Abstract
SrAl2O4:Eu2+, Dy3+ powder has been successfully prepared via a combustion method and then introduced into the titanium dioxide (TiO2) film photoanode of the dye-sensitized solar cell (DSSC). The influence of the amount of doping on the light-to-electric energy conversion efficiency was discussed. At the concentration 7 wt% of SrAl2O4:Eu2+, Dy3+ (weight ratio of phosphor powder to TiO2), an overall 20 % improvement was achieved compared to that of the DSSC without SrAl2O4:Eu2+, Dy3+ phosphor doping. The enhancement is mainly due to the presence of the long persistent phosphor doping. It can convert ultraviolet to visible luminescence via down-conversion luminescence. Owing to the emission of long persistent light, the DSSC with SrAl2O4:Eu2+, Dy3+ showed an efficiency of 0.0076 % in the dark after illuminated under a simulated solar light for 1 min. This method represents a novel approach to increase the efficiency of DSSC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The supply of non-renewable resources is becoming more serious. Global energy consumption is increasing and is expected to double by the end of 2050. So, the search for alternative renewable energy sources is undoubtedly important [1, 2]. Sunlight is a freely available abundant source of solar energy which can irradiate the Earth’s surface with 120,000 TW of solar power [3].
Dye-sensitized solar cell (DSSC) is a photoelectric power generation device, which can convert light photons into electricity effectively. It was firstly reported by Dr. Gratzel’s group in 1991 [4]. Since then, the research of the DSSC has become a hot topic owing to its environmentally friendly and low-cost character. A conventional DSSC is assembled by a photoelectrode of dye adsorbed on porous nanocrystalline titanium dioxide (TiO2), a counter electrode of platinum [5].
The dye-sensitized TiO2 photoanode plays an important part in the absorbing sunlight, it can load sensitizers and collect and transport electrons. However, it only absorbs visible light in the wavelength range 300–800 nm. The absorption in the ultraviolet spectrum and red region of the visible spectrum is restricted. Thus, in order to improve the harvest of incident light, many new-type dyes have been under observation [6]. An effective way to improve the conversion efficiency is the utilization of a luminescence layer composed of up-conversion (UC) or down-conversion (DC) phosphor materials [7–9]. It can convert ultraviolet or red irradiation into visible light and more solar irradiation will be utilized, the DSSC photocurrent may increase. Recently, Yao et al. [10] applied a DC phosphor layer composed of ZnO:Eu3+, Dy3+ to DSSC. It can broaden absorption region of solar spectrum in DSSC and enhanced carrier transportation is achieved. Meanwhile, a down convertor Lu3Al5O12:Ce3+ (LuAG) phosphor was used as a visible light amplifier for DSSC [11]. LuAG particles emitting green light, which are capable of stimulating Ru-dye molecules, enhanced the light-harvesting properties of a DSSC.
So far, the rare earth luminescent material used in DSSC mainly are fluorescent materials, however, the introduction of long persistent phosphor in the DSSC is rarely reported. Green emitting SrAl2O4 co-doped with Eu2+, Dy3+ ions has been considered one of the best and long lasting phosphorescent materials [12]. In this paper, SrAl2O4:Eu2+, Dy3+ powder has been synthesized by combustion method and applied in the DSSC. The prepared phosphor powder has the characteristic of DC luminescence and light-to-electric energy conversion efficiency is improved. The research result reveals that the long persistent phosphor SrAl2O4:Eu2+, Dy3+ can be a candidate material to improve the efficiency of DSSC.
2 Experimental
2.1 Materials
Tetrabutyl titanate, absolute ethyl alcohol, glacial acetic acid, polyethylene glycol 20,000, 4-tert-butylpyridine (TBP), nitric acid, OP emulsification agent (Triton X-100), iodine, lithium iodide, urea, boric acid, aluminium nitrate and strontium nitrate were analytic purity supplied by Shanghai Chemical Agent Limited, Shanghai, China. The sensitized dye N-719 [RuL2(NCS)2, L = 4,4′-dicarboxylate-2,2′-bipyridine] was from SOLARO-NIX SA (Aubonne, Switzerland). Dysprosium oxide and europium oxide were supplied by Shanghai Yuelong Non-Ferrous Metals Limited, Shanghai, China.
2.2 Preparation of SrAl2O4:Eu2+, Dy3+ powder
The SrAl2O4:Eu2+, Dy3+ phosphor powder was synthesized by combustion technique. Amounts of urea were added as reducer and fuel, small quantities of boric acid were used as a flux, metal nitrates were used as oxidizer. Powders were weighted according to the stoichiometry.
The procedure used to prepare the precursor had the following stages. Firstly, Eu2O3 (0.088 g) and Dy2O3 (0.093 g) were dissolved into concentrated nitric acid (1.4 g/ml) to form nitrate solution. Al(NO3)3·9H2O (7.5 g), Sr(NO3)2 (2.116 g), H3BO3 (0.18 g) and CO(NH2)2 (15 g) were dissolved enough into deionised water to obtain transparent solution, then mixed the two solutions together and stirred at 70 °C for 4 h to make some water evaporate.
After that, the precursor solution was introduced into a muffle furnace maintained at 600 °C. Initially, the solution boiled and underwent dehydration, followed by decomposition with escaping large amounts of gases (oxides of carbon, nitrogen and ammonia) and then spontaneous ignition occurred and underwent smouldering combustion with enormous swelling, producing white foamy and voluminous ash, the whole process was over within less than 5 min, after the product was cooled to room temperature, milled the ash slightly. The SrAl2O4:Eu2+, Dy3+ phosphor was obtained.
2.3 Preparation of SrAl2O4:Eu2+, Dy3+/TiO2 photoanode
First, the TiO2 colloid was prepared as reported in our previous work [13–15]. Then a different amount of SrAl2O4:Eu2+, Dy3+ powders (0, 3, …, 9 wt%) were dispersed in the TiO2 colloid by stirring and ultrasonic treatment for 30 min respectively. After that, keep the colloid stirring under 105 °C until it becomes thicken and homogeneous. The resulting colloid was deposited on the FTO electrode by a doctor blade coating. The first layer with about 10 μm thickness was coated with pure TiO2. After drying under an infrared lamp, the TiO2 film was sintered at 450 °C for 30 min and cooled down to 80 °C. The second layer with about 4 μm thickness was coated with SrAl2O4:Eu2+, Dy3+/TiO2 colloid by the same method. Finally, SrAl2O4:Eu2+, Dy3+/TiO2 photoanode was obtained. Figure 1 shows a sketch of the structure of the DSSC. For comparison, the DSSC without SrAl2O4:Eu2+, Dy3+ containing layer was assembled.
2.4 Fabrication of the DSSC
The SrAl2O4:Eu2+, Dy3+/TiO2 photoelectrode was immersed into the N719 dye-ethanol solution at room temperature in the dark for 24 h. Then a DSSC was assembled by injecting the redox electrolyte [0.1 M I2, 0.1 M LiI, 0.6 M N(n-C4H9)4I and 0.5 M TBP in acetonitrile] into interspace between the SrAl2O4:Eu2+, Dy3+/TiO2 photoelectrode and a platinum counter electrode. The DSSC based on a pure TiO2 photoelectrode was prepared using the same method.
2.5 Characterization
Field emission scanning electron microscopy (FESEM, SU8000, Hitachi, Japan) was used to study the phosphor morphology. Energy Dispersive Spectrometer (EDS, INCA Energy, OXFORD, UK) was performed to analyze the chemical elements. The crystal structure of the powder was identified by an X-ray diffractometer (XRD, Bruker D8 Advance, Germany) using Cu Kα radiation (λ = 1.5405 Å). The 2 θ angle of the XRD spectra was recorded at a scanning rate of 5° min−1. The photovoltaic testing of the DSSCs was performed by measuring the photocurrent density-cell potential curves under a simulated solar light coming from a 3A solar simulator (94043A, Newport Corporation, America) equipped with a Xe lamp (450 W) and an AM1.5G filter. The impedance measurements were carried out in dark atmosphere and studied on CHI660E (Shanghai Chenhua Device Company, China). The excitation and emission spectra were obtained from a fluorescence spectrometer (Lumina, Thermo Scientific, America). The decay curve was recorded using a fluorescence spectrophotometer (FLS980, Edinburgh, UK) fitted with a xenon lamp after the sample was sufficiently excited for about 15 min. UV–Vis diffuse reflection tests were performed with an UV/Vis–NIR spectrometer (Lambda 950, PerkinElmer, America).
3 Results and discussions
3.1 Morphology and structure characterizations
Field emission scanning electron microscopy (FESEM) measurements were carried out to observe the morphology and size of the sample. Figure 2a shows the FESEM image of the prepared strontium aluminate crystal. Figure 2b shows the surface morphology of the SrAl2O4:Eu2+, Dy3+/TiO2 photoelectrode.
From Fig. 2a, we can see there are many cracks, voids and pores formed on the surface of the foams due to the escaping gases during combustion reaction. The large amount of escaping gases can dissipate heat so as to prevent the material from sintering and contribute to the formation of nanocrystalline phase. The phosphor particles are irregular. The obvious phenomenon of agglomeration suggests the crystal activity of the generated phosphor powder is very high. Figure 2b indicates the successful doping of SrAl2O4:Eu2+, Dy3. Energy-dispersive X-ray Spectroscopy (EDS) was carried to analyse the composition of the SrAl2O4:Eu2+, Dy3/TiO2 photoelectrode, as exhibited in Fig. 2c, which shows that as-synthesized sample composed of Sr, Al, O, Ti, Dy and Eu Elements and no other spurious impurity elements were detected. The results further confirm the existence of TiO2 and SrAl2O4:Eu2+, Dy3.
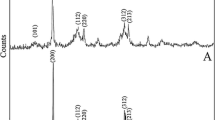
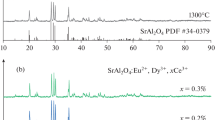
Figure 3 displays a typical X-ray diffraction pattern of the resultant TiO2, SrAl2O4:Eu2+, Dy3+ and SrAl2O4:Eu2+, Dy3/TiO2 respectively. The pure monoclinic phase diffraction peaks of SrAl2O4 are predominant in the XRD pattern. Each diffraction peak in the pattern is similar with the standard pattern of SrAl2O4 JCPDS data (No. 34-0379). It means SrAl2O4:Eu2+, Dy3+ phosphor belongs to a P21 space group and a monoclinic system (a = 8.442 Å, b = 8.822 Å, c = 5.160 Å, β = 93.415°). The result indicates the crystalline phase of SrAl2O4 when doped with Eu2+ and Dy3+ rare earth ions. The small amount of Eu2+ and Dy3+ doped ions enter the host lattice and replace Sr2+ ion partly, they have almost no effect on the SrAl2O4 phase composition [16]. Besides, other obvious diffraction peaks can’t be found in the XRD pattern, proving the resultant material doesn’t contains other crystalline phases. The phase diffraction peaks of pure TiO2 are also presented in the XRD pattern. It is clear to see that the diffraction peaks of TiO2 located at 25.3°, 37.2°, 48.0°, 53.7°, 55.0°, 63.0°, 69.0°, 70.2° and 75.0° can be respectively indexed to the (101), (004), (112), (200), (105), (211), (204), (116), (220), and (215) crystal planes of anatase TiO2 (JCPDS card 21-1272). Compared with the pure TiO2 and single phosphor powder, all the major diffraction peaks of TiO2 and SrAl2O4 can be found and no extra peak was detected in the XRD pattern of SrAl2O4:Eu2+, Dy3/TiO2, which suggests that the SrAl2O4:Eu2+, Dy3+ particles have combined with the TiO2 colloid.
3.2 The properties of SrAl2O4:Eu2+, Dy3+ powder
Figure 4a shows the excitation and emission spectra spectrums of SrAl2O4:Eu2+, Dy3+. It shows a broad band and at maximum excitation peak the corresponding excitation wavelength of is 370 nm which is assigned to the characteristic 4f7 → 4f65d1 transition of the Eu2+ ion. The emission spectra at room temperature of SrAl2O4:Eu2+, Dy3+ was shown in the Fig. 4b at excitation wavelength of 370 nm, it yields high bright green luminescence (λmax = 520 nm) with only one broad band which is due to the typical 4f65d1 → 4f7 transition of luminescent center Eu2+ ion [17]. Dy3+ ion is an auxiliary active ion and work as a hole trap which generates the trap energy level in the original matrix. It is the hole trapped–transported–detrapped process that results in the properties of long afterglow of SrAl2O4:Eu2+, Dy3+ phosphor [18]. The luminescence 520 nm is just within the absorption wavelength range of commonly used dye N719. In addition, the ultraviolet irradiation from the sun can be reabsorbed by the dye N719 via the DC luminescence of SrAl2O4:Eu2+, Dy3+ and contributes to the solar light harvest of DSSC. Besides, from the UV–Vis diffuse reflection spectroscopy shown in Fig. 4c, an obvious absorption was exhibited in the 200–400 nm region which improved the light scattering capabilities of the dye, resulting in an improved light harvesting ability.
The decay characteristics of SrAl2O4:Eu2+, Dy3+ phosphor was tested by continuous exciting with 370 nm UV light for about 15 min. The measurements started in the darkness after the excited source was cut off. As we can see in the Fig. 5, the decay processes of SrAl2O4:Eu2+, Dy3+ phosphor contain the rapid-decaying process and the slow-decaying one. The initial intensity of phosphor is very high and then the intensity of the afterglow decreases rapidly and finally formed a stable long persistent emission. As we know, the roles of Eu2+ ions are the luminescent centers and Dy3+ ions serve as the luminescent centers in aluminate phosphors. The first decay process could be attributed to the short survival time of the electron in Eu2+ while the slow decay process results from the deep trap energy center of Dy3+. It can be seen from the afterglow decay curve that the SrAl2O4:Eu2+, Dy3+ phosphor we have prepared exhibited a good afterglow property.
3.3 The photoelectric performance of the DSSC
The photovoltaic parameters including the open-circuit voltage (VOC), short-circuit density (JSC), fill factor (FF) and overall light-to-electrical energy conversion efficiency (η) of these DSSCs under a simulated solar light irradiation of 100 mW cm−2 are summarized in Table 1 and the photocurrent-voltage curves of the DSSC with and without SrAl2O4:Eu2+, Dy3+ dopant are showed in Fig. 6. Table 1 shows that JSC and increased with the SrAl2O4:Eu2+, Dy3+ dopant level up to 7 wt%, beyond which JSC decreased. The increase in JSC comes mainly from the introduction of long persistent phosphors, which caused more sunlight to be harvested, thus increasing JSC. After that, the large amounts of phosphors influence the TiO2 crystal gap, which cause the block of electronic transmission and the decrease of JSC [19, 20]. While FF increased gradually, from 0.662 to 0.729 V.
As we can see in Fig. 6, an obvious increasement of the sunlight conversion efficiency for DSSC with SrAl2O4:Eu2+, Dy3+ dopant. The DSSC without dopant displays the photovoltaic parameters: VOC = 0.797 V, JSC = 12.54 mA cm−2, FF = 0.662 η = 6.62 %. And DSSC with SrAl2O4:Eu2+, Dy3+ dopant demonstrates enhanced photovoltaic parameters: VOC = 0.828 V, JSC = 13.41 mA cm−2, FF = 0.715, η = 7.94 %. The enhancement mainly due to the DC luminescence response of SrAl2O4:Eu2+, Dy3+ which transfers the ultraviolet light to visible light and broadens the absorption spectrum of the DSSC. As a result, more photoinduced electrons can be produced to enhance the photocurrent, increasing the harvested sunlight and efficiency of DSSC was improved.
The DSSC with SrAl2O4:Eu2+, Dy3+ dopant has a unique property compared with the pure TiO2 electrode for its emission of long persistent light. After the DSSCs were illuminated under a simulated solar light for 1 min, the light source was turned off and the photovoltaic performance of the cells was measured in the dark. Table 2 lists the different data between the cells containing the TiO2 and SrAl2O4:Eu2+/TiO2 electrodes. The efficiency of the DSSC with SrAl2O4:Eu2+, Dy3+ dopant is 0.0076 % in the dark, while the efficiency of the DSSC containing the pure TiO2 electrode is almost zero. The emission of long persistent light from the long afterglow phosphor makes it possible for a cell works in the dark. This method represents a novel approach to improve the DSSC.
As shown in Fig. 7, the photocurrent action (IPCE) spectra show that the absolute IPCE of the cell containing the SrAl2O4:Eu2+, Dy3+ phosphor is higher than the cell containing the pure TiO2 electrode in both the UV and visible regions, which is in good agreement with the observed higher JSC. The improvement is mainly due to the introduction of SrAl2O4:Eu2+, Dy3+ which could absorb near UV-photons and causes a visible green emission. The excited dye N719 emits green light and excess electrons generate. As a result light harvesting ability within the electrode is enhanced and the quantum efficiency increases.
3.4 The impedance spectra of the DSSC
The charge transfer and recombination behavior in the DSSC were studied by analyzing the EIS spectra in the dark. The EIS spectra in Fig. 8 are characterized by the presence of two semicircles in a Nyquist plot [21, 22]. The smaller semicircle in high frequency region represents the electron ejection at the platinum counter electrode/electrolyte interface (R1), and the larger one in medial frequency region is related to chemical capacitance of nanostructured TiO2 and the charge recombination resistance (R2) which reveals the charger transfer at the TiO2/Dye/electrolyte interface and transport in the TiO2 films.
The detailed results are showed in Table 3. R1 values are similarly obtained for the DSSC with different amount of SrAl2O4:Eu2+, Dy3+ dopant. While R2 value increased as the doping amount of SrAl2O4:Eu2+, Dy3+ varies from 0 to 7 wt%. The raised value of R2 is corresponding to the circumstances for charge recombination from the conduction band of the anode materials to the redox I−/I3 − couple. The increasing SrAl2O4:Eu2+, Dy3+ in TiO2 produced some crystal defects, causing electron transport resistance. It means that the charge recombination at the interface of TiO2/Dye/electrolyte within the DSSC is hindered because of the difficulties in charge transferring in dark condition [23, 24], which is favorable for the improvement of photovoltaic performance.
4 Conclusion
In summary, long persistent phosphor SrAl2O4:Eu2+, Dy3+ was successfully synthesized by combustion method and introduced into a DSSC. As a luminescence medium, SrAl2O4:Eu2+, Dy3+ improved light harvesting via a conversion luminescence process and increased photocurrent. SrAl2O4:Eu2+, Dy3+ elevated the energy level of the oxide film and increased the photovoltage. The solar conversion efficiency for a DSSC with SrAl2O4:Eu2+, Dy3+ doping (7:100) reached 7.938 % under a simulated solar light irradiation of 100 mW cm−2, increased by a factor of 1.32 compared to that of the DSSC without SrAl2O4:Eu2+, Dy3+ phosphor doping. This work demonstrates the feasibility of the long persistent phosphor doping in a DSSC and provides a novel way to enhance the sunlight conversion efficiency for solar cells.
References
A.J. Nozik, J. Miller, Chem. Rev. 110, 6443 (2010)
K. Kalyanasundaram, M. Grätzel, J. Mater. Chem. 22, 24190 (2012)
H. Águas, S.K. Ram, A. Araújo, D. Gaspar, A. Vicente, S.A. Filonovich, E. Fortunato, R. Martins, I. Ferreira, Energy Environ. Sci. 4, 4620 (2011)
B. O’Regan, M. Grätzel, Nature 353, 737 (1991)
J. Zhang, X. Li, W. Guo, T. Hreid, J. Hou, H. Su, Z. Yuan, Electrochim. Acta 56, 3147 (2011)
M. Gratzel, Nature 414, 338 (2001)
J. Wu, J. Wang, J. Lin, Z. Lan, Q. Tang, M. Huang, Y. Huang, L. Fan, Q. Li, Z. Tang, Adv. Energy Mater. 2, 78 (2012)
G. Xie, J. Lin, J. Wu, Z. Lan, Q. Li, Y. Xiao, G. Yue, H. Yue, M. Huang, Chin. Sci. Bull. 56, 96 (2011)
J. Zhang, H. Shen, W. Guo, S. Wang, C. Zhu, F. Xue, J. Hou, H. Su, Z. Yuan, J. Power Sources 226, 47 (2013)
N. Yao, J. Huang, K. Fu, S. Liu, E. Dong, Y. Wang, X. Xu, M. Zhu, B. Cao, J. Power Sources 267, 405 (2014)
G.S. Han, Y.H. Song, D.H. Kim, M.-J. Lee, D.G. Lee, S.-H. Han, Y. Kim, M.-K. Jung, D.-H. Yoon, H.S. Jung, RSC Adv. 5, 24737 (2015)
F. Clabau, X. Rocquefelte, S. Jobic, P. Deniard, M.H. Whangbo, A. Garcia, T. Le Mercier, Chem. Mater. 17, 3904 (2005)
J.H. Wu, S.C. Hao, Z. Lan, J.M. Lin, M.L. Huang, Y.F. Huang, L.Q. Fang, S. Yin, T. Sato, Adv. Funct. Mater. 17, 2645 (2007)
J. Wu, S. Hao, Z. Lan, J. Lin, M. Huang, Y. Huang, P. Li, S. Yin, T. Sato, J. Am. Chem. Soc. 130, 11568 (2008)
J.H. Wu, Z. Lan, J.M. Lin, M.L. Huang, S.C. Hao, T. Sato, S. Yin, Adv. Mater. 19, 4006 (2007)
N.M. Son, L.T.T. Vien, L.V.K. Bao, N.N. Trac, J. Phys, Conf. Ser. 187, 012 (2009)
T. Matsuzawa, J. Electrochem. Soc. 143, 2670 (1996)
G. Swati, S. Chawla, S. Mishra, B. Rajesh, N. Vijayan, B. Sivaiah, A. Dhar, D. Haranath, Appl. Surf. Sci. 333, 178 (2015)
K.H. Ko, Y.C. Lee, Y.J. Jung, J. Colloid Interface Sci. 283, 482 (2005)
D. Aberdam, R. Durand, R. Faure, F. Gloaguen, J.L. Hazemann, E. Herrero, A. Kabbabi, O. Ulrich, J. Electroanal. Chem. 398, 43 (1995)
X. Yan, L. Feng, J. Jia, X. Zhou, Y. Lin, J. Mater. Chem. A 1, 5347 (2013)
L.-W. Chong, H.-T. Chien, Y.-L. Lee, J. Power Sources 195, 5109 (2010)
R. Gao, Z. Liang, J. Tian, Q. Zhang, L. Wang, G. Cao, Nano Energy 2, 40 (2013)
Z. Huo, S. Dai, K. Wang, F. Kong, C. Zhang, X. Pan, X. Fang, Sol. Energy Mater. Sol. Cells 91, 1959 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, J., Lin, J., Wu, J. et al. Preparation of long persistent phosphor SrAl2O4:Eu2+, Dy3+ and its application in dye-sensitized solar cells. J Mater Sci: Mater Electron 27, 1350–1356 (2016). https://doi.org/10.1007/s10854-015-3896-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3896-0