Abstract
In this report, we have synthesized nanocrystalline of TiO2 thin films by simple chemical bath deposition technique. The as-deposited films were annealed at different temperatures (300, 500 and 700 °C) for 5 h in ambient atmosphere in order to improve crystallinity and structural perfection. The structural, morphological and optical properties were analyzed by using X-ray diffraction (XRD), Atomic force micrograph, Fourier Infrared spectra, UV–Vis transmission spectra and photoluminescence (PL) spectra analysis. XRD results showed that formation of anatase phase for all the annealed samples. The average crystalline size was found to be increased from 35 nm to 46 nm with increasing the annealing temperature. Band gap energy of the 300 °C sample was found to be 3.42 eV, further it was decrease to 3.33, 3.27 eV for 500 and 700 °C annealed samples respectively. The decreasing in the band gap energy and oxygen related vacancies were analyzed by PL analysis. The photocatalytic activity of the TiO2 films were studied by monitoring the degradation of methylene blue rhodamine B in an aqueous solution under visible light irradiation and were empirical that TiO2 film 700 °C samples exhibited excellent photocatalytic activities, when compared with other TiO2 film.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transition metal oxides (TCOs) are extensively studied, has attracted much attention in the past two decades owing to their various practical applications due to the broad potential to modify their electrical, catalytic and optical properties. Among the various TCOs (SnO2, ZnO, WO3, NiO etc.,) TiO2 is one of the most widely studied materials because of the growing interest in its applications and research in the last few years is due to its exclusive and outstanding structural, optical and electrical properties [1]. Generally, TiO2 exist in three different crystalline forms such as anatase, rutile and brookite, but most common application used only anatase phase [2]. TiO2 is a wide band gap semiconductor material, which has been under extensive investigations due to its applications in a variety of fields such as dye-sensitized electrodes, solar cell, gas sensors, photocatalysts, wave guiding, antireflective coatings and integrated optical amplifiers [3], etc. Many synthesized methods have been adapted for preparation of TiO2 thin films such as sputtering [4], laser ablation [5], electrophoretic deposition [6], anodic oxidation [7], sol–gel [8], screen printing [9], and dip coating [10] methods.
Among them, the chemical bath deposition (CBD) method yields stable, adherent, uniform and hard films with good reproducibility by a relatively simple process [11]. We already synthesized C-doped TiO2 thin films by using CBD technique and studied their structural, optical and photocatalytic properties [11]. In addition, the process parameters like deposition rate and thickness can be easily controlled through this method. In the present work consist of synthesis and efficient investigation of structural, optical and photocatalytic activity of TiO2 thin films by simple CBD technique. A sufficient degree of crystallinity is required to attain the desired electronic properties necessary for photocatalytic applications. This can be achieved through annealing process. Moreover the stability of surface features of the nanocrystalline TiO2 thin films on thermal annealing are of key role because they decide the technological impact of surface interaction with the surroundings. So the as-deposited films were annealed at different temperatures in order to achieve the sufficient crystanality and surface interaction for various applications aspects. To the best of our knowledge, this is the first preliminary report about structural, optical and photocatalytic activity of TiO2 thin films by CBD method.
2 Experimental procedure
2.1 Preparation of TiO2 thin films
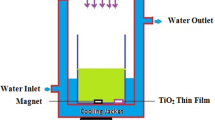
All chemicals used in this study were of analytical grade and were used without further purification. TiO2 films were synthesized by the CBD method. The starting solution was prepared by mixing of titanium isopropoxide, ethyl alcohol in molar ratio 5:50, in a closed beaker stirred for 30 min. pH of the sol was adjusted to 8 with the addition of 2 ml of NH4OH. The dropping rate must be well controlled for the chemical homogeneity. Distilled water (1.5 mol) was added and the solution was refluxed under dry atmosphere for 2 h at 60 °C. For completion of the hydrolysis process, the solution was kept stirred at room temperature for overnight. Films were grown on 76 mm × 26 mm × 1 mm glass microscope slides (ITO glass) which were used as depositing substrates. The glass slides as the substrates for depositing TiO2 films were prepared by ultrasonically cleaned by acetone, followed by ethanol and finally, deionized water for 10 min respectively and allowed to air dry. The substrate was suspended vertically in the reaction bath after stirring the solution properly for homogeneity. The thin film samples were deposited at the temperature between 60 and 70 °C for 1 h. The as-deposited thin films were annealed at 300, 500 and 700 °C for 2 h in muffle furnace, in order to improve the crystanility.
2.2 Characterization techniques
All the samples of TiO2 thin films were successfully characterized by the following techniques. Structural properties of the films were analyzed by using X-ray diffraction (XRD, JEOL diffractometer) with monochromatized Cu Kα radiation (λ = 1.54056 Å) in the range of 10°–80° with the step size of 0.1°. Fourier transform infrared (FT-IR) spectra were recorded on a Shimadzu-8400S spectrometer in range of 400–4000 cm−1 using KBr pellets. The optical transmittance of the thin films was recorded at room temperature by a Perkin Elmer UV/VIS/NIR Lambda 19 spectrophotometer in the wavelength range of 300–900 nm. The morphology of the TNO thin films is observed by Atomic force microscope (ParkXE100 AFM non-contact mode). Photoluminescence (PL) spectra of the samples were recorded using PerkinElmer LS 55 Spectrometer equipped with a 40 W Xenon lamp, Excitation length used was 325 nm.
3 Results and discussion
3.1 XRD analysis
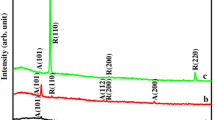
X ray diffraction is a useful technique to determine the phase purity and crystallinity of the samples. The XRD patterns of films annealed at different temperatures are shown in Fig. 1. All the investigated samples were corresponds to tetragonal anatase phase and the results are well matched with the already reported literature values [12]. No peaks corresponding to rutile phase even annealed at 700 °C. This results good in agreement with the already reported that the phase transformation from anatase to rutile phase occurred at 1000 °C when HCL was used as catalyst in sol gel formation by Kim et al. [13]. The average crystallites size was calculated from Scherrer’s equation [14] and was found to increase from 35 to 46 nm as the annealing temperature increases from 300 to 700 °C. The calculated lattice parameters (for 300 °C, a = 4.7356 (Å) and c = 3.3278 (Å) and volumes [74.62 (Å3)] of the TiO2 films are increases with the increase of annealing temperature (Table 1). Moreover the intensity of the peak increases with increase of the annealing temperature (500 and 700 °C). This result reveals that the crystanality of film improved with the increase of annealing temperature. At annealing temperature of 1000 °C a phase transformation is occurred from anatase to rutile phase [2].
3.2 Atomic force microscope analysis
Atomic force microscopy (AFM) is a useful technique to determine the surface morphology and particle size of the samples. Figure 2 shows the 2D and 3D AFM image of TiO2 thin films with different annealed temperature. It can be observed that all the films have smooth surface with uniform particle distribution. In AFM images, a root mean square roughness (Rrms) value is the most important parameter to determine the surface roughness values of the thin films. It was determined by using the formula [15], and the calculated rms value of 300 °C TiO2 thin film was found to be 33 nm, further it was increases to 37 and 43 nm for 500 and 700 °C thin films respectively. These results suggest that rms value increases with increase of annealing temperature as well as increase in the grain size. The increase in grain size due to increase of annealing temperature also confirmed by XRD results.
3.3 UV–Vis transmission spectra analysis
In order to confirm the optical property and band gap energy of the TiO2 films were characterized by UV–Vis transmission spectra analysis. Figure 3a shows the UV–Vis transmission spectra analysis of TiO2 thin films with different annealing temperatures. It was noted that, an increase in the annealing temperature improves the films optical transmission. Evidently, the films are completely transparent in the visible region and a sharp fall in the UV region 300–400 nm. By increasing the annealing temperature from 300 to 700 °C, the absorption edge shifts towards the lower energy side, indicating the decrease in the band gap energy. The absorption coefficient (α) was calculated from the transmission spectra using equation [16],
where T is the optical transmission and t is the film thickness. The direct band gap of thin films was calculated from Fig. 3b using the formula [17],
where α is the absorption coefficient, h is the Planck’s constant, ν is the frequency of incident light, Eg is the energy band gap of material and m is the factor governing the direct/indirect, etc. transition of electron from the valance band to the conduction band. The band gap energy was calculated as 3.42, 3.33 and 3.27 eV for TiO2 thin films of 300, 500 and 700 °C respectively (Fig. 3b). Similar findings were reported for TiO2 thin films synthesized by sol–gel method [3].
3.4 Photoluminescence spectra analysis
Photoluminescence (PL) emission spectra are a useful technique to find out the defect in crystal, oxygen vacancies, charge trapping, immigration, transfer and to understand the fate of electron–hole pairs in semiconductor since [18]. Figure 4 shows the PL emission spectra of TiO2 thin films with different annealing temperatures measured from 300 to 600 nm using a 325 nm He–Cd laser. In all the films, series of peaks were observed at 371, 404, 486 and 556 nm. The UV emission (371 nm) for pure TiO2 could be attributed to oxygen vacancies, Ti interstitials or dangling bond and structural defects. The emissions in the blue-green range (bands at 404 and 486 nm) were defect-related to electron transition mediated by defect levels in the band gap, such as oxygen vacancies. Different types of oxygen vacancies exist, among them the 556 nm is related to V+o oxygen vacancies. Moreover the PL peak intensity decreases with increase in annealing temperature. In addition, all the peaks shift towards the higher wavelength side (red shift), which indicates the decreasing the band gap energy, this is also confirmed by UV–Vis transmission spectra analysis.
3.5 FTIR spectra analysis
FTIR spectrum is an important record, which gives sufficient information about the structure of a compound. Figure 5 shows FTIR spectra of TiO2 thin films with different annealing temperatures recorded in room temperature. All the samples showed the broad band in the range 400–800 cm−1 according to literature sources [19] is due to the stretching vibration of the Ti–O–Ti vibrations. The absorption bands at 1526.13 cm−1 is attributed to –OH bond stretching [20] responsible for hydrophilic nature of TiO2 thin films. The absorption bands at 3414.15 cm−1 is attributed to stretching vibration of surface hydroxyl group or adsorbed water, which has been observed due to the re adsorption of water molecules from ambient atmosphere [21]. Upon these peak intensities are decreases with increase the annealing temperature of 700 °C.
3.6 Photocatalytic activity set up and measurements
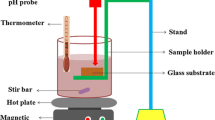
The photocatalytic activity set up was constructed by Vadivel et al. [22]. The detailed experimental set up and sample preparation were done by our previous work [11]. The photocatalytic activities of TiO2 thin films with different annealing temperatures were evaluated by degradation of various dyes (MB and RHB) under visible light irradiation. The results showed that the degradation of the reactive dyes greatly improved by time of the illumination and the quantity of the photocatalyst. In general, the effect of the photocatalyst is more evidently at a definite range with the increasing photocatalyst quantity, the results showed in the present work are agreement with the rule [23]. In order to demonstrate the degradation profile, the spectrum was recorded at regular time intervals. Figures 6 and 7 depicted the degradation profiles of MB and RHB in the presence of TiO2 with different annealing temperatures. At the end of reaction time of 120 min, the MB degradation efficiencies of TiO2 film with different annealing temperatures (300, 500 and 700 °C) are about 59, 68 and 89 %, respectively. Similarly the degradation efficiency of RHB was found to be 56, 61 and 78 %, respectively. Hence, the higher annealing temperature at 700 °C film is an excellent photocatalyst in our experiment when compared to other TiO2 thin films. The repurpose of the TiO2 film (700 °C) as photocatalyst is also investigated by collecting and reusing the same photocatalyst for multiple cycles. As shown in Fig. 8, after 7 runs of photodegradation of MB, the photocatalytic activity of the TiO2 films shows a minor degeneration due to incomplete recollection and loss during washing. As a result, the TiO2 films used as photocatalyst are quite stable and have great potential application in water treatment. The photocatalytic mechanism of TiO2 is illustrated in Fig. 9.
The enhanced photocatalytic activity of TiO2 was attributed to the formation of increasing the number of hydroxyl groups on the surface of TiO2. It can be react with photo excited holes on the surface of TiO2 and produce hydroxyl radicals, which in turn are powerful oxidants in the photocatalytic reaction [24]. Generally, photocatalytic activity depends on several parameters such as crystalline structure, specific surface area and band gap energy. The defect state of the thin film, as well as its crystal phase, influences its electronic nature. As a result the observed optical band gap and the charge TiO2 thin films for photocatalytic applications. In the present work, annealed samples of TiO2 (700 °C) have better photocatalytic performance as compared to other TiO2 films (300 and 500 °C). This could be attributed to smaller band gap as compared to others. At the same time, more MB molecules are adsorbed on the surface of TiO2 films, enhancing the photo excited electron to the conduction band and simultaneously increasing the electron transfer to the adsorbed O2, which results increasing the photocatalytic performance.
4 Conclusions
In summary, we have successfully synthesized nanothin films of TiO2 on a ITO glass substrate by using a facile and novel CBD route, followed by annealing at temperatures of 300–700 °C for 2 h. XRD results reveals that formation of anatase phase for all the samples. Both XRD and AFM images indicated that, crystalline size and surface roughness values (33–43 nm) significantly increasing with the increase of annealing temperature. The absorption edge was red shifted (towards longer wavelength) and the bang gap energy was found to decreases from 3.42 to 3.27 eV with increase of annealing temperatures. The photocatalytic activity of the TiO2 films was evaluated by monitoring the degradation of methylene blue (MB) rhodamine B (RHB) in an aqueous solution under visible light irradiation. The results summarize that, the annealed samples of TiO2 (700 °C) have better photocatalytic performance as compared to other TiO2 films (300 and 500 °C). The synergetic annealing effect plays a another crucial role in producing new states, narrowing the band gap, and reducing the recombination effectively thereby significantly improving the visible light absorption and photocatalytic activity of TiO2 thin films. So our newly prepared TiO2 nanothin films with higher visible light absorption and photocatalytic activity are promising materials for visible light photocatalysts.
References
P. Singh, A. Kumar, D. Kaur, Phys. B 403, 3769 (2008)
J. Ben, M. Naceur, F. Gaidi, R. Bousbih, R. Mechiakh, Chtourou. Curr. Appl. Phys. 12, 422–428 (2012)
A. Ranjitha, N. Muthukumarasamy, M. Thambidurai, R. Balasundaraprabhu, S. Agilan, Optik 124, 6201–6204 (2013)
Y. Yasuda, K. Kamikuri, M. Tobisaka, Y. Hoshi, Thin Solid Films 520, 3736–3740 (2012)
S.J. Wang, W.T. Chang, J.Y. Ciou, M.K. Wei, M.S. Wong, J. Vac. Sci. Technol. A 26, 898–902 (2008)
N. Ghrairi, M. Bouaicha, Nanoscale Res. Lett. 7, 1–17 (2012)
L. Zheng, H. Cheng, F. Liang, S. Shu, C.K. Tsang, H. Li et al., J. Phys. Chem. C 116, 5509–5515 (2012)
J. Tian, H. Deng, L. Sun, H. Kong, P. Yang, J. Chu, Thin Solid Films 520, 5179–5183 (2012)
D.S. Tsoukleris, A.I. Kontos, P. Aloupogiannis, P. Falaras, Catal. Today 124, 110–117 (2007)
M. Behpour, V. Atouf, Appl. Surf. Sci. 258, 6595–6601 (2012)
A.T. Rajamanickam, K. Dhanakodi, P. Thirunavukkarasu, J. Mater. Sci. Mater. Electron. 26, 4038–4045 (2015)
C.-P. Lin, H. Chen, A. Nakaruk, P. Koshy, C.C. Sorrell, Energy Procedia 34, 627–636 (2013)
D.J. Kim, S.H. Hahn, S.H. Oh, E.J. Kim, Mater. Lett. 57, 355–360 (2002)
M. Parthibavarman, K. Vallalperuman, S. Sathishkumar, M. Durairaj, K. Thavamani, J. Mater. Sci. Mater. Electron. 25, 730 (2014)
J.M. Bennett, L. Mattson, Introduction to surface roughness and scattering (Optical Society of America, Washington, 1989)
S.S. Roy, J. Podder Gilberto, J. Optoelect. Adv. Mater. 12, 479 (2010)
R.K. Nath, S.S. Nath, J. Anal. Sci. Technol. 3, 85 (2012)
X.Z. Li, F.B. Li, Environ. Sci. Technol. 35, 2381 (2001)
S. Liu, X. Chen, X. Chen, Chin. J. Catal. 27, 697–702 (2006)
N.T. McDevitt, W.L. Ban, Spectrochim. Acta 20, 799–808 (1964)
T. Krishnakumar, R. Jayaprakash, M. Parthibavarman, A.R. Phani, V.N. Singh, Mater. Lett. 63, 896–898 (2009)
S. Vadivel, G. Rajarajan, J. Mater. Sci. Mater. Electron. 26, 3155–3162 (2015)
W. Wu, X. Xiao, S. Zhang, F. Ren, C. Jiang, Nanoscale. Res. Lett. 6, 533 (2011)
J. Yu, H. Yu, B. Cheng, M. Zhou, X. Zhao, J. Mol. Catal. A Chem. 253, 112–118 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rajamanickam, A.T., Thirunavukkarasu, P. & Dhanakodi, K. Effect of annealing temperature on the structural, optical and photocatalytic activity of TiO2 thin films by chemical bath deposition technique. J Mater Sci: Mater Electron 26, 8933–8938 (2015). https://doi.org/10.1007/s10854-015-3575-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3575-1