Abstract
In this work, the effect of visible light on the structural, morphological and optical properties of \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) thin films prepared by thermal evaporation was studied. Deposited thin films were exposed to visible light of intensity \(10^{4}\) and \(10^{5}\) Lux for the duration of 2, 4, 8 and 20 h. Prepared samples were characterized with X-ray diffraction (XRD), Scanning electron microscope (SEM) and UV–Vis–NIR spectrophotometer. The amorphous nature of as-deposited and visible light exposed thin films was confirmed by XRD. The SEM images did not show significant change in the morphology of thin films after visible light exposure. The optical transmission of as-deposited and exposed thin films was measured by UV–Vis–NIR spectrophotometer in the wavelength range of 500–3300 nm. Optical transmission was used to calculate the absorption coefficient and optical band gap. Photo-darkening (decrease in optical band gap) with light exposure was observed in \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) thin films. Photo-darkening may be attributed to the enhanced lone pair-lone pair interactions which broaden the valence band and hence decrease the optical band gap. Thus, change in optical parameters is because of change in local structure of deposited thin films.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Chalcogenide (ChG) are potential candidates in various technological applications as optoelectronic [1,2,3], IR-fiber [4, 5] and photonic [6,7,8,9,10] devices. Phase change memory materials are the centre of attraction among researchers [8, 9]. Among phase change materials, \(\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5\) (GST) is an excellent candidate because of high thermal stability, fast crystallization speed, good endurance, scalability and reliability [8,9,10]. Doping of different elements [11,12,13,14,15,16] in GST has been done to optimize its properties for various technological applications.
Chalcogenide glasses, when exposed to light or heavy ion radiation, show variety of changes in structural, electronic and optical properties [17,18,19,20]. Chalcogenide glasses consist of a disordered structure having some wrong bonds as defects, also known as dangling bonds. Bond breaking and bond rearrangement of atoms take place, when these glasses are irradiated with heavy ions or light, which results in the change in local structure. Exposure of light to the chalcogenide amorphous thin films [21] which excite electron hole pairs, produces change in atomic configuration which results in structural changes. This further results in change in the physical and chemical properties of these materials [22, 23]. For various technological applications, the operating mode of these materials is based on the change of electronic and optical properties as a result of some change in the microscopic structure.
Photo induced changes (structural and optical properties) in ChG are one of the technologically important phenomena such as photo-darkening, photo-bleaching and photo-structural change. According to Zakery et al. [24] light induced changes in ChG are due to the presence of lone pair p-states in their valence band and structural flexibility (low coordination of chalcogens). Vlcek et al. [25] proposed that change in optical properties upon photo-irradiation and annealing is due to change in density of homopolar bonds. These photo-induced changes in ChG may be either reversible or irreversible, which favour these as potential candidates in various technological applications.
In the present study, \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) thin films were fabricated using thermal evaporation. The effect of visible light (different intensity and duration) on structural and optical properties was discussed.
2 Experimental
\((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) bulk alloys and thin films have been prepared by melt quenching [7, 26] and thermal evaporation method [7], respectively. Thin films were exposed in an inert environment for different time (2, 4, 8 and 20 h) by visible light (25W LED lamp) of intensity 10\(^{4}\) and 10\(^{5}\) Lux measured using Lux meter (HTC-LX-102A). The amorphous nature of deposited and light exposed thin films was checked by X-ray diffraction (XRD) using X-ray diffractometer (X’Pert PRO PANalytical) with radiation of Cu K\(_{\alpha }\)(\(\lambda\) = 1.5406 Å). Scanning electron microscopic (SEM) images were taken from JEOL JSM-6510 LV. The optical transmission of as-deposited and visible light exposed thin films was measured by UV–Vis–NIR spectrophotometer (Perkin Elmer Lambda 750) in the wavelength range of 500-3300 nm.
3 Results and discussion
3.1 Structural and morphological study
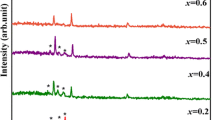
XRD patterns of \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) as-deposited and visible light (intensity 10\(^{5}\) Lux for 8 h) exposed thin films are shown in Fig. 1. The absence of sharp peaks in XRD patterns confirm the amorphous nature of films. All other films show similar XRD patterns (results not shown here).
Figure 2a–d show SEM images of \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0\) and 3) as-deposited and visible light (intensity \(10^{5}\) Lux for 8 h) exposed thin films, respectively. SEM images confirm the uniformity and smoothness of GST thin films. SEM images do not show any growth of the particles which also confirms the amorphous nature of thin films. Other films also show the similar SEM images (results not shown here).
3.1.1 Optical study
Optical absorption coefficient (\(\alpha\)) of \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) as-deposited and light exposed thin films is calculated from optical transmission (T) using Eq. (1):
where d is the thickness of deposited thin films measured using DTM-101 and was \(\sim\) 700 nm. In high absorption region (where \(\alpha \ge 10^{4}\hbox { cm}^{-1}\)), optical band gap \((E_{g})\) is calculated using Tauc’s relation [27] given as follow:
where \(h\nu\) is the incident photon energy, B is band tailing parameter and m indicates the nature of transition. Ge–Sb–Te chalcogenide alloys are direct band gap materials, so m has value of 2 [28]. The optical band gap can be obtained by extrapolating the straight-line portion of \((\alpha h\nu )^{1/2}\) versus \({h\nu }\) plot to the energy axis.
The relationship between \(({\alpha h\nu })^{1/2}\) and \(h \nu\) for all samples is shown in Fig. 3. The values of optical band gap for all the samples are listed in Table 1 with maximum uncertainty of 0.01 eV. The change of optical band gap in chalcogenide may be due to structural changes through strong coupling of photo-generated carriers with lattice [29].
Figure 4 shows the variation of optical band gap of \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) thin films exposed to the light intensity of 10\(^{4}\) and 10\(^{5}\) Lux with time, respectively. From the figure it is clear that the optical band gap changes with Ag incorporation in GST thin film and it also varies with exposure time and intensity of light. Singh et al. [30] studied the effect of Ag doping on optical band gap of GST thin films. They reported that the optical band gap increases with Ag incorporation upto 3% Ag content and this increase in optical band gap is due to decrease in density of localized states in mobility gap. At higher Ag content, the optical band gap started decreasing because of distortion in the host GST matrix. The large optical band gap reduces the threshold current which further increase the switching speed. It is also observed that the optical band gap for all thin films decreases with light exposure upto 8 h and after that it does not show any significant change. Osman et al. [31] also found decrease in optical band gap in a-Ge\(_{x}\)Se\(_{90-x}\)Sb\(_{10}\) thin films with illumination time. Light intensity also effects optical band gap, low intensity causes more photo-darkening which can be understood from charge exchange between localized states representing intrinsic or impurity defects [32,33,34]. The decrease in optical band gap with light exposure can be understood in terms of the interaction of band gap light with lone pair of electrons of chalcogen atoms. Thus photo-darkening may be due to the enhanced lone pair-lone pair interactions which broaden the valence band and decrease the optical band gap [35].
The photo-darkening can further be understood from change in local structure upon light exposure. There is a tendency of bond breaking and bond rearrangement in all chalcogenide alloys with light exposure. It is assumed that the variation of optical band gap with light exposure is a result of non-radiative recombination of electron–hole pairs through transient self trapped excitons [36]. During this recombination, excess energy is released which overcomes the activation barriers to form defect pairs, resulting in structural changes in the films relative to as-deposited films. According to Street [36], the exciton recombination in amorphous chalcogenide thin films can occur through two different ways: (1) directly to the ground state through radiative path, or (2) via non-radiative transition through defect creation. However, the probability of non-radiative recombination is more because of low photoluminescence efficiency of chalcogenide alloys [37]. These defects (\(\hbox {D}^{+}\) and \(\hbox {D}^{-}\)) are also known as valence alternation pair, where D and the superscript denote the chalcogen atom and its charge state, respectively. The radiative and non-radiative paths can be understood from the schematic diagram [36] shown in Fig. 5. In chalcogenide, strong electron–phonon coupling was observed due to non-bonding p-orbital. This coupling strongly favors the formation of low energy defect pairs but the formation energy of these defect pairs is significantly less than the bandgap energy and the exciton energy. As a consequence, highly unstable excitons interact with the lattice to form a \(\hbox {D}^{+}\hbox {D}^{-}\) pair, which corresponds to self trapping of excitons. During self trapping process of exciton, energy is released and this energy modifies the local structure, which gives rise to the permanent change in optical properties.
4 Conclusion
\((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x\) (\(\hbox {x} = 0, 1\) and 3) thin films, transparent, smooth and uniform, are prepared using thermal evaporation technique. Effect of visible light is studied for different exposure time and light intensity on \((\hbox {Ge}_2\hbox {Sb}_2\hbox {Te}_5)_{100-x}\hbox {Ag}_x (\hbox {x} = 0, 1\) and 3) thin films. Photo-darkening is observed in all thin films. The decrease in the optical band gap (Photo-darkening) is more prominent for smaller exposure time and lower light intensity. This may be due to the interaction of band gap light with lone pair electrons of chalcogen atom, which results in broadening of the valence band.
References
M.P. Abubacker, G. Selvan, A.R. Balu, Optoelectronic, magnetic and antifungal properties of CdS thin films co-doped with zinc and bromine. J. Mater. Sci. 28, 10433–10440 (2017)
E.R. Shaaban, M.N.A. Salam, M. Mohamed, M.A. Abdel-Rahim, A.Y. Abdel-Latief, Determination of the optical constants of AsSeAg chalcogenide thick films with high precision for optoelectronics applications. J. Mater. Sci. (2017). doi:10.1007/s10854-017-7175-0
J.J. Carey, J.P. Allen, D.O. Scanlon, G.W. Watson, The electronic structure of the antimony chalcogenide series: Prospects for optoelectronic applications. J. Solid State Chem. 213, 116–125 (2014)
V.S. Shiryaev, M.F. Churbanov, G.E. Snopatin, V.G. Plotnichenko, E.V. Karaksina, T.V. Kotereva, V.V. Koltashev, A.D. Plekhovich, Preparation of core-clad arsenic rich As–Se glass fiber. J. Non-Cryst. Solids 448, 11–15 (2016)
R. Chahal, F. Starecki, C. Boussard-Pldel, J.L. Doualanb, K. Michel, L. Brillandd, A. Braudb, P. Camy, B. Bureaua, V. Nazabal, Fiber evanescent wave spectroscopy based on IR fluorescent chalcogenide fibers. Sens. Actuators B 229, 209–216 (2016)
J. Sharma, R. Singh, H. Singh, T. Singh, P. Singh, A. Thakur, S.K. Tripathi, Synthesis of SnSe\(_{2}\) thin films by thermally induced phase transition in SnSe. J. Alloys Compd. 724, 62–66 (2017)
P. Singh, P. Sharma, V. Sharma, A. Thakur, Linear and non-linear optical properties of Ag doped GeSbTe thin films estimated by single transmission spectra. Semicond. Sci. Technol. 32, 045015 (2017)
M. Wuttig, N. Yamada, Phase change materials for rewriteable data storage. Nat. Mater. 6, 824–832 (2007)
S. Raoux, W. Welnic, D. Ielmini, Phase change materials and their application to nonvolatile memories. Chem. Rev. 110, 240–267 (2010)
Y.J. Park, J.Y. Lee, Y.T. Kim, Surface energy and the equilibrium shape of hexagonal structured \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) grain. Appl. Phys. Lett. 88, 201905 (2006)
K. Darmawikarta, S. Raoux, P. Tchoulfian, T. Li, J.R. Abelson, S.G. Bishop, Evolution of subcritical nuclei in nitrogen-alloyed \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\). J. Appl. Phys. 112, 124907 (2012)
J. Fu, X. Shen, Q. Nie, G. Wang, L. Wu, S. Dai, T. Xu, R.P. Wang, Crystallization characteristics of Mg-doped \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) films for phase change memory applications. Appl. Surf. Sci. 264, 269272 (2013)
Q. Wang, B. Liu, Y. Xia, Y. Zheng, R. Huo, Q. Zhang, S. Song, Y. Cheng, Z. Song, S. Feng, Cr-doped \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) for ultra-long data retention phase change memory. Appl. Phys. Lett. 107, 222101 (2015)
Y. Zhu, Z. Zhang, S. Song, H. Xie, Z. Song, X. Li, L. Shen, L. Li, L. Wu, B. Liu, Ni-doped GST materials for high speed phase change memory applications. Mater. Res. Bull. 64, 333–336 (2015)
G. Wang, Q. Nie, X. Shen, R.P. Wang, L. Wu, J. Fu, T. Xu, S. Dai, Phase change behaviors of Zn-doped \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) films. Appl. Phys. Lett. 101, 051906 (2012)
K.H. Song, S.W. Kim, J.H. Seo, H.Y. Lee, Characteristics of amorphous \(\text{ Ag }_{0.1}(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) )\(_{0.9}\) thin film and its ultrafast crystallization. J. Appl. Phys. 104, 103516 (2008)
V. Sharma, A. Thakur, Phase transition in Se-Te thin film on UV illumination. J. Optoelectron. Adv. Mater. 9, 3097–3099 (2007)
F. Qiu, T. Narusawa, J. Zheng, Swift and heavy ion implanted chalcogenide laser glass waveguides and their different refractive index distributions. Appl. Opt. 50, 733–737 (2011)
F. Xia, S. Baccaro, H. Wang, W. Hua, H. Zeng, X. Zhang, G. Chen, ray irradiation induced multiple effects on GeAsSe chalcogenide glasses. J. Non-Cryst. Solids 354, 1365–1368 (2008)
S.K. Tripathi, A. Thakur, G. Singh, J. Sharma, V. Sharma, K.P. Singh, G.S.S. Saini, N. Goyal, Proton induced changes on the optical parameters of a-(\(\text{ Ge }_{20}\text{ Se }_{80})_{0.96}\text{ Ag }_{0.04}\) thin films. J. Mater. Sci. Lett. 41, 1847–1850 (2006)
K. Tanaka, N. Terakado, A. Saitoh, Photo-induced anisotropic deformations in covalent chalcogenide glasses. J. Optoelectron. Adv. Mater. 10, 124–130 (2008)
M. Stabl, L. Tichy, Photo-induced changes of the short wavelength absorption edge in some GeAsS amorphous thin films. Opt. Mater. 27, 549–557 (2004)
A.V. Kolobov, H. Oyanagi, M. Kondo, A. Matsud, A. Roy, K. Tanaka, Photo-induced bond switching in amorphous chalcogenide. J. Lumin. 83–84, 205–208 (1999)
A. Zakery, S.R. Elliott, Optical properties and applications of chalcogenide glasses: a review. J. Non-Cryst. Solid 330, 1–12 (2003)
M. Vlcek, C. Raptis, T. Wagner, A. Vidourek, M. Frumar, I.P. Kotsalas, Photo and thermally induced phenomena in amorphous GeSbS films. J. Non-Cryst. Solid 192–193, 669–673 (1995)
R. Kaur, P. Singh, K. Singh, A. Kumar, A. Thakur, Optical band gap tuning of Sb-Se thin films for xerographic based applications. Superlatt. Microstruct. 98, 187–193 (2016)
J. Tauc, The optical properties of solids (North-Holland, Amsterdam, 1970)
B.S. Lee, J.R. Abelson, S.G. Bishop, D.H. Kang, B.K. Cheong, K.B. Kim, Investigation of the optical and electronic properties of \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) phase change material in its amorphous, cubic, and hexagonal phases. J. Appl. Phys. 97, 093509 (2005)
P. Khan, T. Saxena, H. Jain, K.V. Adarsh, Nanosecond light induced, thermally tunable transient dual absorption bands in \(\text{ a }-\text{ Ge }_{5}\text{ As }_{30}\text{ Se }_{65}\) thin film. Sci. Rep. 4, 6573 (2014)
P. Singh, R. Kaur, P. Sharma, V. Sharma, M. Mishra, G. Gupta, A. Thakur, Optical band gap tuning of Ag doped \(\text{ Ge }_{2}\text{ Sb }_{2}\text{ Te }_{5}\) thin films. J. Mater. Sci. 28, 11300–11305 (2017)
M.A. Osman, A.M. Abosehly, A.A. Othman, K.A. Aly, Photoinduced phenomena in thermally evaporated \(\text{ a }-\text{ Ge }_{{x}}\text{ Se }_{90-x}\text{ Sb }_{10}\) thin films. J. Adv. Therm. Sci. Res. 2, 64–70 (2015)
A.M. Andriesh, I.P. Kulyak, V.V. Ponomar, Z.I. Kanchiev, Photostimulated absorption of light in chalcogenide glass fibers. Sov. J. Quantum Electron. 17, 372–373 (1987)
K. Tanaka, Mechanism of photo darkening in amorphous chalcogenide. J. Non-Cryst. Solids 59–60, 925–928 (1983)
N. Kastner, Bonding Bands, Lone-Pair Bands, and Impurity States in Chalcogenide Semiconductors. Phys. Rev. Lett. 28, 355–357 (1972)
H. Fritzsche, Towards understanding the photo-induced changes in chalcogenide glasses. Semiconductors 32, 850–854 (1998)
R.A. Street, Non-radiative recombination in chalcogenide glasses. Solid State Commun. 24, 363–365 (1977)
K. Shimakawa, A. Kolobov, S.R. Elliott, Photo-induced effects and metastability in amorphous semiconductors and insulators. Adv. Phys. 44, 475–588 (1995)
Acknowledgements
This work is financially supported by Department of Science and Technology, New Delhi under Research Project (Sanction No. SB/FTP/PS-075/2013 dated 29/05/2014). PS is thankful to Department of Science and Technology, New Delhi for providing financial support as SRF under above mentioned project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P., Kaur, R., Sharma, P. et al. Effect of visible light on the structural and optical properties of (Ge2Sb2Te5)100−x Ag x (x = 0, 1 and 3) thin films. J Mater Sci: Mater Electron 29, 1042–1047 (2018). https://doi.org/10.1007/s10854-017-8004-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8004-1