in the range of 4–20 ppm. In 2006, the International Agency for Research on Cancer (IARC) of the WHO and in 2011 the National Toxicology Program of the US Department of health and human services categorized HCHO as a human carcinogen (Guzman et al. 2018). During the last decades, highly sensitive yet low-cost, HCHO sensors with broad detection range and fast response had drawn considerable attention to the scientific community. Traditional chemiresistive HCHO sensors were fabricated using semiconductor oxides sensing film with high sensitivity and detection range (Afzal et al. 2012). Recently Khampho et al. have been invented an HCHO sensor with AgOx doped SnO2 sensing film for good response at high temperature (350 °C) (Khamfoo et al. 2020). However, these sensors work at high operating temperatures (200–600 °C), leading to high power consumption, flammability risk, and requirement of rigid, costly substrates like quartz or glass (Liu 2014; Yamazoe 2005). For obvious reasons, detecting HCHO at room temperature with low power consumption and an absence of flammability risk on flexible and environment-friendly sensing platforms needs further investigation.
Nowadays, room temperature HCHO sensors using various nanomaterials are investigated due to large surface to volume ratio, and edge functionalization properties (Cheng et al. 2010; Robinson et al. 2008; Huang 2012). The discovery of graphene opens up plenty of research opportunities with different new nanomaterials in fundamental and application domains, including flexible electronics, bio-degradable sensors, non-toxic biomedical drug delivery, etc. Graphene quantum dots (GQD) (Shen et al. 2012), a derivative of graphene, is the newest member of the graphene family with three-dimensional confinement of carbon domains, possessing excellent properties like low toxicity, size-dependent electronic properties, and excellent biocompatibility (Ponomarenko 2008). The existence of functional groups at the edges of GQDs helps test gas molecules to interact, leading to potential candidates for sensing applications (Shen et al. 2012; Dey et al. 2018). A recent study has shown that the composition of Ag-doped perovskite-type oxide LaFeO3 sensing film with GQD reduces the operating temperature from 90 to 55 °C and provides greater selectivity in VOC sensing by functionalizing GQDs with different dopants (Zhang et al. 2018). Another report demonstrated the room temperature isopropanol sensor with GQD-TiO2 heterojunction sensing film and showed a response value of 13.8 at 50 ppm (Shao et al. 2016). However, the Silicon substrate with gold finger electrodes makes the device fabrication process difficult, time-consuming, expensive and demands industrial machinery support. Furthermore, GQDs embedded in a conjugated polymer matrix has been used for room temperature VOC sensors on industrially fabricated SiO2/Silicon substrate (Gavgani 2015). However, inexpensive, eco-friendly paper-based sensors might solve the problems related to the cost and hazardous industrially processed silicon-based sensors and offer great potential for flexible e-skin sensors (Wang 2009). Since the extraction of environmentally benign cellulose paper from wood pulp, different cellulose derivatives have found tremendous applications in various low-cost, flexible sensors. However, a challenging key point in the paper-based sensor device fabrication is the porous nature of the paper substrate that hinders the making of a continuous, smooth film of nanomaterials on it. Thus, various electrode design techniques on cellulose papers have been developed to block the papers’ porous structures, including inkjet printing, drawing with pencils or ball pens filled with conductive ink, etc. (Seekaew et al. 2014; Wu et al. 2014). Earlier, some groups have reported commercially available conducting polymer poly3,4-ethylenedioxy thiophene: polystyrenesulphonate (PEDOT:PSS) based solution process-able electrodes for optoelectronic applications such as solar cell (Tang et al. 2015; Lee 2017), LED (Kim et al. 2013), and photodetectors (Liu et al. 2015; Aga et al. 2014) due to their high electrical conductivity, aqueous solution process-ability, superior environmental stability and biocompatibility, superior film-forming properties, and fine-tuning of their physicochemical properties (Kim et al. 2011). PEDOT:PSS film possesses a unique structure of entangled conductive PEDOT domains with an insulating PSS matrix that can be used to tailor its electronic properties (Dehsari et al. 2014; Kim et al. 2011), which makes it a promising candidate as electrodes in paper sensors.
In this work, we have fabricated a novel disposable, biocompatible, all solution-processed, room temperature, paper-based HCHO sensor using conducting ink of PEDOT:PSS as an electrode and GQDs-based colloidal ink as the sensing material. Graphene Quantum Dots are synthesized from graphite powder using modified Hummers’ method followed by amiditive cutting in conc. HNO3/H2SO4 solution and subsequent reduction by hydrothermal reaction (Dey et al. 2020). Further, a continuous film of desirable conductance is achieved as electrodes on the porous paper substrate by successive spin-coating of PEDOT:PSS. Our experiment reveals that GQD ink on PEDOT:PSS electrodes shows the response from 7 ppm exposure of HCHO under dry air conditions even at room temperature. The study shows that the GQD ink on conductive PEDOT:PSS electrode on the paper substrate can be effectively utilized to overcome the limitation of expensive industrial-made HCHO sensors.
2 Experimental Section
2.1 Graphene Quantum Dot Synthesis
Synthesis of graphene quantum dots in bottom-up technique from graphite flakes is followed by three different steps: a) Preparation of graphene oxide using modified hammer’s method; b) Acid cutting of GO to form graphene oxide quantum dots; c) Hydrothermal reduction to form GQDs.
Chemical oxidation of Graphite flakes: Graphene oxide is synthesized by a modified hammer’s method as reported earlier. Shortly, 750 mg of graphite powder and 750 mg of NaNO3 were added with 37.5 mg of H2SO4 and stirred constantly on an ice bath till the temperature reaches 0 °C. Next, at this very low temperature, 4.5 g KMnO4 was added to the mixture dropwise under stirring to avoid an explosion. After that, the mixture was transferred to a 40˚C water bath and allowed to stir for 1 h. Then 60 ml water was added to the mixture and stirred for 30 min at 95 °C operation temperature. After that the mixture was removed from the heater and150 ml water is added to dilute the mixture. Finally, 4.5 ml H2O2 was added for the quenching of the reaction. Leave the mixture for about 5 h to settle down and then filter the mixture using 4.5 µm pore-sized Whatman filter paper. Then wash the sample with DI water by centrifuging it at 20,000 rpm 4–5 times until the solution reached pH 7. Finally, 250 ml of DI water is added to make a graphene oxide solution.
Acid cutting of graphene oxide sheets: To reduce the size of graphene oxide sheets, as-synthesized GO sheets were treated with H2SO4 and HNO3 mixture (1:3 ratio) and the whole solution was ultra-sonicated for 24 h. Finally, the mixture was washed up to pH 7 by centrifuging 5–6 times. The resultant solution is graphene oxide quantum dots (GOQDs).
Hydrothermal reduction of graphene oxide quantum dots: GQDs were synthesized by the hydrothermal method using GOQDs as precursor material. 10 ml of uniformly dispersed GOQD solution was transferred into a Teflon lined autoclave (100 ml) and heated at 200 °C for 12 h. After removing from the hydrothermal chamber, GQDs were transferred into DMF to make a uniformly dispersed solution.
2.2 Sensor Device Fabrication
Sensor devices were fabricated on flexible disposable Whatman cellulose chromatography papers as substrate. To fabricate electrodes on cellulose paper substrate, PEDOT:PSS was drop casted at a separation distance of 1 mm. The sensing layer was prepared by bridging the gap between the two electrodes by GQD ink from DMF solvent. The remnant DMF solvent was completely removed keeping in a vacuum chamber. Further, multiple sequential drop-casting steps were performed to acquire resistance of the order of KΩ at a separation distance of 3 mm.
2.3 Sensing Unit
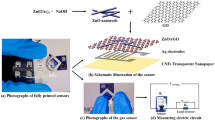
The gas sensing unit (GSU) used in our experiment is a custom-made setup consists of mass flow controllers (MFCs), gas mixers, gas cylinders, an airtight chamber, a data acquisition (DAQ) system, and a computer as shown in Fig. 1b. The sample chamber is made up of stainless steel maintaining great isolation between the inner and outer environments. Air and H2 gas flow rates were controlled through MFCs from Alicat. Steel pipes were connected with the different parts of the sensors system. By varying the different flow rates of air and hydrogen, the different concentrations of H2 were achieved. In the gas mixer, two gases were mixed properly before coming into the test chamber. HCHO vapor is created by flowing dry air inside a container containing HCHO solution. Inside the test chambers, there were four probes, capable of measuring two devices simultaneously. The outputs of the sensors were connected in Agilent 349,721 LXI DAQ unit fitted with 34901A 20 channel multiplexer switches and a digital ohmmeter. BenchLink Data Logger Pro software was used for sensor response data acquisition. In this study, the change of sensor resistance due to different concentrations of formaldehyde was measured using DAQ system at room temperature. The outlet gas test chamber was connected through a bubbler up to the outside of the room so that the gases can be thrown out from the room.
3 Result and Discussion
3.1 Sample Characterization
The structural characterization of as-synthesized GQDs was performed using high-resolution transmission electron microscopy (HRTEM) [JEOL, JEM-2100F]. Atomic force microscopy (AFM) [Agilent Technology, 5500 AFM] was used to predict the height of the GQDs in tapping mode for further knowledge about the structure of synthesized GQDs. Further, Raman spectroscopy was performed with a 514 nm wavelength Argon laser and a spectrometer [JobinYvon Horiba T64000] for confirmation of molecular bonding present in the material. For optical characterization of the material, ultraviolet–visible (UV–Vis) absorption spectroscopy with a fiber probe-based Avaspec-3648 spectrometer combined with broadband Deuterium and Halogen excitation sources, is performed on GQDs in the solution phase. For steady-state Photoluminescence studies, the GQD sample was analyzed in solution using a He-Cd Laser of excitation wavelength of 325 nm and a monochromator [TRIAX-320] equipped with a photomultiplier detector [Hamamatsu R928].
The TEM image of as-prepared GQDs from graphite powder indicates mono-dispersed GQDs with an average diameter of 4 nm, distributed on the TEM grid as depicted in Fig. 2a. The AFM image with the corresponding height profile in the inset (Fig. 2b) provides a height measurement of an average of ~4 nm. AFM image of Fig. 2b shows a uniform distribution of GQD nanoparticles over a large area.
a TEM image of GQDs showing particles dispersed uniformly over the area. b AFM image of mono-dispersed GQDs with average height 4 nm showing in height profile along the line drawn in the image. c Raman spectrum of as synthesised GQDs showing distinct G and D bands. d Optical absorption spectra and photoluminescence spectra of GQDs
Figure 2c shows Raman spectra of as-synthesized GQDs under 514 nm laser excitation. Two distinct Raman peaks are observed at ~1342 cm−1 and ~1590 cm−1 representing the D and G band, respectively, which are the characteristic peaks of graphene-based materials. The ‘crystalline’ G band is attributed to the primary in-plane vibrational mode at Γ point of the Brillouine zone. Moreover, the disordered ‘D’ band arises due to the existence of defects and functional groups in chemical synthesized GQDs and represents the breathing mode of clustering hexagonal aromatic rings. The characteristic 2D.
The 2D Raman peak of graphene at ~2700 cm−1 is reduced for GQDs due to three-dimensional confinement of carbon domains. Figure 2d represents a typical UV absorption spectrum (blue curve) and photoluminescence emission spectrum (red curve) of as-synthesized GQDs in an aqueous solution. An intense absorption peak is observed at ~295 nm, which contributes to the π–π* transition in the molecular orbital of sp2 hybridized carbon domains from the hexagonal core of GQDs. The photoluminescence spectrum of GQDs (the red curve) depicts two peaks at ~440 nm and ~520 nm. The intense peak at 440 nm is due to the electronic transition from sp2 hybridized confined carbon core states, and the relatively weaker one is due to the emission from radiative shallow trap states arises due to the presence of surface functional groups.
3.2 Gas Sensing Performance Evaluation
The working principle of the chemiresistive sensor is hinged on the resistance change due to the interaction between the gas molecules and the surface of the sensing material. The fabricated device is mounted inside a custom-made gas sensing unit and exposed to dry air for about 1 h to get a stable baseline. After achieving a stable baseline, the sensing device is exposed to different concentrations of HCHO for a constant time of 10 min, followed by restricting the HCHO flow and allowing dry air inside the chamber for another 10 min for recovery of the sensing film. After achieving a stable baseline under dry air exposure, upon test gas molecules exposure, the resistance of the devices increases or decreases due to interaction between the gas molecules and the sensing layer. Again, in the absence of test gas at dry air condition, the resistance of the devices recoils to its initial position, which results in dynamic sensor response. The response of the device is calculated using the equation (Liu et al. 2015)
where \({R}_{\mathrm{Test gas}}\) is the resistance of the sensing film at respective ppm exposure of test gas and \({R}_{\mathrm{Baseline}}\) is the resistance of the same in dry air condition.
The fabricated device is mounted on the sensing chamber and exposed to different ppm of formaldehyde concentrations starting from 7 ppm. Under HCHO exposure the sensing film resistance starts to increase and gets saturated, but after stopping the HCHO flow the resistance starts to decrease. The response of the device at four concentrations of HCHO is depicted in Fig. 3. The sensor response is calculated from experimental data using Eq. 1 and shown in Table 1. The device shows 0.15% response on a minimum of 7 ppm under HCHO exposure. The chemical synthesis route incorporated oxygen-containing defect sites on GQD carbon domains, which withdraws electrons from the material, making it a p-type semiconducting material with majority carrier holes. HCHO is a reducing gas with electron donating character. Adsorption of HCHO molecules on the surface of GQD leads to electron transfer into the material from HCHO molecules and a decrease in hole concentration in the intrinsically p-type GQD layer occurs. The decrement of majority carrier concentration results in the lower conductivity of the material which leads to an increase of resistance of the sensing film.
In the case of the recovery process, after restricting the HCHO flow, dry air is started to flow into the chamber which accelerates the desorption process of HCHO molecules from GQD surface. This desorption process disrupts the electron transfer into GQD film, leading to the recovery of the film resistance. Additionally, an anomalous behavior has been observed in the sensor response for every concentration of gas flow. However, the initial decrease in resistance can be attributed to experimental error due to very low ppm of HCHO flow inside the chamber which starts to increase after a few seconds.
The performance of our device is comparable with available literature as shown in Table 2.
4 Conclusion
Graphene quantum dots are synthesised successfully from graphite powder using a top-down method followed by characterization with optical spectroscopic techniques. For device fabrication, we have chosen low-cost disposable cellulose paper substrate and solution-processed PEDOT:PSS conducting ink as electrodes. Graphene quantum dot ink has been drop cast between the PEDOT:PSS electrodes as the sensing material for formaldehyde sensing. The novel fabricated devices are completely solution-processed, low cost, and biocompatible. Formaldehyde sensing has been performed using the devices at the lowest concentration of 7 ppm with responsivity 0.15%. The fabricated low-cost novel disposable sensors may lead to new opportunities in the room temperature flexible formaldehyde sensors for healthcare applications with very simple fabrication techniques.
References
Afzal A, Cioffi N, Sabbatini L, Torsi L (2012) NOx sensors based on semiconducting metal oxide nanostructures: progress and perspectives. Sens Actuators, B Chem 171–172(2012):25–42
Aga RS, Lombardi JP, Bartsch CM, Heckman EM (2014) Performance of a printed photodetector on a paper substrate. IEEE Photonics Technol Lett 26(3):305–308
Cheng Z, Li Q, Li Z, Zhou Q, Fang Y (2010) Suspended graphene sensors with improved signal and reduced noise. Nano Lett 10(5):1864–1868
Chung FC, Wu RJ, Cheng FC (2014) Fabrication of a Au@SnO2 core-shell structure for gaseous formaldehyde sensing at room temperature. Sens Actuators B Chem 190:1–7
Dehsari HS, Shalamzari EK, Gavgani JN, Taromi FA, Ghanbary S (2014) Efficient preparation of ultralarge graphene oxide using a PEDOT:PSS/GO composite layer as hole transport layer in polymer-based optoelectronic devices. RSC Adv 4(98):55067–55076
Dey T, Mukherjee S, Ghorai A, Das S, Ray SK (2018) Surface state selective tunable emission of graphene quantum dots exhibiting novel thermal quenching characteristics. Carbon NY 140:394–403
Dey T, Mukherjee S, Ghorai A, Das S, Ray SK (2020) Effects of size and localized states in charge carrier dynamics and performance of solution-processed graphene quantum dots/silicon heterojunction near-UV photodetectors. J Phys Chem C 124(22):12161–12167
Gavgani JN (2015) A room temperature volatile organic compound sensor with enhanced performance, fast response and recovery based on N-doped graphene quantum dots and poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) nanocomposite. RSC Adv 5(71):57559–57567
Gogurla N, Sinha AK, Santra S, Manna S, Ray SK (2014) Multifunctional Au-ZnO plasmonic nanostructures for enhanced UV photodetector and room temperature NO sensing devices. Sci Rep 4
Guzman JMCC, Tayo LL, Liu CC, Wang YN, Fu LM (2018) Rapid microfluidic paper-based platform for low concentration formaldehyde detection. Sens Actuators B Chem 255:3623–3629
Huang X (2012) Reduced graphene oxide-polyaniline hybrid: preparation, characterization and its applications for ammonia gas sensing. J. Mater Chem 22(42):22488–22495
Khamfoo K, Inyawilert K, Wisitsoraat A, Tuantranont A, Phanichphant S, Liewhiran C (2020) Formaldehyde sensor based on FSP-made AgOx-doped SnO2 nanoparticulate sensing films. Sens Actuators B Chem 309
Kim YH, Sachse C, MacHala ML, May C, Müller-Meskamp L, Leo K (2011) Highly conductive PEDOT:PSS electrode with optimized solvent and thermal post-treatment for ITO-free organic solar cells. Adv Funct Mater 21(6):1076–1081
Kim YH, Lee J, Hofmann S, Gather MC, Müller-Meskamp L, Leo K (2013) Achieving high efficiency and improved stability in ITO-free transparent organic light-emitting diodes with conductive polymer electrodes. Adv Funct Mater 23(30):3763–3769
Lee CP (2017) A paper-based electrode using a graphene dot/PEDOT:PSS composite for flexible solar cells. Nano Energy 36:260–267
Li X (2017) Flexible room-temperature formaldehyde sensors based on rGO film and rGo/MoS2 hybrid film. Nanotechnology 28(32)
Li N, Fan Y, Shi Y, Xiang Q, Wang X, Xu J (2019) A low temperature formaldehyde gas sensor based on hierarchical SnO/SnO2 nano-flowers assembled from ultrathin nanosheets: synthesis, sensing performance and mechanism. Sens Actuators B Chem 294:106–115
Liu H (2014) Physically flexible, rapid-response gas sensor based on colloidal quantum dot solids. Adv Mater 26(17):2718–2724
Liu Z, Parvez K, Li R, Dong R, Feng X, Müllen K (2015) Transparent conductive electrodes from graphene/PEDOT:PSS hybrid inks for ultrathin organic photodetectors. Adv Mater 27(4):669–675
Liu CC, Wang YN, Fu LM, Huang YH (2018) Microfluidic paper-based chip platform for formaldehyde concentration detection. Chem Eng J 332:695–701
Ponomarenko LA (2008) Chaotic dirac billiard in graphene quantum dots. Science 320(5874):356–358
Robinson JT, Perkins FK, Snow ES, Wei Z, Sheehan PE (2008) Reduced graphene oxide molecular sensors. Nano Lett 8(10):3137–3140
Rong Q (2018) Design of ultrasensitive Ag-LaFeO3 methanol gas sensor based on quasi molecular imprinting technology. Sci Rep 8(1):1–12
Seekaew Y, Lokavee S, Phokharatkul D, Wisitsoraat A, Kerdcharoen T, Wongchoosuk C (2014) Low-cost and flexible printed graphene-PEDOT:PSS gas sensor for ammonia detection. Org Electron 15(11):2971–2981
Shao S, Wang W, Zhou K, Jiang F, Wu H, Koehn R (2017) GQDs-TiO2 heterojunction based thin films for volatile organic compounds sensor with excellent performance at room temperature. Mater Lett 186:193–197
Shen J, Zhu Y, Yang X, Li C (2012) Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Commun 48(31):3686–3699
Srivastava PK, Pandit GG, Sharma S, Rao AM (2000) Volatile organic compounds in indoor environments in Mumbai, India. Sci Total Environ 255:161–168
Tang Z, Elfwing A, Melianas A, Bergqvist J, Bao Q, Inganäs O (2015) Fully-solution-processed organic solar cells with a highly efficient paper-based light trapping element. J Mater Chem A 3(48):24289–24296
Wang L (2009) Simple, rapid, sensitive, and versatile SWNT-paper sensor for environmental toxin detection competitive with ELISA. Nano Lett 9(12):4147–4152
Wang B, Yu J, Li X, Yin J, Chen M (2019) Synthesis and high formaldehyde sensing properties of quasi two-dimensional mesoporous ZnSnO3 nanomaterials. RSC Adv 9(26):14809–14816
Wu X, Li F, Wu W, Guo T (2014) Flexible organic light emitting diodes based on double-layered graphene/PEDOT:PSS conductive film formed by spray-coating. Vacuum 101:53–56
Yamazoe N (2005) Toward innovations of gas sensor technology. Sens Actuators B Chem 108(1–2) (SPEC. ISS., 2–14, 2005)
Zhang YM, Lin YT, Chen JL, Zhang J, Zhu ZQ, Liu QJ (2014) A high sensitivity gas sensor for formaldehyde based on silver doped lanthanum ferrite. Sens Actuators, B Chem 190:171–176
Zhang Y, Zhao J, Sun H, Zhu Z, Zhang J, Liu Q (2018) B, N, S, Cl doped graphene quantum dots and their effects on gas-sensing properties of Ag-LaFeO3. Sens Actuators B Chem 266:364–374
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this paper
Cite this paper
Das, S., Chakrabarty, P., Dey, T., Santra, S., Das, S., Ray, S.K. (2021). Room Temperature Detection of Formaldehyde with Economical and Ecofriendly Graphene Quantum Dot Ink Treated Paper-Based Sensor. In: Mukherjee, M., Mandal, J., Bhattacharyya, S., Huck, C., Biswas, S. (eds) Advances in Medical Physics and Healthcare Engineering. Lecture Notes in Bioengineering. Springer, Singapore. https://doi.org/10.1007/978-981-33-6915-3_28
Download citation
DOI: https://doi.org/10.1007/978-981-33-6915-3_28
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-33-6914-6
Online ISBN: 978-981-33-6915-3
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)