Abstract
The microstructures and microwave dielectric properties of the (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 samples (where Ln stands for Nd, Pr and Sm, x = 0.2–0.3) were studied. The XRD result showed that the (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 (Ln = Nd, Pr and Sm) solid-solutions with single orthorhombic perovskite phase could be formed in the componential range of 0.2 ≤ x ≤ 0.3. With an increasing of Ln3+ content, the dielectric constant (ε r ) decreased due to the smaller dielectric polarizability of LnAlO3 than that of Sr0.2Na0.4Sm0.4TiO3. With increasing x from 0.2 to 0.3, the quality factor (Q·f) firstly increased and then decreased for the specimens with PrAlO3, because the Q·f values were strongly depended on grain sizes. Moreover, either increasing the A-site bond valence or decreasing the B-site bond valence led to a decrease in the temperature coefficient of the resonant frequency (τ f). Typical values of ε r ~ 52.7, Q·f ~ 9700 GHz (at 4.33 GHz) and τ f ~ 1.5 ppm/°C were obtained for the (1 − x)Sr0.2Na0.4Sm0.4TiO3–xSmAlO3 (x = 0.25) ceramic sintered at 1470 °C for 4 h.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of the mobile and satellite communication, microwave electronic devices are required to be developed and fabricated for miniaturization and integration. Materials with the perovskite structure are widely employed as these microwave electronic devices. Furthermore, perovskites materials are characterised by the chemical formula ABO3, and a structure which is very tolerant to substitution for ions of various sizes on both A- and B-site cation sublattices [1, 2]. In order to fulfill the materials with a high dielectric constant (ε r), a moderate quality factor (Q·f) and a near zero temperature coefficient of resonant frequency (τ f), two compounds, one with SrTiO3-based or CaTiO3-based [3–6] materials and the other with those materials of negative τ f values [7–14], have been mixed to form a solid solution or composite.
In our previous work, the Sr2+ substitutions for (Ca0.61Nd0.26)2+ and (Ca0.4Sm0.4)2+ could form a solid solution in (1 − x)SrTiO3–xCa0.61Nd0.26TiO3 [15] and (1 − x)SrTiO3–xCa0.4Sm0.4TiO3 [16] ceramics, respectively. These SrTiO3-based materials were similar with the most of interesting CaTiO3-based materials [17–23] for microwave communication and passive component applications. In the present work, similarly, the Sr1−x Na x/2Sm x/2TiO3 ceramics (Abbreviation as SNSTx) could also from a solid solution in the componential range of 0.7 ≤ x ≤ 0.9. The preferable microwave dielectric properties with τ f ~ 297 ppm/°C, ε r ~ 125 and Q·f ~ 3500 GHz (at 2.76 GHz) were obtained for the SNSTx (x = 0.8) samples sintered at 1350 °C for 4 h, which was briefly analysed below. Moreover, the high positive τ f of the SNST0.8 ceramics could be suppressed to small or zero τ f by addition of a high negative τ f microwave ceramics, which made it a suitable candidate for microwave applications. It was reported that LnAlO3 (Ln = La, Pr, Nd, Sm Dy, Y and Er) materials had low dielectric loss and a high negative τ f [9, 10, 24, 25]. The SNST0.8 and LnAlO3 (Ln = Nd, Pr and Sm) ceramics belonged to a same or distorted perovskite structure. Thus, the microstructures and microwave dielectric properties of the (1 − x)(Sr0.2Na0.4Sm0.4)TiO3–xLnAlO3 (Ln = Nd, Pr and Sm, 0.2 ≤ x ≤ 0.3) ceramic systems were researched in the present work (referred to hereafter as SNST-NAx, SNST-PAx and SNST-SAx).
2 Experimental
The (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 (Ln = Nd, Pr and Sm, x = 0.2, 0.25, 0.3) specimens were prepared by the conventional solid state reaction method. The starting materials are Nd2O3, Pr6O11, Sm2O3, SrCO3, Na2CO3, Al2O3 and TiO2 powders with high-purity reagent-grade (more than 99.9 %). These powders are mixed according to the desired compositions and ground in distilled water for 24 h with ZrO2 balls. The mixtures are dried and calcined at 1200 °C for 2 h. The calcined powders mixed with an appropriate amount of PVA (5 wt%) as a binder were ground and sieved through 100-mesh screen, then pressed into pellets with 10–11 mm in diameter and 4.5–5.0 mm in thickness. All samples were prepared using an automatic uniaxial hydraulic press at 150 MPa. These pellets were preheated at 650 °C for 2 h to expel the binder, then sintered at 1400–1520 °C for 4 h in air.
Ceramic densification was assessed from mass and dimension measurements. Structural analysis was undertaken by X-ray diffraction (XRD) using a Brukerd8-advance system. Microstructures were examined by scanning electron microscopy (SEM) (JSM-5610LV). The dielectric behaviors at microwave frequency of the samples were measured by the TE01δ shielded cavity method [26] using a network analyzer and a temperature chamber (Agilent E5230C). The quality factor was characterized by Q·f (Q = 1/dielectric loss, f = resonant frequency) value at 2.5–4.7 GHz. The τ f value was calculated by the following formula [27]:
where f75 and f25 represent the resonant frequencies at 75 and 25 °C, respectively.
3 Results and discussions
3.1 Sr1−x Na x/2Sm x/2TiO3 ceramics
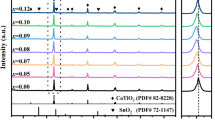
Figure 1a shows the typical XRD patterns of the SNST0.9, SNST0.8 and SNST0.7 ceramics sintered at 1350 °C for 4 h. It can be seen that the solid solution based on pure orthorhombic perovskite phase, belonging to the space group Pnma(62), is identified for all samples. The similar results have also been confirmed in the (Sr, Ca, Ln)TiO3 (Ln = Nd and Sm) ceramics [15, 16]. Moreover, all diffraction peaks of the orthorhombic phase slightly shift to the smaller angles with increasing Sr2+ content due to the substitution of larger Sr2+ (1.44 Å, CN = 12) for (Sm1/2Na1/2)2+ (1.37 Å, CN = 12) at A-site ion [28]. Figure 1b shows the magnified (121) peak shifting to the lower angles, which accords with the Vegard’s law. The surface morphologies of SNST0.9, SNST0.8 and SNST0.7 ceramics sintered at 1350 °C for 4 h are detected by SEM, as shown in Fig. 1c–e, respectively. It can be observed that the surfaces of the sintered samples exhibit a dense grain structure, and very small grains of 1–3 μm are observed at Fig. 1c, then the average grain sizes increase with increasing Sr2+ content (see Fig. 1d, e).
The microwave dielectric properties (ε r, Q·f value and τ f value) of the SNSTx ceramics sintered at 1350 °C/4 h as a function of x are shown in Fig. 2. The dielectric constant (ε r) decreases continually from 143 to 108 and the τ f values shift observably from 433.7 to 127.1 ppm/°C with the decrease in Sr2+ content. It indicates that the ε r and τ f value are sensitive to the Sr/(Sm, Na) ratio in the SNSTx (0.7 ≤ x ≤ 0.9) ceramics. Similar result was also confirmed in our previous work [15, 16]. For the quality factor (Q·f) of the SNSTx ceramics, the maximum [~3500 GHz (at 2.76 GHz)] is obtained at x = 0.8. It seems likely that the low Q·f values of ceramics are mostly due to the large number of grain boundaries existed in the SNST0.9 sample and the abnormal large grains detected at the SNST0.7 sample (see Fig. 1c, e). These phenomenons can affect the dielectric loss of ceramics [29, 30]. The initial goal of our studies is to find a new dielectric material for microwave application. Consequently, the SNST0.8 ceramics with τ f ~ 297 ppm/°C, ε r ~ 125 and Q·f ~ 3500 GHz (at 2.76 GHz) are selected to combine with LnAlO3 (Ln = Nd, Pr and Sm) ceramics for a detailed investigation below.
3.2 (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 ceramics
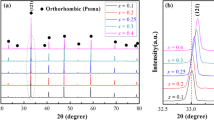
The XRD patterns of the SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.3) ceramics sintered at 1470 °C for 4 h are shown in Fig. 3a–c, respectively. For all specimens, a single phase with orthorhombic perovskite, belonging to the space group Pnma (62), is obtained in the range of 0.2 ≤ x ≤ 0.3. These results are similar with that of the pure SNSTx (0.7 ≤ x ≤ 0.9) specimens, as shown in Fig. 1a. In addition, the insets of Fig. 3a–c show a magnified (121) peak. This suggests that the (121) peak slightly shift to a higher angle with increasing LnAlO3 content due to the incorporation of the smaller Al3+ (0.535 Å) in place of Ti4+ (0.605 Å) for B-site ion and the partly substitution of smaller Ln3+ (Nd = 1.27 Å, Pr = 1.17 Å and Sm = 1.24 Å) for (Sr0.2Na0.4Sm0.4)2+ (1.38 Å) at A-site ion [28]. The lattice parameters and unit cell volumes of the SNST-NAx, SNST-PAx and SNST-SAx ceramics are calculated from the XRD patterns and the results are illustrated in Table 1. It can be seen that the unit cell volume gradually decreases with an increase in LnAlO3 content (Ln = Nd, Pr and Sm), respectively. This phenomenon obeys the general known Vegard’s law as well.
Typical grain microstructural photographs of the SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.25) ceramics sintered at 1470 °C for 4 h are shown in Fig. 4. The average grain sizes of the SNST-NAx (x = 0.2, 0.25) specimens are small compared with that of the specimen with x = 0.3, and a small number of imperfect grain boundaries are observed in Fig. 4a1–a3. In addition, the SNST-PAx (x = 0.2, 0.25) specimens exhibit very small grains of 2–4 μm and very big grains over 10 μm, as shown in Fig. 4b1, b2. And it is clear that the abnormal larger grains exceeded 35 μm are observed for the SNST-PAx (x = 0.3) specimens in Fig. 4b3. Moreover, a well-developed and uniform microstructure can be achieved at the SNST-SA0.2 specimens (see Fig. 4c1). Also the average grain size slowly increases and no obvious pores are shown in microscopic surface of the SNST-SA0.25 and SNST-SA0.3 specimens, as illustrated in Fig. 4c2, c3, respectively.
Figure 5a1–a3 show the relative densities of the SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.3) ceramics at different sintering temperatures for 4 h, respectively. For all samples, the densities initially increase with increasing sintering temperature and reach a maximum, then slightly decrease at a higher sintering temperature. The changes of dielectric constant (ε r) for the SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.3) ceramics as functions of various sintering temperatures are demonstrated in Fig. 5b1–b3, respectively. The ε r of all the samples increases to a maximum value in a certain sintering temperature, corresponding to the optimum densification temperature. According to the graphs, the variation of ε r with sintering temperatures is generally consistent with the change trend of the relative densities at the same x, which also confirms that the ε r is significantly dependent upon the compactness. On the other hand, the ε r decreases with the increase of LnAlO3 (Ln = Sm, Nd) content due to the smaller dielectric polarizability of NdAlO3 (11.83 Å3), PrAlO3 (12.14 Å3) and SmAlO3 (11.56 Å3) than that of (Sr0.2Na0.4Sm0.4)TiO3 (12.51 Å3) [31]. Also, as seen in Fig. 5b1–b3, the ε r in the specimens with PrAlO3 is slightly higher than that of the specimens with NdAlO3 and SmAlO3. It can be explained by the larger ionic polarizability of Pr3+ (5.32 Å3) than that of Nd3+ (5.01 Å3) and Sm3+(4.74 Å3) [31]. Moreover, a maximum ε r value ~63.7 is obtained for the SNST-PA0.2 specimen sintered at 1450 °C for 4 h. Therefore, in addition to the relative density, the variance in the dielectric constant (ε r) can be attributed to the change of dielectric and ionic polarizability.
The room-temperature Q·f values of the SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.3) ceramics sintered at different temperatures for 4 h are demonstrated in Fig. 6. It is observed that the Q·f values of all the specimens are insensitive to the sintering temperatures with an increasing of LnAlO3 content due to the fact that the relative densities of all the specimens are higher than 96 % and the compactness of ceramics is closely related with the sintering temperature. In addition, the effects of secondary phase on the Q·f value have been neglected because the secondary phase has not been detected, as illustrated in Fig. 3. Consequently, we infer that the Q·f value is strongly dependent on grain sizes and grain boundaries in these ceramics systems. The Q·f value of the SNST-NAx and SNST-SAx specimens increases with the rise of x, as shown in Fig. 6a, c, respectively. However, in Fig. 6b, the Q·f value increases firstly with x up to 0.25 and then decreases for the SNST-PAx specimens. This is due to the large irregular grains existing in the SNST-PA0.3 specimen (see Fig. 4b3). Based on the classical damped oscillator model, the Q·f value is inversely proportional to the dielectric constant (ε r) in the infrared frequency range. For the SNST-PAx (0.2 ≤ x ≤ 0.3) specimens, the variation of Q·f value is not consistent with that of ε r. These results obviously break the mentioned rule and can be attributed to influences from external factors, which also confirms that the Q·f values of these ceramics systems are largely depended on grain sizes and grain boundaries. Similar results in the (1 − x)(Ca0.7Nd0.2)TiO3–x(Li0.5Nd0.5)TiO3 ceramics are also reported by Kim et al. [32]. Furthermore, it can be perceived that the optimal Q·f value (~9700 GHz) is obtained in the SNST-SA0.25 specimen sintered at 1470 °C for 4 h, the reason can be related to the more uniform grain size and the less imperfect grain boundaries in the SNST-SA0.25 specimen.
Replacing (Sr0.2Na0.4Sm0.4)2+ and Ti4+ with Ln3+ and Al3+ in the (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 (Ln = Nd, Pr and Sm, 0.2 ≤ x ≤ 0.3) ceramics leads to the variation in atomic interactions, which results in a change of bond valence for the materials. The bond valence of the SNST-NAx, SNST-PAx and SNST-SAx ceramics is calculated using the following equations [33]:
where R ij is the bond valence parameter, d ij is the length of a bond between atom i and j, and b is a universal constant (0.37 Å) [33]. The results are recorded in Table 2. With an increase in x, the A-site bond valence increases while the B-site bond valence decreases for all the specimens, as shown in Table 2. It has been reported that the B-site bond valence of the perovskite structure is closely related to the temperature coefficient of the resonant frequency (τ f). Kim and Yoon [34] suggest that the τ f values of the (1 − x)CaTiO3–xLi1/2Sm1/2TiO3 (0.0 ≤ x ≤ 1.0) ceramics decrease with an increasing of B-site bond valence, and Yang et al. [30] have reported the similar results in the (Zn1−x Mg x )3Nb2O8 (x = 0.02–1.0) specimens. However, it has been shown the decrease of the τ f values with decreasing of B-site bond valence in (1 − x)CaTiO3–x(Li0.5La0.5)TiO3 (0.2 ≤ x ≤ 0.8) ceramics by Li et al. [20]. The common characteristic of these ceramic systems is that the τ f values gradually shift to near zero or negative direction with the decrease in bond length between the B-site cation and oxygen. The same results are confirmed in the present work (Table 2). Thus we suggest that, according to the bond valence theory [33], the shorter the bond length is, the stronger the bond energy become. It also illustrates that the bond strength between B-site ion and oxygen will be stronger increasingly, which results in the decrease of the rattling effect in oxygen octahedra, and the τ f values gradually reduce. Furthermore, τ f values can be controlled by the substitution for the A-site ion. An increasing A-site bond valence gives rise to the increase in degree of oxygen octahedral tilting due to the change of ionic radius at the A-site, it also has some influence in a decreasing τ f value [17]. To sum up, Fig. 7a–c shows the τ f values of the SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.3) ceramics as a function of A- and B-site bond valences. As increasing of the A-site bond valence, the τ f values decrease, while the τ f values decrease with the decrease in B-site bond valence as well.
4 Conclusions
The microstructures and microwave dielectric properties of the SNSTx (0.7 ≤ x ≤ 0.9), SNST-NAx, SNST-PAx and SNST-SAx (0.2 ≤ x ≤ 0.3) ceramics have been investigated in this paper. The XRD results show that the (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 (Ln = Nd, Pr and Sm) solid-solutions with the single orthorhombic perovskite phase can be formed in the componential range of 0.2 ≤ x ≤ 0.3. The dielectric constant (ε r) decreases with LnAlO3 (Ln = Nd, Pr and Sm) content due to the smaller dielectric polarizability of LnAlO3 than that of (Sr0.2Na0.4Sm0.4)TiO3, and the ε r is closely related to the compactness of ceramics as well. The quality factor (Q·f) of all the specimens is strongly depended on grain sizes and grain boundaries. Moreover, the increasing A-site bond valence and the decreasing B-site bond valence lead to a decrease in the temperature coefficient of the resonant frequency (τ f) in these ceramic specimens. The resultant dielectric properties have made the SNST-SA0.25 (ε r ~ 52.7, Q·f ~ 9700 GHz (at 4.33 GHz) and τ f ~ 1.5 ppm/°C) ceramics as very interesting materials for microwave communication and passive component applications.
References
C.F. Tseng, C.C. Huang, Microwave dielectric properties of (1 − x)Nd(Co1/2Ti1/2)O3–x(Ca0.8Sr0.2)TiO3 composite ceramics. J. Mater. Sci. 47, 3982–3988 (2012)
Changlai Yuan, Guohua Chen, Tao Yang et al., Microstructures and microwave dielectric properties of low-temperature fired Ca0.8Sr0.2TiO3–Li0.5Sm0.5TiO3 ceramics with Bi2O3–2B2O3 addition. J. Electron. Mater. (2014). doi:10.1007/s11664-014-3422-9
C.H. Hsun, S.H. Tsai, Dielectric characteristics of Sr substitution on Ca0.4Sm0.4TiO3 ceramics at microwave frequency. Ceram. Int. 40, 10111–10114 (2014)
I.M. Reaney, E.L. Colla, N. Setter, Dielectric and structural characteristics of Ba- and Sr-based complex perovskites as a function of tolerance factor. Jpn. J. Appl. Phys. 33, 3984–3990 (1994)
H. Kagata, J. Kato, Dielelctric properties of Ca-based complex perovskite at microwave frequencies. Jpn. J. Appl. Phys. 33, 5463–5465 (1994)
A. Manan, I. Qazi, A. Ullah, Preparation, characterization, and microwave dielectric properties of Sr2La3Nb1−x Ta x Ti4O17 (0 ≤ x ≤ 1) ceramics. J. Electron. Mater. 42, 138–142 (2013)
J. Li, Y. Han, T. Qiu, C. Jin, Effect of bond valence on microwave dielectric properties of (1 − x)CaTiO3–x(Li0.5La0.5)TiO3 ceramics. J Mater. Res. Bull. 47, 2375 (2012)
D.A. Abdel Aziz, I. Sterianou, I.M. Reaney, (1 − x)CaTiO3–x(Li0.5Nd0.5)TiO3 for ultra-small dielectrically loaded antennas. J. Mater. Sci. 44, 6247 (2009)
B. Jancar, D. Suvorov, M. Valant, G. Drazic, Characterization of CaTiO3–NdAlO3 dielectric ceramics. J. Eur. Ceram. Soc. 23, 1391–1400 (2003)
G.A. Ravi, F. Azough, R. Freer, Effect of Al2O3 on the structure and microwave dielectric properties of Ca0.7Ti0.7La0.3Al0.3O3. J. Eur. Ceram. Soc. 27, 2855–2859 (2007)
C.L. Huang, H.L. Chen, C.C. Wu, Improved high Q value of CaTiO3–Ca(Mg1/3Nb2/3)O3 solid solution with near zero temperature coefficient of resonant frequency. Mater. Res. Bull. 36, 1645–1652 (2001)
C.H. Hsu, H.A. Ho, Microwave dielectric in the Sm(Co1/2Ti1/2)O3–CaTiO3 ceramic system with near-zero temperature coefficient with resonant frequency. Mater. Lett. 64, 396–398 (2010)
E.A. Nenasheva, L.P. Mudroliubova, N.F. Kartenko, Microwave dielectric properties of ceramics based on CaTiO3–LnMO3 system (Ln–La, Nd; M-Al, Ga). J. Eur. Ceram. Soc. 23, 2443–2448 (2003)
C.F. Tseng, C.L. Huang, W.R. Yang, Microwave dielectric properties of xNd(Zn1/2Ti1/2)O3–(1 − x)CaTiO3 ceramics. Mater. Lett. 61, 4054–4057 (2007)
F. Liu, X.Y. Liu, C.L. Yuan et al., Crystal structure and dielectric properties of (1 − x)SrTiO3–xCa0.4Sm0.4TiO3 ceramic system at microwave frequencies. Mater. Chem. Phys. 148, 1083–1088 (2014)
F. Liu, C.L. Yuan, X.Y. Liu et al., Microstructures and dielectric properties of (1 − x)SrTiO3–xCa0.61Nd0.26TiO3 ceramic system at microwave frequencies. J. Mater. Sci. Mater. Electron. (2014). doi:10.1007/s10854-014-2373-5
E.S. Kim, E.S. Chun, D.H. Kang, Effects of structural characteristics on microwave dielectric properties of (1 − x)Ca0.85Nd0.1TiO3–xLnAlO3 (Ln = Sm, Er and Dy) ceramics. J. Eur. Ceram. Soc. 27, 3005–3010 (2007)
H.J. Kim, S. Kucheiko, S.J. Yoon et al., Microwave dielectrics in the (La1/2Na1/2)TiO3–Ca(Fe1/2Nb1/2)O3 system. J. Am. Ceram. Soc. 80, 1316–1318 (1997)
M.H. Kim, S. Nahm, C.H. Choi et al., Dielectric properties of (1 − x)NdGaO3–xCaTiO3 solid solution at microwave frequencies. Jpn. J. Appl. Phys. 41, 717–721 (2002)
J.M. Li, Y.X. Han, T. Qiu et al., Effect of bond valence on microwave dielectric properties of (1 − x)CaTiO3–x(Li0.5La0.5)TiO3 ceramics. J Mater. Res. Bull. 47, 2375 (2012)
P.L. Wise, I.M. Reaney, W.E. Lee et al., Structure microwave property relations in (Sr x Ca1−x ) n+1Ti n O3n+1. J. Eur. Ceram. Soc. 21, 1723–1726 (2001)
Y. Konishi, Novel dielectric wave guide components-microwave applications of new ceramic materials. Proc. IEEE 79, 726–740 (1991)
N. Ichinose, N. Chida, Microwave dielectric properties of (Li1/2Nd1/2)TiO3–(Na1/2Ln1/2)TiO3 (Ln = La, Nd, Sm) ceramic system. Proc. IEEE Int. Symp. 196, 513–514 (1998)
C. Zuccaro, W. Winter, N. Klein et al., Microwave absorption in single crystals of lanthanum aluminate. J. Appl. Phys. 82, 5695–5704 (1997)
D. Mateika, H. Kohler, H. Landern et al., Mixed perovskite substrate for high Tc superconductors. J. Cryst. Growth 109, 447–456 (1991)
J. Krupka, K. Derzakowski, B. Riddle et al., A dielectric resonator for measurements of complex permittivity of low loss materials as a function of temperature. Meas. Sci. Technol. 9, 1751–1756 (1998)
T. Nishikawa, K. Wakino, H. Tamura et al., Precise measurement method for temperature coefficient of microwave dielectric resonator material. IEEE. MTT-S. Int. Microwave. Symp. Dig. 3, 277–280 (1987)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. A. 32, 751–767 (1976)
D.M. Iddle, A.J. Bell, A.J. Moulson, Relationships between dopants, microstructure and the microwave dielectric properties of ZrO2–TiO2–SnO2 ceramic. J. Mater. Sci. 27, 6303 (1992)
W.R. Yang, C.C. Pan, C.L. Huang, Influence of Mg substitutions for Zn on the phase relation and microwave dielectric properties of (Zn1−x Mg x )3Nb2O8 (x = 0.02–1.0) system. J. Alloys Comp. 581, 257–262 (2013)
R.D. Shannon, Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 73, 348–366 (1993)
E.S. Kim, B.S. Chun, D.W. Yoo et al., Microwave dielectric properties of (1 − x)(Ca0.7Nd0.2)TiO3–x(Li0.5Nd0.5)TiO3 ceramics. Mater. Sci. Eng. B 99, 247–251 (2003)
N.E. Brese, M. O’Keeffe, Bond-valence parameters for solids. Acta. Cryst. B47, 192–197 (1991)
E.S. Kim, K.H. Yoon, Microwave dielectric properties of (1–x)CaTiO3–xLi1/2Sm1/2TiO3 ceramics. J. Eur. Ceram. Soc. 23, 2397–2401 (2003)
Acknowledgments
Financial supports of the National Natural Science Foundation of China (Grant No. 11464006), the Natural Science Foundation of Guangxi (Grant No. 2014GXNSFBA118254), the research fund of Guangxi Key Laboratory of Information Materials through 131018-Z, 131004-Z and Guangxi Experiment Center of Information Science through 20130115 are gratefully acknowledged by the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, J., Yuan, C., Liu, F. et al. Microstructures and microwave dielectric properties of (1 − x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 (Ln = Nd, Pr and Sm) ceramic systems. J Mater Sci: Mater Electron 26, 4862–4869 (2015). https://doi.org/10.1007/s10854-015-2994-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2994-3