Abstract
Microwave dielectric ceramics (1 − x)(Sr0.3Ca0.427Nd0.182)TiO3–xNdAlO3 (abbreviated as SCNTAx hereafter, 0.1 ≤ x ≤ 0.4) were prepared by conventional mixed oxide route, and their phase composition, microstructure and microwave dielectric properties were investigated as a function of the x value and sintering temperatures. A single tilted orthorhombic perovskite structure in space group Pnma was refined in the studied composition range. For microware dielectric properties, the decreasing relative permittivity was strongly affected by the ionic polarizability of Nd3+ and Al3+ in SCNTAx ceramic systems. Also, the quality factor of SCNTAx solid solution had strongly depended on apparent densities and average grain sizes. As expected, the promising ceramic of SCNTAx (x = 0.25) sintered at 1520 °C for 4 h was found to possess good microwave dielectric properties: a relative permittivity (ε r) of 55.6, a quality factor (Q × f) of 25,600 GHz (at 4.249 GHz) and a temperature coefficient of resonant frequency (τ f ) of 6.7 ppm/°C. Especially, the τ f values of SCNTAx ceramics were not strongly depended on tolerance factor (t) with increasing of the NdAlO3 content, while these τ f values were essentially correlated with the B-site bond valence and octahedral tilting. Wherein, either decreasing the B-site bond valence or increasing the octahedral tiltings (θ and φ) led to a decrease in τ f value for the present ceramics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of mobile and satellite communication, microwave electronic devices are required to be developed and fabricated for miniaturization and integration. Particularly, as the typical representative of perovskite paraelectric titanates (ABO3 structure), CaTiO3 has been attracted much attention as microwave dielectric because of its high relative permittivity (~170) [1, 2]. However, its dielectric loss (1/Q) is rather high, compared with the complex perovskites. Thus, much work has been performed to control the microwave dielectric loss in CaTiO3 through A-site substitution, such as Ca1–x Ln2x/3TiO3 (Ln = La, Nd and Sm, Q × f ~ 8000–12,000 GHz) [3–5]. But their temperature coefficient of resonant frequency (τ f ~ 190–440 ppm/°C) are too large to apply those microwave frequency devices, so that much attention has to paid to improve their temperature stability by mixing CaTiO3-based materials with those materials (ABO3) of negative τ f value. Among these candidates, CaTiO3–NdGaO3 and CaTiO3–LaAlO3 ceramic systems are attracting great interests due to their better microwave dielectric properties (ε r ~ 35–45, Q × f ≥ 33,000 GHz and τ f ≈ 0 ppm/°C) [6–8]. However, it is well known that a higher ε r can facilitate circuit miniaturization because the wavelength inside the dielectric resonators (DRs) is inversely proportional to the square root of its ε r. Although the good temperature stability and the lower dielectric loss can be obtained in those ceramics, the relative permittivity (ε r) is not still high enough and can not meet the miniaturization for the high-demand DRs. Therefore, it should be an important issue to further enhance the ε r through the preparation of new dielectric ceramic materials.
SrTiO3 is a well-known incipient ferroelectric with cubic perovskite structure at room temperature, and it possesses a higher relative permittivity (ε r ~ 270), a modest quality factor (Q × f ~ 3,000 GHz) and a larger temperature coefficient of resonant frequency (τ f ~ 1650 ppm/°C) [2, 9], compared with that of CaTiO3. High relative permittivity makes it possible to noticeably miniaturize passive microwave components. Therefore, for practical application, some researchers have become increasingly interested in microwave dielectric properties of the SrTiO3 series materials [10, 11]. In our previous work [12], the (Sr, Ca, Nd)TiO3 ceramic systems were synthesized to investigate their structure and microwave dielectric properties, wherein the Sr2+ substitutions for (Ca0.61Nd0.26)2+ in (1 − x)SrTiO3–xCa0.61Nd0.26TiO3 (SCNTx) ceramics could form solid solution. Especially, the (Sr0.3Ca0.427Nd0.182)TiO3 (SCNT) specimen sintered at 1400 °C for 4 h was an orthorhombic distorted perovskite-type microwave dielectric material with a moderate ε r ~ 145 and a very high Q × f ~ 7900 GHz. In addition, a relatively lower positive τ f ~ 415.6 ppm/°C, compared with that of SrTiO3 and/or CaTiO3, can also be obtained for this sample. This means that the SCNTx ceramics can ensure the higher ε r, at the same time achieve a temperature-stable material by forming solid solutions with the microwave ceramics of high negative τ f values. On the other hand, NdAlO3 belongs to a distorted perovskite structure at room temperature with low ε r ~ 22.3, but high Q × f ~ 58,000 and negative τ f ~ −33 ppm/°C [13]. In that way, the higher positive τ f of SCNTx can be suppressed to low or near-zero τ f value with the addition of NdAlO3. Therefore, the aim of this work was to obtain a relative high ε r (≥55), good Q × f (≥25,000 GHz) and near-zero τ f by synthesizing (1 − x)(Sr0.3Ca0.427Nd0.182)TiO3–xNdAlO3 (referred to hereafter as SCNTAx hereafter, 0.1 ≤ x ≤ 0.4) ceramic systems. Moreover, the phase composition, microstructure, and sintering behavior of the SCNTAx ceramics were also investigated in composition range with x = 0.1–0.4. Specially, regulatory mechanism of the τ f values with the change of tolerance factor (t), B-site bond valence (\( \overline{V}_{B} \)) and octahedral tiltings (θ and φ) were discussed in details as well.
2 Experimental
Specimens with x = 0.1, 0.2, 0.25, 0.3 and 0.4 of (1 − x)(Sr0.3Ca0.427Nd0.182)TiO3–xNdAlO3 (SCNTAx) ceramics were prepared by the conventional solid state reaction method using high-purity reagent-grade raw materials (more than 99.9 %) of CaCO3, SrCO3, Nd2O3, Al2O3 and TiO2. These materials were mixed according to the desired compositions and ground in distilled water for 24 h with ZrO2 balls. The mixtures were dried and calcined at 1150 °C (x = 0.1, 0.2, 0.25, 0.3) and 1250 °C (x = 0.4) for 2 h. The calcined powders were then sieved by using a 100 and 200 mesh screen. The sieved powders with 5 wt% PVA as a binder were pressed into pellets 11.5 mm in diameter and 5.5–6.0 mm in thickness under a pressure of 150 MPa. Additionally, these pellets were preheated at 650 °C for 2 h to expel the binder, then sintered at 1450–1620 °C for 4 h in air.
The crystalline structure of the prepared samples was analyzed by an X-ray diffractometer (XRD, D8-Advance, Bruker, Germany). After spraying the surfaces, the microstructures of these sintered specimens were examined by scanning electron microscopy (SEM, S-4800, Hitachi, Japan) coupled with energy dispersive spectroscopy (EDS). Additionally, the bulk density of the sintered ceramics was evaluated from the dimensions and weight. The relative permittivity (ε r) was measured using the Hakki-Coleman dielectric resonator method with TE01δ resonant modes (Agilent E5230C) [14], and the quality factor was characterized by Q × f (Q = 1/dielectric loss, f = resonant frequency) value at 3.8 = 6.1 GHz, which were measured through the parallel plate method, as modified and improved by Courtney [15]. In addition, the temperature coefficient of the resonant frequency (τ f ) was measured by using a temperature chamber. Accordingly, these τ f values were calculated by the following equation (Eq. 1) [16]:
where f 75 and f 25 represent the resonant frequencies at 75 and 25 °C, respectively.
3 Results and discussions
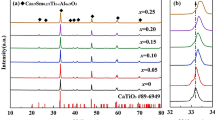
The room-temperature X-ray diffraction (XRD) patterns recorded from the (1 − x)(Sr0.3Ca0.427Nd0.182)TiO3–xNdAlO3 (SCNTAx, 0.1 ≤ x ≤ 0.4) ceramics sintered at 1550 °C for 4 h are illustrated in Fig. 1a. As shown, all peaks can be indexed based on CaTiO3 (PDF#42-0423) with an orthorhombic Pbnm structure and no second phase is detected for all the specimens, indicating the formation of SCNTAx solid solution, even in the sample with x = 0.4. In addition, as shown in the Fig. 1b, the main diffraction peak (121) of the orthorhombic phase slightly shifts to a higher angle with increasing NdAlO3 content. This is due to the fact that the ionic radii of Nd3+ (1.27 Å, CN = 12) is slightly larger than that of (Sr0.3Ca0.427Nd0.182)2+ (1.235 Å, CN = 12) at A-site, but the incorporation of the much smaller Al3+ (0.535 Å, CN = 6) in place of Ti4+ (0.605 Å, CN = 6) at B-site [17]. Thus, with an increase in the substitution of smaller Al3+, all diffraction peaks gradually shift to the higher angles and the lattice parameters and unit cell volumes (Table 1) of the SCNTAx ceramics gradually decrease as well, which obeys the general known Vegard’s law.
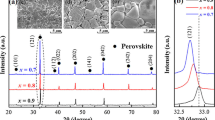
Figure 2 shows the back-scattered electron images (BEIs) of as-sintered surfaces of the SCNTAx (0.1 ≤ x ≤ 0.4) ceramics sintered at 1550 °C for 4 h. As shown in Fig. 2a–e, the color of all the grains is basically the same, which suggests that a single phase exists in the SCNTAx ceramics within the studied composition range, and this is a well agreement with the results of the XRD patterns (see Fig. 1a). In addition, a small amount of porosity and very small grains of 1–4 μm can be observed in Fig. 2a–c, and the small average grain size will result in a large amount of grain boundary, causing the low relative densities of the ceramics. Moreover, the average grain sizes increase with increasing NdAlO3 content (see Fig. 2d, e), wherein a well-developed and uniform microstructure can be achieved by sintering the specimen with x = 0.3 at 1550 °C, it also means that 1550 °C may be the best densification sintering temperature for the SCNTAx (x = 0.3) sample.
Generally, the tolerance factor (t) is believed to be closely related with the temperature coefficient of resonant frequency (τ f ) in ABO3 perovskite solid solutions, and the τ f value increases linearly with an increasing of the tolerance factor if t < 1 [18, 19]. Wherein, the tolerance factor has been proposed and also defined as [20, 21]:
where R A is the radius of the A-site ion, R B is the radius of B-site ion, and R O is the radius of oxygen ion. However, our previous work (not be published) showed that the τ f value in (1 − x)(Sr0.3Ca0.427Nd0.182)TiO3–xSmAlO3 (SCNSTAx, 0.1 ≤ x ≤ 0.4) ceramics sintered at 1480 °C for 4 h gradually decreased, while tolerance factor (t) of the SCNSTAx ceramics increased from 0.9307 to 0.9409 with increasing x value. This is due to the fact that the similarity of ionic radii between Sm3+ (1.24 Å, CN = 12) and (Sr0.3Ca0.427Nd0.182)2+ (1.235 Å, CN = 12) at A-site is not enough to change the bending of B–O–B bond in adjacent BO6 octahedra, as shown in Fig. 3a. When the size of A-site substitution is small enough, the tolerance factor (t) can effectively reflect the structural stability in ABO3 structure. Moreover, for SCNSTAx ceramic systems, the increasing t is attributed to the incorporation of smaller Al3+ (0.535 Å, CN = 6) in place of Ti4+ (0.605 Å, CN = 6) at B-site, but the substitution of Al3+ for B-site just results in an increase in the intensity of oxygen and B-site ions at single BO6 octahedra, which hardly impacts on the thermal stability of ceramic systems. An increase in tolerance factor (t) merely indicates that the volume of the BO6 octahedron is better matched to the size of the AO12 polyhedron. In the present work, for the SCNTAx ceramics, the Eq. (2) becomes:
By calculating, the tolerance factor (t) gradually increases from 0.9317 at x = 0.1 to 0.9452 at x = 0.4, while the τ f value also gradually declines according to the analysis below. These results are similar with that of SCNSTAx ceramics, and it also illustrates that the τ f values are not strongly depended on tolerance factor (t) with increasing x value in SCNTAx ceramic systems. Thus, we consider that, without the phase transitions [22, 23], the τ f value is closely related with the the size of A-site ions substitution and the bending of B–O–B bonds. It can be concluded that the increasement of the bending in B–O–B bonds means the higher stability and lower symmetry in ABO3 perovskite structure. Also, it also indicates that the rattling effect in single BO6 octahedra decreases, giving rise to a decrease in τ f value.
On the other hand, the temperature coefficient of the resonant frequency (τ f ) strongly depends on B-site bond valence or octahedral tilting for ABO3 perovskite structure [24–27]. The bond valences of B-site (\( \overline{V}_{B} \)) for the SCNTAx ceramics are calculated using the following equations [24, 28]:
where \( \overline{\text{R}}_{\text{ij}} \) is the average bond valence parameter, d ij is the length of a bond between atom i and j, and b is a universal constant (0.37 Å). In this work, the SCNTAx is an distorted orthorhombic perovskite with a space group of Pnma on a \( \sqrt 2 \) × \( \sqrt 2 \) × 2 cell in the investigational range with x = 0.1–0.4, and its d B–O is approximately equal to \( \frac{1}{4} \) b (lattice parameter) in an unit cell volume [29], as shown in Fig. 3b. Additionally, replacing Ti4+ with Al3+ in the SCNTAx ceramics will lead to a variation in the atomic interactions, which results in a change in bond valence of this material. Table 1 summarizes the B-site bond valences calculated using Eqs. (4), (5) and the relevant lattice parameters for the present SCNTAx ceramics. Meanwhile, Fig. 4 displays the τ f value of the SCNTAx (0.1 ≤ x ≤ 0.4) ceramics sintered at 1550 °C for 4 h as a function of B-site bond valences. As can be seen from Fig. 4, the τ f value gradually shifts to near zero or negative direction with a decrease in \( \overline{V}_{B} \) as x value increases from 0.1 to 0.4. According to the bond valence theory [28], the shorter the bond length is, the stronger the bond energy become, which also explains that the rattling effect of B-site cation is decreased, thus causing τ f value to decrease. Moreover, the tilting of octahedra is another important parameters related to the τ f value of ABO3 perovskites that significantly affect the stability of crystal structure. The orthorhombic Pnma in these SCNTAx systems gives a tilt system of a − b + a − by using Glazer’s notation [21], meaning that there are two approximately equal anti-phase tilts about the a axis and an in-phase tilt about the b axis. It can also be conceived as the result of tiltings (θ and φ) about the [101] and [010] orientations of the regular octahedra (see Fig. 3b). Additionally, the unit cell parameters are related to the octahedral tiltings and bond valence parameters through the following relationships [30]:
where \( \overline{\text{R}}_{\text{ij}} \) is the same as that of Eq. (2). Figure 4 also shows that the NdAlO3-addition increases octahedral tiltings (θ and φ) from 21.09° to 24.90°, and from 20.77° to 24.97°, affecting a reduction in τ f value of the SCNTAx ceramics from 132.8 to −19.5 ppm/°C. These results can be attributed to the fact that the increasing octahedral tilting results in a corresponding increase in the restoring force for recovering the tilting, and this stronger restoring force can inhibit the increase of heat effect and suppress the ionic rattling of A- and B-sites in a certain degree [27, 31], then the τ f value accordingly decreases. Therefore, the temperature coefficient of the resonant frequency (τ f ) can be effectively evaluated by the B-site bond valences and octahedral tilting for these SCNTAx (0.1 ≤ x ≤ 0.4) perovskite ceramics.
Figure 5 illustrates the relative permittivity (ε r) of SCNTAx (0.1 ≤ x ≤ 0.4) ceramics sintered at various sintering temperatures (1450–1620 °C) for 4 h with different x value. It can be found that the ε r decreases steadily from 62.2 to 41.2 with the increase of NdAlO3 content when sintering temperature is 1550 °C. We approve that the relative permittivity is strongly dependent on the ionic polarizability for ABO3 perovskite solid solutions materials [25, 32]. For the present SCNTAx (0.1 ≤ x ≤ 0.4) solid solutions, although (Sr0.3Ca0.427Nd0.182)2+ has a similar ionic polarizability (3.53 Å3) than that of Nd3+ (5.01 Å3), the ionic polarizability of Al3+ (0.79 Å3), however, is much lower than that of Ti4+ (2.93 Å3). Therefore, the ε r decreases gradually with an increasing of x value on account of the fact that the integral dielectric polarizability of NdAlO3 (11.83 Å3) is smaller than that of SCNT (12.49 Å3) [33]. In addition, as shown, the ε r are not affected greatly by sintering temperatures in the same x value, and a maximum ε r value of ~55.6 is obtained for the specimen using SCNTAx (x = 0.25) sintered at 1520 °C for 4 h. Moreover, thses ranges in sintering temperature of the SCNTAx ceramics increase from 1450–1550 °C at x = 0.1 to 1550–1620 °C at x = 0.4 because NdAlO3 requires a higher sintering temperature of ~1650 °C [13].
Figure 6a shows the apparent density of SCNTAx (0.1 ≤ x ≤ 0.4) ceramics sintered at different sintering temperature for 4 h. It can be observed that, for the same x value, the specimens’ apparent densities initially increase with increasing sintering temperature and reach a maximum, then slightly decrease at a further increasing sintering temperature. On the other hand, the room-temperature Q × f values of the SCNTAx (0.1 ≤ x ≤ 0.4) ceramics sintered at 1450–1620 °C for 4 h are demonstrated in Fig. 6b. As reported, the Q × f value strongly depends on the extrinsic factors, such as second phase, density, and grain size [34, 35]. In these SCNTAx ceramic systems, the effects of the secondary phase can be neglected, because the secondary phase in SCNTAx solid solution is not detected through the investigated composition range with x = 0.1–0.4, as confirmed in Fig. 1. As expected, an ordinary behavior is found that the Q × f value is inside the range exhibited by the end members SCNT with a Q × f value of 7900 GHz and NdAlO3 with a Q × f value of 58,000 GHz. In addition, the variation in Q × f value of SCNTAx (except x = 0.1) ceramics is basically consistent to that of the apparent density with the increasing sintering temperature, and the higher density means the lower porosity, producing a decrease in dielectric loss of the ceramic systems [36]. As a result, the Q × f value is also mainly controlled by the microstructure in the present ceramics. Although the Q × f value increases gradually with an increase in x value due to the higher Q × f value of NdAlO3, a slow increase in Q × f values for the samples with x = 0.3 and 0.4 is shown in Fig. 6b. It can be explained by that large grains in dielectric ceramics usually contain more defects, such as dislocations, planar defects, and even the dislocation networks, which can lead to a decrease in the Q × f value to some extent [31, 37]. Therefore, the increasing extent of Q × f value in SCNTAx (0.1 ≤ x ≤ 0.4) ceramics is decreased due to the increasing average grain sizes, as shown in Fig. 2a–e.
4 Conclusions
The microstructure, phase composition and microwave dielectric properties of (1 − x)(Sr0.3Ca0.427Nd0.182)TiO3–xNdAlO3 (SCNTAx, 0.1 ≤ x ≤ 0.4) ceramics were investigated in this work. The XRD results showed that the single orthorhombic perovskite solid solution could be formed in the composition range of x = 0.1–0.4. Also, a temperature-stable mechanism of these SCNTAx ceramics at microwave frequency were also explained through the tolerance factor (t), B-site bond valence and octahedral tiltings. Wherein, the τ f values of SCNTAx ceramics were not strongly depended on tolerance factor (t < 1) with increasing of the NdAlO3 content, but on the bending of B–O–B bond at adjacent BO6 octahedron for ABO3 perovskite structure. On the other hand, these τ f values were essentially correlated with the B-site bond valence and octahedral tilting, and either decreasing the B-site bond valence or increasing the octahedral tiltings (θ and φ) led to a decrease in τ f value for these ceramic systems. Moreover, ε r decreased with increasing x value due to the smaller dielectric polarizability of NdAlO3 than that of SCNT, and the Q × f value in SCNTAx solid solution had strongly depended on the apparent density and average grain sizes. As a result, excellent microwave dielectric properties of ε r ~ 55.6, Q × f ~ 25,600 GHz (at 4.249 GHz) and τ f ~ 6.7 ppm/°C were obtained for SCNTAx (x = 0.25) ceramic sintered at 1520 °C for 4 h, which is a promising candidate for miniaturization components in developing wireless communication systems.
References
F. Liang, M. Ni, W.Z. Lu et al., Crystal structure and microwave dielectric properties of CaTiO3–La[Ga(1−δ)Al δ ]O3 ceramics system. Mater. Res. Bull. 57, 140–145 (2014)
P.L. Wise, I.M. Reaney, W.E. Lee et al., Structure microwave property relations in (Sr x Ca1–x ) n+1Ti n O3n+1. J. Eur. Ceram. Soc. 21, 1723–1726 (2001)
I.S. Kim, W.H. Jung, Y. Inaguma et al., Dielectric properties of A site deficient perovskite type lanthanum-calcium-titanium oxide solid solution system (1 − x)La2/3TiO3–xCaTiO3 (0.1 ≤ x ≤ 0.96). Mater. Res. Bull. 30, 307–316 (1995)
M.S. Fu, X.Q. Liu, X.M. Chen, Structure and microwave dielectric characteristics of Ca1−x Nd2x/3TiO3 ceramics. J. Eur. Ceram. Soc. 28, 585–590 (2008)
W.S. Kim, E.S. Kim, K.H. Yoon, Effects of Sm3+ substitution on dielectric properties of Ca1−x Sm2x/3TiO3 ceramics at microwave frequencies. J. Am. Ceram. Soc. 82, 2111–2115 (1999)
M.H. Kim, S. Nahm, C.H. Choi et al., Dielectric properties of (1 − x)NdGaO3–xCaTiO3 solid solution at microwave frequencies. Jpn. J. Appl. Phys. 41, 717–721 (2002)
H.X. Yuan, X.M. Chen, M.M. Mao, Structure and microwave dielectric characteristics of Ca1+x Nd1−x Al1−x Ti x O4 ceramics. J. Am. Ceram. Soc. 92(10), 2286–2290 (2009)
D.D. Khalyavin, A.N. Salak, A.M.R. Senos et al., Ferreira Structure sequence in the CaTiO3–LaAlO3 microwave ceramics-revised. J. Am. Ceram. Soc. 89(5), 1721–1723 (2006)
R.C. Kell, A.C. Greenham, G.C.E. Olds, High-permittivity temperature-stable ceramic dielectrics with low microwave loss. J. Am. Ceram. Soc. 56(7), 352–354 (1974)
C.J. Howard, G.R. Lumpkin, R.I. Smith et al., Crystal structures and phase transition in the system SrTiO3–La2/3TiO3. J. Solid. State. Chem. 177, 2726–2732 (2004)
C.L. Huang, K.H. Chiang, Dielectric properties of B2O3 doped (1–x)LaAlO3–xSrTiO3 ceramic system at microwave frequency range. Mater. Res. Bull. 37, 1941–1948 (2002)
F. Liu, C.L. Yuan, X.Y. Liu et al., Microstructures and dielectric properties of (1 − x)SrTiO3–xCa0.61Nd0.26TiO3 ceramic system at microwave frequencies. J. Mater. Sci.: Mater. Electron. 26(1), 128–133 (2015)
S.Y. Cho, I.T. Kim, K.S. Hong, Microwave dielectric properties and applications of rare earth aluminates. J. Mater. Res. 14, 114–119 (1999)
B. Hakki, P. Coleman, A dielectric resonator method of measuring inductive capacities in the millimeter range. IEEE Trans. Microwave Theory Tech. MTT 8, 402–410 (1960)
W. Courtney, Analysis and evaluation of a method of measuring complex permittivity and permeability of microwave materials. IEEE Trans. Microwave Theory Tech. MTT 18, 476–485 (1970)
T. Nishikawa, K. Wakino, H. Tamura et al., Precise measurement method for temperature coefficient of microwave dielectric resonator material. IEEE MTT-S Int. Microwave Symp. Dig. 3, 277–280 (1987)
R.D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. A 32, 751–767 (1976)
M. Reaney, E.L. Colla, N. Setter, Dielectric and structural characteristics of Ba and Sr based complex perovskites as a function of tolerance factor. Jpn. J. Appl. Phys. 33, 3984–3990 (1994)
I.M. Reaney, R. Ubic, Dielectric and structural characteristics of perovskites and related materials as a function of tolerance factor. Ferroelectrics 228, 23–38 (1999)
A.M. Glazer, The classification of tilted octahedral perovskites. Acta. Cryst. B28, 3384–3392 (1972)
A.M. Glazer, Simple ways of determining perovskite structures. Acta. Cryst. A31, 756–762 (1975)
L.A. Khalam, M.T. Sebastian, Microwave dielectric properties of Sr(B′1/2Nb1/2)O3 [B′ = La, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Y, Er, Yb and In] ceramics. Int. J. Appl. Ceram. Tech. 3, 364–374 (2006)
L.A. Khalam, M.T. Sebastian, Low loss dielectrics in the Ca(B′1/2B″1/2)O3 [B′ = lanthanides, y] system. J. Am. Ceram. Soc. 90, 1467–1474 (2007)
W.R. Yang, C.C. Pan, C.L. Huang, Influence of Mg substitutions for Zn on the phase relation and microwave dielectric properties of (Zn1−x Mg x )3Nb2O8 (x = 0.02–1.0) system. J. Alloys. Comp. 581, 257–262 (2013)
E.S. Kim, B.S. Chun, D.W. Yoo et al., Microwave dielectric properties of (1 − x)(Ca0.7Nd0.2)TiO3–x(Li0.5Nd0.5)TiO3 ceramics. Mater. Sci. Eng. B 99, 247–251 (2003)
J. Qu, C. Yuan, F. Liu et al., Microstructures and microwave dielectric properties of (1−x)Sr0.2Na0.4Sm0.4TiO3–xLnAlO3 (Ln = Nd, Pr and Sm) ceramic systems. J. Mater. Sci.: Mater. Electron. 26(7), 4862–4869 (2015)
E.S. Kim, E.S. Chun, D.H. Kang, Effects of structural characteristics on microwave dielectric properties of (1 − x)Ca0.85Nd0.1TiO3–xLnAlO3 (Ln = Sm, Er and Dy) ceramics. J. Eur. Ceram. Soc. 27, 3005–3010 (2007)
N.E. Brese, M. O’Keeffe, Bond-valence parameters for solids. Acta. Cryst. B47, 192–197 (1991)
M. Yashima, R. Ali, Structural phase transition and octahedral tilting in the calcium titanate perovskite CaTiO3. Solid State Ionics 180, 120–126 (2009)
Y.S. Zhao, Crystal chemistry and phase transitions of perovskite in P–T–X space: data for (K x Na1−x )MgF3 perovskite. J. Solid State Chem. 141, 121–132 (1998)
J.M. Li, Y.X. Han, T. Qiu et al., Effect of bond valence on microwave dielectric properties of (1 − x)CaTiO3–x(Li0.5La0.5)TiO3 ceramics. Mater. Res. Bull. 47, 2375 (2012)
J.J. Qu, F. Liu, X. Wei et al., New dielectric material systems of Sr x Nd2(1−x)/3TiO3 perovskites-like at microwave frequencies. Mater. Chem. Phys. 173, 309–316 (2016)
R.D. Shannon, Dielectric polarizabilities of ions in oxides and fluorides. J. Appl. Phys. 73(1), 348–366 (1993)
F. Zhao, Z.X. Yue, Y.Z. Lin et al., Phase relation and microwave dielectric properties of xCaTiO3–(1–x)TiO2–3ZnTiO3 multiphase ceramics. Ceram. Int. 33, 895–900 (2007)
C.H. Hsun, C.H. Chang, A temperature-stable and high-Q microwave dielectric ceramic of the MgTiO3–(Ca0.8Sr0.2)(Zr0.1Ti0.9)O3 system. Ceram. Int. 41, 6965–6969 (2015)
C.H. Hsun, S.H. Tsai, Dielectric characteristics of Sr substitution on Ca0.4Sm0.4TiO3 ceramics at microwave frequency. Ceram. Int. 40, 10111–10114 (2014)
J.M. Li, T. Qiu, Microwave dielectric properties of (1–x)Ca0.6La0.267TiO3–xCa(Sm0.5Nb0.5)O3 ceramics. Ceram. Int. 38, 4331–4335 (2012)
Acknowledgments
Financial supports of the National Natural Science Foundation of China (Grant No. 11464006), the Natural Science Foundation of Guangxi (Grant No. 2014GXNSFBA118254).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qu, J., Huang, D., Wei, X. et al. Effect of NdAlO3 on microstructure, dielectric properties and temperature-stable mechanism of (Sr, Ca, Nd)TiO3 ceramics at microwave frequency. J Mater Sci: Mater Electron 27, 11110–11117 (2016). https://doi.org/10.1007/s10854-016-5228-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5228-4