Abstract
The effect of GdVO4:Dy3+ luminescent film as down converter material on the efficiency of dye sensitized solar cell (DSSC) was investigated. For this purpose, GdVO4 nanoparticles doped with lanthanide were synthesized by hydrothermal method and the effect of different parameters such as temperature, reaction time, concentration of dopant and annealing regime on their morphology and photoluminescence (PL) properties were studied. The ultraviolet–visible (UV–Vis) and PL spectroscopy studies indicated that GdVO4 nanoparticles could be used as luminescent down conversion (DC) and UV filters. Considering the decrease of chemical stability of dye sensitized solar cells under UV radiation, nanoparticles of GdVO4:Dy3+, constructed as a UV filter and the luminescent DC were used to increase the cell efficiency of DSSC. The efficiency of DSSC increased by 10.30 % using a film of nanoparticles on the back surface of the photo electrode (PE) and by 2.69 %, when the nanoparticles were used to prepare the PE of DSSC, compared with a DSSC lacking GdVO4:Dy3+.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, dye-sensitized solar cells (DSSCs) have attracted much attention for their practical application as a promising candidate for the development of next generation solar cells because of their low fabrication cost and relatively high efficiency [1–6]. However, how to enhance its efficiency is still a crucial problem. In general, the cell consists of a porous nanocrystalline TiO2 film sensitized by a dye for absorbing incident light, a redox electrolyte, and a platinized counter electrode to catalyze the redox couple regeneration reaction [3, 7]. To improve the harvest of incident light and increase the photocurrent of the DSSC, many dyes have been synthesized. However, even the best (N-719, N-749) of these absorb only visible light in the wavelength range 300–800 nm [3, 7].
For the practical use of DSSCs, the chemical stability is as important as its conversion efficiency. Irreversible electrochemical and thermal degradation of the dye or electrolyte components, originating from UV irradiation affect the chemical stability of DSSCs [8, 9]. An effective way to reduce the energy losses is modifying the solar energy spectrum via luminescence phenomenon to the wavelength range in which the solar cells have high absorption probability [9, 10]. The solar spectrum modification results in efficiency enhancement of solar cell through down shifting (DS), down conversion (DC) and up conversion (UC) [9].
Rare earth (RE) doped luminescence materials are of significance for their wide potential applications as anode materials in lithium ion batteries, ferroelectrics, laser host materials, luminescent materials [11] and down converters in dye sensitized solar cells (DSSCs) [9]. Common strategy to avoid the UV light is using an additional UV filter specially coated to absorb ultraviolet light. The gadolinium orthovanadate (GdVO4) rare-earth doped luminescence nanocrystals (NCs) are among the most important functional materials, which can act as UV filter [12]. Synthesis of GdVO4 powders has been previously accomplished using high-temperature solid state reaction between Gd2O3 and NH4VO3 [12]. Hydrothermal method is amongst the most promising solution chemical methods, in which the particle size and distribution, phase homogeneity, and morphology can be well-controlled [13]. By far, GdVO4 nanoparticles (NPs) of different shapes, such as wire like, rod like and cube like have been successfully synthesized by the hydrothermal method [14–16]. These works bring us lots of interesting results and fabrication techniques. Among lanthanide doped GdVO4 NPs, Dy-doped GdVO4 have attracted a great deal of interest [17, 18].
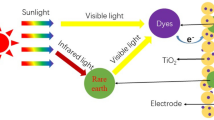
Due to capability to absorb the UV light and emit the visible light, employing GdVO4:Dy3+ as down converter material, increases the chemical stability of DSSC. Making use of this material in outer surface of DSSC, causes reflection of the incoming light. Since the photoluminescent materials usually have a refractive index in the range between 1 and 10, more than half of the PL emission is lost in the sun direction, resulting in a net loss of light current generated by the solar cell instead of an increase and therefore there is possibility of decrease of the photocurrent in DSSC [19].
Sheet-like GdVO4:Dy3+ NPs were successfully fabricated via a simple hydrothermal method. The as-prepared samples were characterized by using powder X-ray diffraction (XRD), scanning electron microscopy (SEM), UV–vis diffuse reflectance spectroscopy (UV–vis) and PL spectroscopy. For the first time, the effect of the produced nanostructures as down conversion film on the efficiency of DSSC was investigated. In order to retain the energy of UV light and yet keep up the chemical stability of DSSC, the use of GdVO4:Dy3+ NPs as a PE of DSSC can be a suitable strategy, since the absorption of reflected photons by DC material increases the photocurrent of DSSC by photoanode.
2 Experimental
2.1 Synthesis of GdVO4 nanoparticles
Chemical reagents including Gadolinium (III) nitrate hexahydrate (Gd(NO3)3·6H2O, Aldrich), Dysprosium (III) nitrate pentahydrate (Dy(NO3)3·5H2O, Alfa Aesar), ammonium metavanadate (NH4VO3, Merck), Ethanol (Merck), Oleic acid (Merck), NaOH (Merck), deionized (DI) water (Merck) were analytical reagents. GdVO4:Dy3+ nanoparticles were prepared using the simple hydrothermal method. In a typical synthesis, a solution of 5.5 ml deionized water containing 0.03 g of NH4VO3 and 0.3 g of NaOH form a Na3VO4 aqueous solution. 10 ml surfactant solution containing Oleic acid and Ethanol with proportion of 1:1 was slowly added to initial solution. Then a solution of 0.22 g precursor of Gd(NO3)3·6H2O and Dy(NO3)3·5H2O (1 mmol total) in 1 ml deionized water were slowly added to the above solution at room temperature, while was stirred for 15 min. The mixture was transferred to a 50 mL autoclave and maintained at 160 °C for 12 h. When the autoclave was cooled to room temperature naturally, white precipitate was obtained. The precipitate was washed with ethanol, centrifuged and dried at 60 °C in air. Then, GdVO4 nanoparticles with different molar ratios of Dy3+ ion were synthesized by the above mentioned method and the effect of various parameters such as temperature, synthesis duration and annealing procedure on the morphology and PL properties were studied. The synthesis temperature and duration for 6 produced samples are shown in Table 1.
2.2 Fabrication of DSSCs
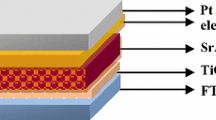
Electrophoresis deposition (EPD) was utilized to prepare working electrode used in DSSCs. EPD solution was prepared by mixing TiO2 nanopowders (P25), acetone, acetyl acetone, I2, DI water, and ethanol. This mixture was stirred for 24 h. During EPD, the cleaned FTO glass maintained at a positive potential (anode) while a pure steel mesh was used as the counter (cathode) electrode. The distance between the two electrodes was about 1 cm with an applied voltage of 10 V. Six deposition cycles, each with duration of 5 s were accomplished. The coated substrates were dried at 150 °C in air for 5 min. The thickness of the resulting layer was 15 µm and heat-treated at 500 °C in air for 15 min. For sensitizing, the working electrodes were immersed in a solution of N719 dye in ethanol and acetonitrile (0.03 mM) for 24 h. Counter electrode was prepared by spin coating of a solution of H2PtCl6 on the FTO glass. Two electrodes with a spacer film of Surlyn were joined together then the electrolyte containing \({\text{I}}^{ - } /{\text{I}}_{3}^{ - }\) redox couple was injected into the cell.
The effect of GdVO4:Dy3+ NPs as down converter material on the performance of DSSC was investigated through two ways. Firstly, a uniform thin film of GdVO4:Dy3+ NPs at 0.1 and 0.3 M concentration in cyclohexene was prepared by spin coating method at 3000 rpm for 30 s on the back surface of working electrode and secondly, these NPs introduced into the EPD solution as impurity with different molar ratios in initial layers of working electrode.
2.3 Characterization
X-ray diffraction (XRD) patterns were recorded by a Philips- X’Pert Pro, X ray diffractometer using Ni-filtered Cu Kα radiation at scan range of 2θ (0–70). Scanning electron microscope (SEM) and energy dispersive analysis of X-ray (EDAX) were taken by MIRA 3 FEG Tescan. Room temperature PL (PL) was studied on a Perkin-Elmer (LS 55) fluorescence spectrophotometer. UV–Vis diffuse reflectance spectroscopy analysis (UV–Vis) was measured using Shimadzu UV–Vis scanning spectrometer. The photovoltaic measurements were accomplished by solar simulator (Luzchem) and IVIUMSTAT (IVIUM) under AM 1.5 condition. For measurement of thickness of working electrode a 3D Optical Microscopy Nanofocus was used.
3 Results and discussion
3.1 Structure and morphology
X-ray powder diffraction (XRD) patterns of GdVO4 and GdVO4:Dy3+ nanoparticles at room temperature are shown in Fig. 1. The sharp peaks of this sample at 18°, 24°, 33°, 35°, 49° and 50° can be perfectly assigned to [101], [200], [112], [220], [312] and [213] planes of the tetragonal phase of GdVO4 with lattice constants comparable to the values given in JCPDS 72-0277. No impurity traces were detected, indicating that the product is rather pure. The sharp peaks in XRD pattern indicate the crystalline phase of the sample with the lattice constants of a = b=7.19 Å and c = 6.33 Å. From XRD data the crystallite size of the as-prepared GdVO4:Dy3+ nanocrystal was calculated to be 10–60 nm using the Scherer equation:
where β is the width of the observed diffraction line at its half intensity maximum, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the wavelength of X-ray source used in XRD.
EDAX analysis was employed to investigate the chemical composition of as-synthesized GdVO4:Dy3+ nanocrystalline. EDAX pattern shown in Fig. 2 confirms that Gd, V and O are the major chemical components with approximate atomic ratios of 12.11, 13.02 and 70.10 % respectively. The concentration of Dy is about 0.65 %. The presence of C with concentration of 4.12 % is due to absorption of CO2 from the precursors. The morphology and structural details of GdVO4 nanoparticles are shown in Fig. 3. From the SEM micrograph, we can observe that the as-prepared samples are composed of square shaped nanosheets. As seen, thickness of these nanosheets is about 30 nm.
The influence of time and temperature of synthesis on morphology of Gd0.98VO4:Dy0.02 were also studied. Figure 4 indicates the XRD pattern of prepared GdVO4:Dy3+ at different time and temperature. It is shown the GdVO4 pattern with tetragonal phase for all of the samples. Figure 5 shows the SEM images of samples prepared according to Table 1. As is seen, nanosheets prepared at 140 and 180 °C are agglomerated in Fig. 5a, f. Comparing Figs. 5b–e, it is clear that the sample 4, prepared at 160 °C for 12 h has better morphology.
On the basis of the results above, the formation of GdVO4 nanosheets demonstrated with a possible growth process, as shown schematically in Fig. 6. The oleic acid molecules control the growth of the nanocrystalline particles with formation micelle structures in aqueous solution and ethanol is a component producer of the micelle system. In micelle system, the inner chambers of micelles act as a template and are caused regular structures and special morphologies [20]. During the reaction process, the NH4VO3 first reacted with NaOH to form Na3VO4 solution. VO4 3− anions trapped in the micelle chambers. So the subsequently added Gd3+ ions have a greater chance of reacting with VO4 3− and under treatment in the oven, the reaction process will be completed. As seen in Fig. 6, the particles growth were restricted in one dimension, so the growth occurred in two other dimensions. Thus, the sheet-like morphology were obtained.
For clarifying the effect of annealing regime on XRD pattern, Gd0.98VO4:Dy0.02 NPs were annealed at 300, 500 and 700 °C for 1 h. Figure 7 indicates the tetragonal phase of GdVO4 which can be fitted with JCPDS Card no.72-0277. The peak intensity becomes stronger with increase in temperature from 300 to 500 °C implying better crystallinity. But this peak intensity was decreased in temperature from 500 to 700 °C due to increase of amorphous phase. So increase of temperature is not suitable because of eventuality phase change or oxidation of NPs.
3.2 Optical properties
The room temperature UV–Vis diffuse reflectance spectra of the GdVO4:Dy3+ sample is presented in Fig. 8a. As shown in Figure, this sample could absorb UV light with the maximum absorption at 285 nm and is transparent for visible light. Therefore, the addition of GdVO4 film would reduce the UV absorption of DSSCs and the lifetime of DSSCs could be greatly enhanced due to the filtering of UV light. Since the light absorption relates to the host lattice, this analysis describes GdVO4 without impurity [21].
The optical band gap of the NPs was determined from the optical absorption spectra. The optical absorption of the NPs shows a sudden increase at around 285 nm. However, a more accurate estimate of the band gap Eg was made by assuming a direct transition between valence band and conduction band [22]. The absorption coefficient α is related to the band gap Eg as:
where hν is the energy of the photon. The absorption coefficient α has been calculated from the optical absorption using the relation α = (l/t) log (absorption). The optical band gap Eg was determined by extrapolating the linear portion of the plot relating (αhν)2 and hν to (αhν)2 = 0 [23]. Therefore, according to Fig. 8b the value of 2.6 eV was obtained for the Eg.
PL spectroscopy is important to characterize the optical properties. The effect of concentration of Dy3+ ion as impurity on the PL property was investigated. The room temperature PL spectra of GdVO4 NPs and Gd1−xVO4:Dyx (x = 0, 0.01, 0.02, 0.04, 0.06, 0.08) NPs dispersed in cyclohexane and for excitation wavelength of 285 nm are shown in Fig. 9. According to the emission spectra, PL spectra of GdVO4 NPs show weak emission in 450–550 nm, But the PL spectra of GdVO4:Dy3+ nanosheets have strong peaks at 478 and 571 nm. Therefore, the presence of Dy ions as impurity in lattice site of GdVO4 NPs causes the intensity of PL spectra to increase. Figure 10 shows the PL intensity of GdVO4:Dy3+ nanosheets as a function of impurity concentration. According to the emission spectra, the PL intensity of Gd1−xVO4:Dyx is optimized in 0.02 mmol impurity concentration.
Excitation with wavelength of 285 nm leads to partial electron transfer from the excited states of [VO4]3− to the 4F9/2 band of Dy3+. This process involves fast multiphonon assisted non-radiative (NR) relaxation processes in Dy3+. Subsequently, Dy3+ emits visible photons peaking at 478 and 571 nm respectively, due to transitions from 4F9/2 to 6HJ (J = 15/2, 13/2) [12]. Figures 11 and 12 show the PL spectra of Gd0.98VO4:Dy0.02 NPs prepared at various synthesis durations and temperatures in accordance with Table 1. As is evident, the sample synthesized at 160 °C for 12 h has maximum PL intensity. Therefore, both the PL spectra and SEM results confirm that the optimized synthesis condition for temperature and time are 160 °C and 12 h.
Figure 13 displays the PL spectra of samples annealed at different temperatures and under excitation at 285 nm. As shown in Fig. 13, a small shift to longer wavelengths is evident compared with the non-annealed sample. Also, with raising the annealing temperature from 300 to 500 °C, the emission intensity increases as a result of improved crystallinity and decrease in inherent lattice defects [24]. By further raising the temperature from 500 to 700 °C, the PL spectra decreased due to diminish of crystallinity. However, the PL spectra of non-annealed sample is sharper than those of the annealed samples. Therefore, PL studies confirm that the Gd0.98VO4:Dy0.02 NPs is a good choice for use in DSSC as DC material.
3.3 Photoelectric performance of DSSCs with different PEs
To investigate the effect of a down converter material on the DSSC, the optimized Gd0.98VO4:Dy0.002 NPs (synthesized at 160 °C, 12 h) were used. Figure 14 depicts the photocurrent–voltage characteristics of DSSCs for PE without GdVO4:Dy3+ NPs and with different concentration of GdVO4:Dy3+. The results are summarized in Table 2.
The uncoated solar cell had a short current density (Jsc) of 18.62 mA.cm−2, fill factor (FF) of 0.54, open circuit voltage (Voc) of 0.67 V and with energy efficiency of 6.70 %. Accordingly, the solar cell coated with luminescent film with concentration of 0.1 M, had Jsc of 20.16 mA cm−2, FF of 0.55, Voc of 0.67 V and efficiency of 7.39 %. So, the solar cell coated with luminescent film with concentration of 0.1 M improved the current density and energy conversion efficiency by 8.28 and 10.30 %, respectively. This is because the emitted light by down-converter can be reabsorbed by N719 dye and generates current. Presence of GdVO4:Dy3+ NPs on the surface of DSSC causes the incoming single photons from UV to be converted to two visible photons [10, 12, 25]. This mechanism results in an increase of photon flux and finally an increase in current density which eventually improves the efficiency.
But the current density and energy conversion efficiency in a solar cell coated with luminescent film with concentration of 0.3 M, are reduced respectively to 2.31 and 8.36 % since by increase concentration, the possibility of reflection of incoming light is enhanced. The above obtained result and the possibility of cleaning DC material on cell surface encouraged us to use this material in PE of DSSC and near to the cell surface.
According to the data in Table 2, the DSSC efficiency for the samples with concentrations more than 1 % of DC material are decreased because of exceeding replacement of GdVO4:Dy3+ with TiO2 that causes reduction in dye absorption by TiO2 NPs and rise in recombination of charge carriers due to different morphology of TiO2 NPs and DC NPs .
Therefore, by decrease of concentration of DC NPs to 0.5 %, photovoltaic characteristics were obtained as Jsc of 17.40 mA.cm−2, FF of 0.58, Voc of 0.68 V and η of 6.88 % according to Table 2. Therefore PE with 0.5 % concentration of DC NPs improved the FF and energy conversion efficiency by 7.41 and 2.69 %. This increase of fill factor due to increase in Jmpp, causes an enhancement in efficiency of DSSC. If the concentration of DC material is lower than 0.5 %, the influence of the UV filter is lost.
A decrease in overall energy efficiency has been reported for a DSSC coated with a film consisting LaVO4:Dy3+ NCs prepared by a Lanthanum source [26]. In other research, a DSSC with Eu3+ doped TiO2 nanorods as down converter material was fabricated in a bilayer electrode of Eu3+ doped TiO2 nanorod/TiO2 nanoparticle (Eu-NR/NP) [27]. An increase in light-to-electrical energy conversion efficiency by 1 % has been reported for a cell including Eu-NR/NP bilayer electrode compared with the cell consisting un-doped bilayer electrode. Increase of current density and energy conversion efficiency respectively by 6.7 and 2.6 % was reported for DSSc solar cell coated with LaVO4:Dy NPs as down converter material [9]. Considering the results obtained in this work, the synthesized GdVO4:Dy NPs is recommended as downconversion material and UV filter for DSSCs.
4 Conclusion
The presence of Dy ion as impurity in GdVO4:Dy3+ NPs plays an important role in the optical properties of this material, as mentioned before the pure GdVO4 NPs show weak emission bands. The UV–Visible spectroscopy results show that GdVO4:Dy3+ NPs absorb ultraviolet radiation and can be applied in DSSC as UV filter to prevent DSSC destruction under UV illumination. According to the PL spectroscopy results, GdVO4:Dy3+ luminescent NPs can be applied in DSSC as DC material. In the present study, the use of GdVO4:Dy3+ NPs thin film as DC layer lead to improved fill factor, current density and efficiency of DSSC. Use of a layer of GdVO4:Dy3+ on the back surface of the PE cause the DSSC efficiency to increase by a factor of 2.64 % and with using it inside the PE this increase is 10.30 %. However, overuse of DC material reduces the efficiency of the DSSC.
References
H. Sun, L. Pan, Z. Piao, Z. Sun, Enhanced performance of cadmium selenide quantum dot-sensitized solar cells by incorporating long afterglow europium, dysprosium co-doped strontium aluminate phosphors. J. Colloid Interface Sci. 416, 81–85 (2014)
B. O’regan, M. Grätzel, Low cost and highly efficient solar cells based on the sensitization of colloidal titanium dioxide. Nature 335(24), 737–740 (1991)
M. Grätzel, Photoelectrochemical cells. Nature 414(6861), 338–344 (2001)
N. Sharifi, N. Ghazyani, N. Taghavinia, Morphological dependence of light backscattering from metallic back reflector films: application in dye-sensitized solar cells. Phys. Status Solidi (a) 212(4), 785–790 (2015)
K.H. Ko, Y.C. Lee, Y.J. Jung, Enhanced efficiency of dye-sensitized TiO2 solar cells (DSSC) by doping of metal ions. J. Colloid Interface Sci. 283(2), 482–487 (2005)
A.S. Nair, R. Jose, V. Shengyuan, S. Ramakrishna, A simple recipe for an efficient TiO2 nanofiber-based dye-sensitized solar cell. J. Colloid Interface Sci. 353(1), 39–45 (2011)
Q. Li, J. Lin, J. Wu, Z. Lan, Y. Wang, F. Peng, M. Huang, Enhancing photovoltaic performance of dye-sensitized solar cell by rare-earth doped oxide of Lu2O3:(Tm3+, Yb3+). Electrochim. Acta 56, 4980–4984 (2011)
K. Andreas, M. Grätzel, Dye-sensitized core-shell nanocrystals: improved efficiency of mesoporous tin oxide electrodes coated with a thin layer of an insulating oxide. Chem. Mater. 14, 2930–2935 (2002)
M. Zahedifar, Z. Chamanzadeh, S.M.H. Mashkani, Synthesis of LaVO4:Dy3+ luminescent nanostructure and optimization of its performance as down-converter in dye-sensitized solar cells. J. Lumin. 135, 66–73 (2013)
C. Strümpel, M. McCann, G. Beaucarne, V. Arkhipov, A. Slaoui, V. Švrček, C. del Cañizo, I. Tobias, Modifying the solar spectrum to enhance silicon solar cell efficiency—an overview of available materials. Sol. Energy Mater. Sol. Cells 91, 238–249 (2007)
L. Guocong, D. Xuechen, L. Haibin, D. Hui, Z. Ligang, Novel polyhedron-like t-LaVO4:Dy3+ nanocrystals: hydrothermal synthesis and PL properties. Cryst. Growth 310, 4689 (2008)
D.C. Yu, S. Ye, M.Y. Peng, Q.Y. Zhang, J.R. Qiu, J. Wang, L. Wondraczek, Efficient near-infrared downconversion in GdVO4:Dy3+ phosphors for enhancing the photo-response of solar cells. Sol. Energy Mater. Sol. Cells 95, 1590 (2011)
C. Feng, J. Zhang, R. Lang, Z. Jin, Z. Wu, Z. Zhang, Unusual photo-induced adsorption–desorption behaviour of propylene on Ag/TiO2 nanotube under visible light irradiation. Appl. Surf. Sci. 257, 1864 (2011)
T. Gavrilović, D.J. Jovanovic, V. Lujpur, M.D. Dramicanin, Multifunctional Eu3+-and Er3+/Yb3+-doped GdVO4 nanoparticles synthesized by reverse micelle method. Sci. Rep. 4, 4290 (2014)
Y. Zheng, H. You, G. Jia, K. Liu, Y. Song, M. Yang, H. Zhang, Facile hydrothermal synthesis and luminescent properties of large-scale GdVO4:Eu3+ nanowires. Cryst. Growth Des. 9, 5101–5107 (2009)
L. Yang, G. Li, M. Zhao, J. Zheng, X. Guan, L. Li, Morphology-controllable growth of GdVO4:Eu3+ nano/microstructures for an optimum red luminescence. Nanotechnology 23, 245602 (2012)
J. Wang, Y. Xu, M. Hojamberdiev, J. Peng, G. Zhu, Na2 EDTA-assisted hydrothermal synthesis and luminescent properties of YVO4:Eu3+ with different morphologies in a wide pH range. Mater. Sci. Eng. B 156, 42 (2009)
L.-N. Jin, Q. Liu, W.-Y. Sun, Synthesis and photoluminescence of octahedral GdVO4 microcrystals by hydrothermal conversion of Gd2 (CO3) 3·xH2O nanospheres. Solid State Sci. 19, 45–50 (2013)
A. De Vos, A. Szymanska, V. Badescu, Modelling of solar cells with down-conversion of high energy photons, anti-reflection coatings and light trapping. Energy Convers. Manag. 50, 328–336 (2009)
H. Cui, Y. Feng, W. Ren, T. Zeng, H. Lv, Y. Pan, Strategies of large scale synthesis of monodisperse nanoparticles. Recent Pat. Nanotechnol. 3, 32–41 (2009)
C.R. Ronda, Phosphors for lamps and displays: an applicational view. J. Alloys Compd. 225, 534–538 (2005)
J. Callaway, Quantum theory of the solid state (Academic Press, London, 1974)
M.N. Kamalasanan, N.D. Kumar, S. Chandra, Structural, optical, and dielectric properties of sol-gel derived SrTiO3 thin films. J. Appl. Phys. 74, 679–686 (1993)
X.-C. Jiang, L.-D. Sun, C.-H. Yan, Ordered nanosheet-based YBO3:Eu3+ assemblies: synthesis and tunable luminescent properties. J. Phys. Chem. B 108, 3387–3390 (2004)
B.M. van der Ende, L. Aarts, A. Meijerink, Lanthanide ions as spectral converters for solar cells. J. Phys. Chem. Chem. Phys. 11, 11081–11095 (2009)
J. Liu, Q. Yao, Y. Li, Effects of downconversion luminescent film in dye-sensitized solar cells. Appl. Phys. Lett. 88, 173119 (2006)
H. Hafez, J.H. Wu, Z. Lan, Q.H. Li, G.X. Xie, J.M. Lin, M.L. Huang, Y.F. Huang, M.S. Abdel-Mottaleb, Enhancing the photoelectrical performance of dye-sensitized solar cells using TiO2:Eu3+ nanorods. Nanotechnology 21, 415201 (2010)
Acknowledgments
Authors are grateful to research council of the University of Kashan for providing financial support to undertake this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zahedifar, M., Chamanzadeh, Z., Madani, M. et al. Synthesis and characterization of GdVO4:Dy3+ nanosheets as down converter: application in dye-sensitized solar cells. J Mater Sci: Mater Electron 27, 4447–4456 (2016). https://doi.org/10.1007/s10854-016-4316-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4316-9