Abstract
Octahedron-like and rod-like NaY(MoO4)2 microcrystals with tetragonal scheelite-type structure were successfully synthesized by a low cost molten salt method using NaCl as the reaction medium. The as-obtained products were characterized by powder X-ray diffractometer, thermal field emission scanning electron microscope, and photoluminescence spectrometer. The results show that NaY(MoO4)2 with different morphologies have been controllably obtained via adjusting calcining temperature and reaction time. Under the UV light excitation, the emission intensity of the octahedron-like NaY(MoO4)2:Eu3+/Tb3+ microcrystals is stronger than the rod-like ones. Moreover, the luminescence colors of the Eu3+–Tb3+ co-doped NaY(MoO4)2 octahedron-like microcrystals can be tuned from red, orange, yellow and green-yellow to green by simply adjusting the relative doping concentrations of the activator ions, which might have potential application in the areas such as optoelectronic devices in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As far as we concerned, the physicochemical properties of inorganic functional materials depend on not only the chemical composition, structure and crystal phase but also the morphology factors, including shape, size and dimensionality [1–3]. Therefore, research efforts have focused on developing convenient and efficient approaches for the manufacture of various luminescent materials with tunable microstructures as well as different shapes and sizes [4–6].
Double alkaline rare earth tungstates and molybdates [ALn(MO4)2, where A is an alkali-metal cation, Ln is a rare earth metal cation, M is Mo6+ or W6+] can generate various inorganic compounds having tetragonal and monoclinic symmetries, which have been considered as promising host materials for doping with rare earth ions [7–9]. ALn(MO4)2 have been widely used in various areas such as optoelectronic device, flat plane display and biological imaging [10, 11], by virtue of their sharp emission lines, high mono-chromaticity, long luminescence life-time and high photo-stability [12–14]. Besides, they can be used to prepare all kinds of phosphors that emit a variety of colors by doping different lanthanide ions [15–17].
Recently, several molybdate micro/nano materials have been obtained by many synthesis techniques such as solid state method, molten salt method, hydrothermal or solvothermal method and template method. NaY(MoO4)2 with different morphologies have been synthesized successfully by a hydrothermal method [18–20]. The molten salt method, providing the advantage of simple instrumentation, easy manipulation and effective cost, is available for obtaining purified, crystalline, single-phase powders at a lower temperature [21, 22]. On the basis of the advantages of molten salt method for chemical synthesis of micro/nanocrystals, Yan et al. synthesized sphere-like ZnWO4:Eu3+ powders [23], which presented the homogenous nanometer particle size. And Wu et al. [24] prepared large-scale 1D rod-like KEu(MoO4)2 microcrystals. Nowadays, the materials emitting multiple colors have become a research focus because of their important role in some fields such as large screen electrical display devices [10]. Zeng et al. [25] co-doped Gd2(WO4)2 microstars with different amounts of Eu3+ and Tb3+ giving rise to a variety of colors including warm-white light. Urchin-like NaY(MoO4)2 microarchitectures synthesized by a hydrothermal process were also co-doped with Eu3+ and Tb3+ (to a total concentration of 5 %) by Xu et al. [19].
In this paper, molten salt synthesis technology has been engaged in the synthesis of NaY(MoO4)2 microcrystals in the NaCl medium at different reaction parameters. The morphology of the NaY(MoO4)2 was found to be manipulated by changing the calcining temperature and reaction time. Furthermore, the luminescence properties of Eu3+/Tb3+ doped NaY(MoO4)2 and the tunable emission of Eu3+–Tb3+ co-doped NaY(MoO4)2 have also been investigated.
2 Experimental
2.1 Preparation of samples
Starting chemicals (Na2CO3, MoO3, Y2O3, Eu2O3, Tb4O7, NaCl) purchased from Shanghai Chemical Reagents Co. Ltd. were of analytical reagent grade and used as received without further purification. Tb2O3 was prepared under reducing atmosphere of hydrogen for 12 h at 900 °C with Tb4O7 as the reactant. In order to synthesize NaY(MoO4)2 microcrystals, the reactants Na2CO3, Y2O3 and MoO3 (molar ratios of 1:1:4) were mixed with 40 wt% NaCl medium in absolute ethanol by ball milling for 7 h, and then calcined at 650–850 °C with the heating rate of 10 °C min−1 for 0.5–10 h in an alumina crucible. The as-prepared products were washed thoroughly with deionized water several times until no free chloride ions were detected by the AgNO3 solution. Finally, the obtained products were dried at 80 °C for 24 h and kept for further characterization. Eu3+/Tb3+ doped and co-doped NaY(MoO4)2 microcrystals were prepared in a similar way.
2.2 Characterization
The phase structure of the as-prepared products was identified by powder X-ray diffractometer (XRD, SmartLab-3kw, Rigaku Ltd, Japan) using Cu Ka radiation at a scanning rate of 5° min−1 in a 2θ range of 5–80°. The thermal field emission scanning electron microscope (FE-SEM, Ultra55, ZEISS, German) was employed for the observation of the morphology and size of the products. The X-ray room temperature PL spectra were recorded on FL3-221 fluorescence spectrometer (HORIBA Jobin-Yvon, France).
3 Result and discussion
3.1 Phase and morphology
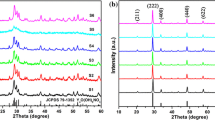
The XRD patterns of NaY(MoO4)2 powders obtained at 650–850 °C for 6 h in the presence of NaCl medium are shown in Fig. 1. All diffraction peaks of the as-obtained white powders can be readily indexed to the pure tetragonal phase of NaY(MoO4)2 [space group: I41/a (88)] according to the JCPDS Card No. 52-1802. The strong and sharp diffraction peaks indicate the high crystallinity of the synthesized products, and no additional peaks of other phases can be found, revealing a high purity of the obtained powders. Evidences above indicate that well-crystallized tetragonal NaY(MoO4)2 crystals are obtained under the present molten salt conditions at 650 °C and above.
The calcining temperature plays an important role in the final morphology and size of the products as shown in Fig. 2. At 650 °C, the products consist of some small particles and rod-like microcrystals (Fig. 2a). The products obtained at 700 °C are composed of rod-like morphologies with an average dimension of 300–600 nm in length and 100 nm in diameter as well as some small particles (Fig. 2b). When the calcining temperature increases to 750 °C (Fig. 2c), the quantities of rod-like crystals decrease and octahedral-like particles preliminary generate. After the calcining temperature increases to 800 °C (Fig. 2d), the products are mainly uniform octahedral-like NaY(MoO4)2 particles with an average size of 200–500 nm, leaving almost no rod-like crystals. When the calcining temperature reaches to 850 °C, some larger particles with irregular morphology generate, these large particles give birth to an inhomogeneous grain distribution of the products (Fig. 2e).
The molten medium is able to promote the effective collision between the small micro/nanoparticles, contributing to the growth of the crystals and inducing the formation of new crystallographic faces on them [26]. In this paper, the melting point of NaCl using as medium is around 800 °C. When the reaction temperature is below 800 °C, the small size particles do not have effective collision between each other because that NaCl medium can not provide a fluid environment for the reactants. Therefore, the length direction becomes the preferential growth direction, which results in forming the rod-like NaY(MoO4)2 crystals. When the calcining temperature reaches to 800 °C, a highly supersaturated solution [27] is formed and the effective collision becomes active for the reason that the temperature is closed to the melting point of NaCl medium. As a result, the new octahedron-like NaY(MoO4)2 particles generate because of the same effective collision rate in each direction.
Furthermore, the time-dependent experiments were conducted by keeping the other synthetic parameters remaining unchanged, including the calcining temperature, the weight of molten salt and the heating rate. The XRD patterns of NaY(MoO4)2 crystals synthesized using NaCl molten salt at 800 °C for different reaction times: (a) 0.5 h, (b) 2 h, (c) 6 h and (d) 10 h are compared (Fig. 3). The tetragonal phase structure of the NaY(MoO4)2 remains when the reaction time is controlled in the range from 0.5 to 10 h. The XRD results reveal that pure scheelite-type NaY(MoO4)2 crystals could be formed by molten salt method even suffering a short reaction time (0.5 h).
Figure 4 presents the FE-SEM images of the tetragonal phase NaY(MoO4)2 crystals obtained at 800 °C for different reaction time. With the reaction time of 0.5 h, tiny particles aggregate together into bulks due to the small dimensions and high surface energy (Fig. 4a). With the reaction time of 2 h (Fig. 4b), the products are mainly octahedron-like NaY(MoO4)2 crystals with the size of 100–300 nm and some small size particles. When the reaction time is prolonged to 6 h, the NaY(MoO4)2 crystals have narrow particle size distribution and an average size of 200–500 nm (Fig. 4c). When the reaction carries out for 10 h, some other morphologies appear and the products have a broad size distribution (Fig. 4d).
The relevant formation mechanism of different double alkaline rare earth tungstates and molybdates has been reported several times in the literature. For example, Liu et al. [18] revealed that the bipyramid-like NaY(MoO4)2 microcrystals were formed by a three-step ‘‘dissolution–renucleation–growth’’ mechanism. Nowadays, the oriented aggregation and preferential growth mechanisms also have been successfully proposed to explain the formation of octahedron-like microcrystals [28]. The well-known Ostwald ripening, in which small crystallites such as ions, atoms, or molecules grow into larger ones, is generally believed to be the main path of crystal growth [18, 29].
On the basis of the above morphological evolution, an oriented aggregation and Ostwald ripening progress can be proposed for the formation of octahedron-like NaY(MoO4)2 crystals. The reactants initially begin to fuse into the ion state with the rise of the reaction temperature in the presence of NaCl molten salt. The mixing of Na+, Y3+ and MoO4 2− ions results in the direct formation of NaY(MoO4)2 nuclei. With the presence of molten salt, Cl− can be absorbed to the surfaces of growing particles, which promotes the formation of amorphous NaY(MoO4)2 nanoparticles and reduces energy consumption. And then, part of the nucleation clusters aggregate together into some tiny octahedral-like microparticles which have unstable facets with relatively high surface energy. The reason for above phenomenon is that the formation of octahedral-like microparticles can greatly reduce the interfacial energy of primary amorphous particles. At the last stage, larger octahedron-like NaY(MoO4)2 crystals eventually generate at the cost of the small ones according to the typical Ostwald ripening process. And Cl− can be desorbed from the surfaces of the NaY(MoO4)2 crystals due to the desorption efficiency [24].
3.2 Photoluminescence properties
NaY(MoO4)2 shows excellent thermal and hydrolytic stability and is considered to be an effective luminescent host for rare earth ions to produce phosphors and laser crystals. Owing to the similar chemical properties of Y3+ and Eu3+/Tb3+ ions [30], little doped Eu3+/Tb3+ into NaY(MoO4)2 has no influence on the morphologies as shown in Fig. 5.
Figure 6 shows the PL excitation and emission spectra of the octahedron-like and rod-like NaY(MoO4)2:Eu3+ phosphors. The excitation spectra (Fig. 6, left) are obtained by monitoring the emission of the Eu3+ 5D0 → 7F2 transition at 615 nm. The excitation spectra of NaY(MoO4)2:Eu3+ with different morphologies have similar profiles and each one consists of two parts. One part is a strong broad band ranging from 200 to 350 nm with a maximum at about 298 nm, which is attributed to the Mo6+–O2− and O2−–Eu3+ charge transfer band. The other part in the excitation spectra is a series of sharp excitation peaks in the longer region (350–450 nm) associated with the general f–f transitions of Eu3+ ions. It is indicated that an energy transfer process occurs from the MoO4 2− groups to Eu3+ ions in the crystal because of the presence of the excitation band of the MoO4 2− groups in the excitation spectrum of Eu3+ ions. Upon excitation into the MoO4 2− at 298 nm, the most intensive peak at 615 nm arises from the 5D0 → 7F2 transition, while the other peaks at 537, 591, 657 and 702 nm correspond to the 5D1 → 7F1, 5D0 → 7F1, 5D0 → 7F3, 5D0 → 7F4 transitions of Eu3+ ions, respectively (Fig. 6, right). Moreover, according to Stark energy splitting [16], the (2 J + 1) Stark components of J-degeneracy splitting lead to the two subpeaks at 615 nm. The electric dipole transition 5D0 → 7F2 is a hypersensitive transition and the emission intensity is strongly influenced by local environment surrounding Eu3+ ions. If Eu3+ is located at a low symmetry site, the 5D0 → 7F2 transition should be dominant, while in a site with an inversion center, the 5D0 → 7F1 magnetic dipole transition will be preponderant. In the present case, the emission intensity of 5D0 → 7F2 is evidently stronger than the other one, which indicates that the non-inversion center site is occupied by Eu3+ in the NaY(MoO4)2 host lattice. Besides, the morphologies have no effect on the peak locations but can affect the red emission intensity strongly. And the PL intensity of octahedron-like microcrystals is stronger than the rod-like ones. It is believed that the improvement of the PL intensity may be attributed to the uniform morphology, average size and narrow particle size distribution.
The excitation spectra of the octahedron-like and rod-like NaY(MoO4)2:Tb3+ phosphors monitored with Tb3+ 5D4 → 7F5 at 545 nm exhibit intensive and broad band from 200 to 350 nm peaked at 298 nm, which correspond to the charge-transform transitions within the MoO4 2− groups (Fig. 7, left). The emission spectra consist of a group of lines centered at about 488, 545, 587, and 621 nm, which correspond to the 5D4 → 7FJ (J = 6, 5, 4, 3) transitions of the Tb3+ ions, respectively (Fig. 7, right). By comparison of the emission spectra of NaY(MoO4)2:Tb3+ with different morphologies, the emission intensity of the octahedron-like microcrystals is also stronger than the rod-like ones. The PL excitation of Tb3+ doped NaY(MoO4)2 crystals is much similar to that of the Eu3+ doped samples from 200 to 350 nm, implying that these samples can be rationally mixed for many practical applications due to their equal excitation wavelength.
The emission and energy transfer process in Eu3+ and Tb3+ doped NaY(MoO4)2 is shown schematically in Fig. 8a. Upon excitation at 298 nm, electrons of MoO4 2− excite from the ground state to the highest state. Then the excited electrons either relax to the lowest vibrational level of the excited state of MoO4 2−, or transfer non-radiatively to higher levels of Eu3+, from which the energy relax to the 5D0 or 5D1 level by the cross-relaxation process (Fig. 8a, left). Red emission is obtained as a result of 5D1 → 7F1 and 5D0 → 7FJ (J = 1, 2, 3, 4) transitions. The excitation energy of MoO4 2− is mainly transferred to the higher levels of Tb3+ (Fig. 8b, right). And the energy from the higher levels relaxes to 5D4 level of Tb3+ by the same process compared with that of Eu3+.
To investigate the tunable PL properties of the NaY(MoO4)2 crystals, we have co-doped Eu3+ and Tb3+ ions with different relative concentrations into the octahedron-like NaY(MoO4)2 host lattice, as the octahedron-like NaY(MoO4)2 give stronger emissions in the progress of this research. The emission spectra of the Eu3+–Tb3+ co-doped NaY(MoO4)2 samples under the excitation at 298 nm are shown in Fig. 9. It can be seen that the as-prepared pure Eu3+-doped NaY(MoO4)2 crystals show strong red emission under UV light excitation. With the doping of Tb3+ ions into the NaY(MoO4)2 host lattice, the characteristic emission of the Tb3+ ions can be found besides the Eu3+ emission. Green emissions gradually rise along with the increasing of Tb3+ concentration.
The emission and energy transfer process in Eu3+–Tb3+ co-doped NaY(MoO4)2 is shown schematically in Fig. 8b. Firstly, electrons on MoO4 2− relax to the higher levels of Tb3+ upon excitation at 298 nm. The electrons relax to the 5D4 level, and then either return to the ground state to produce the Tb3+ emissions (5D4–7F6, 5, 4, 3), or transfer excitation energy from 5D4 (Tb3+) level to the higher excited energy levels of Eu3+ (4f6) through cross relaxation, which relax to the 5D0 or 5D1 (Eu3+) level, where the red emissions take place. The energy transfers from Tb3+ to Eu3+ could be very efficient due to large spectral overlap between Tb3+ emission and Eu3+ absorption [25].
The commission international Del’Eclairage (CIE) chromaticity coordinates of the Eu3+–Tb3+ co-doped NaY(MoO4)2 phosphors was calculated based on the CIE 1931 standards (Table 1). The color of these samples can be tuned from red, orange, yellow and green-yellow to green by adjusting the associative doping ratio of Eu3+ and Tb3+ ions (Fig. 10, from a to f).This result indicates that the as-obtained phosphors could show merits of multicolor emissions in the visible region under UV light excitation, which might make it possible that desired colors produced with NaY(MoO4)2 host lattice could have potential application in the areas such as optoelectronic devices in the future.
4 Conclusion
The NaY(MoO4)2 microcrystals with different morphologies have been synthesized with different reaction conditions via a low cost molten salt method. The calcining temperature and reaction time play critical roles in controlling the morphology and the size of the NaY(MoO4)2 samples. The octahedron-like crystals could be obtained at the calcining temperature of 800 °C for 6 h. The Eu3+/Tb3+ ions doped octahedron-like NaY(MoO4)2 phosphors show stronger red/green emission under UV light excitation than the rod-like ones. Moreover, when co-doping with Eu3+ and Tb3+ in an appropriate ratio, the PL emission colors of the NaY(MoO4)2:Eu3+/Tb3+ phosphors can be tuned from red, orange, yellow and green-yellow to green, which might have potential application in the areas such as optoelectronic devices in the future.
References
H. Jung, D. Hwang, E. Kim, B.J. Kim, W.B. Lee, J.E. Poelma, J. Kim, C.J. Hawker, J. Huh, D. Ryu, J. Bang, ACS Nano 5, 6164–6173 (2011)
C.T. Dinh, T.D. Nguyen, F. Kleitz, T.O. Do, ACS Nano 3, 3737–3743 (2009)
Y. Tian, B. Chen, B. Tian, R. Hua, J. Sun, L. Cheng, H. Zhong, X. Li, J. Zhang, Y. Zheng, T. Yu, L. Huang, Q. Meng, J. Alloys Compd. 509, 6096–6101 (2011)
J.C. Sczancoski, M.D.R. Bomio, L.S. Cavalcante, M.R.B. Joya, P.S. Pizani, J.A. Varela, E. Longo, M.S. Li, J.A. Andre´s, J. Phys. Chem. C 113, 5812–5822 (2009)
J. Thirumalai, R. Krishnan, I.B. Shameem Banu, R. Chandramohan, J. Mater. Sci.: Mater. Electron. 24, 253–259 (2013)
B.K. Lim, M.J. Jiang, J. Tao, H.C. Camargo, Y.M. Zhu, Y.N. Xia, Adv. Funct. Mater. 19, 189–200 (2009)
B.A. Marinkovic, M. Ari, R.R. de Avillez, F. Rizzo, F.F. Ferreira, K.J. Miller, M.B. Johnson, M.A. White, Chem. Mater. 21, 2886–2894 (2009)
M.W. Stoltzfus, P.M. Woodward, R. Seshadri, J.H. Klepeis, B. Bursten, Inorg. Chem. 46, 3839–3850 (2007)
R. Krishnan, J. Thirumalai, I.B. Shameem Banu, R. Chandramohan, J. Mater. Sci.: Mater. Electron. 24, 4774–4781 (2013)
Y.H. Wang, Y.S. Liu, Q.B. Xiao, H.M. Zhu, R.F. Li, X.Y. Chen, Nanoscale 3, 3164–3169 (2011)
C.X. Li, J. Lin, J. Mater. Chem. 20, 6831–6847 (2010)
C. Bouzigues, T. Gacoin, A. Alexandrou, ACS Nano 5, 8488–8505 (2011)
F. Yang, Y. Liu, Y. Lu, H. Chen, D. Zhang, H. Wu, J. Mater. Sci.: Mater. Electron. 25, 3608–3613 (2014)
L. Armelao, S. Quici, F. Barigelletti, G. Accorsi, G. Bottaro, M. Cavazzini, E. Tondello, Coord. Chem. Rev. 254, 487–505 (2010)
W. Bu, Y. Xu, N. Zhang, H. Chen, Z. Hua, J. Shi, Langmuir 23, 9002–9007 (2007)
G.S. Yi, B.Q. Sun, F.Z. Yang, D.P. Chen, Y.X. Zhou, J. Cheng, Chem. Mater. 14, 2910–2914 (2002)
G.H. Li, L.L. Li, M.M. Li, W.W. Bao, Y.H. Song, S.C. Gan, H.F. Zou, X.C. Xu, J. Alloys Compd. 550, 1–8 (2013)
J. Liu, B. Xu, C. Song, H.D. Luo, X. Zou, L.X. Han, X.B. Yu, CrystEngComm 14, 2936–2943 (2012)
Z.H. Xu, C.X. Li, G.G. Li, R.T. Chai, C. Peng, D.M. Yang, J. Lin, J. Phys. Chem. C 114, 2573–2582 (2010)
Y. Huang, L.Q. Zhou, L. Yang, Z.W. Tang, Opt. Mater. 33, 777–782 (2011)
X. Wang, L.S. Gao, F. Zhou, Z.D. Zhang, M.R. Ji, C.M. Tang, T. Shen, H.G. Zheng, J. Cryst. Growth 265, 220–223 (2004)
L.J. Xie, J.F. Ma, J. Zhou, Z.Q. Zhao, H. Tian, Y.G. Wang, J.T. Tao, X.Y. Zhu, J. Am. Ceram. Soc. 89, 1717–1720 (2006)
B. Yan, F. Lei, J. Alloys Compd. 507, 460–464 (2010)
T. Wu, Y.F. Liu, Y.N. Lu, L. Wei, H. Gao, H. Chen, CrystEngComm 15, 2761–2768 (2013)
Y. Zeng, Z. Li, L. Wang, Y. Xiong, CrystEngComm 14, 7043–7048 (2012)
C.J. Mao, J. Geng, X.C. Wu, J.J. Zhu, J. Phys. Chem. C 114, 1982–1988 (2010)
S.D. Hutagalung, Materials Science and Technology, Materials Science (InTech, Croatia, 2012)
Y. Li, G.F. Wang, K. Pan, W. Zhou, C. Wang, N.Y. Fan, Y.J. Chen, Q.M. Feng, B.B. Zhao, CrystEngComm 14, 5015–5020 (2012)
J.F. Banfield, S.A. Welch, H.Z. Zhang, T.T. Ebert, R.L. Penn, Science 289, 751–754 (2000)
L. Xu, X. Yang, Z. Zhai, X. Chao, Z. Zhang, W. Hou, CrystEngComm 13, 4921–4929 (2011)
Acknowledgments
This work is financially supported by Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions, Major Program for the Natural Scientific Research of Jiangsu Higher Education Institutions (12KJA430002) and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT), IRT1146.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X., Liu, Y., Lu, Y. et al. Molten salt synthesis and tunable photoluminescent properties of Eu3+–Tb3+ doped NaY(MoO4)2 microcrystals. J Mater Sci: Mater Electron 26, 2987–2994 (2015). https://doi.org/10.1007/s10854-015-2787-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-2787-8