Abstract
Using CoCl2 and SbCl3 as precursors and NaBH4 as reducing agent, we obtained nano CoSb3 particles of about 10 nm with the assistance of microwave radiation. Different Sb/Co ratio and different microwave radiation time were used to prepare CoSb3. The results show that single CoSb3 phase was synthesized successfully under the condition of Sb/Co ratio with 5:1 and microwave radiation time with 5 min. The samples with microstructure and nanostructure were synthesized respectively by cold isostatic pressing and sintering method, and their thermoelectric properties were studied. Due to its powder preparation method and its small grain size, the sample with nanostructure has lower electrical resistivity and thermal conductivity than the sample with microstructure. The maximum ZT value was found to be 0.11 at 650 K in the sample with nanostructure, which is about 10 times higher than that of the sample with microstructure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thermoelectric materials have received much attention in power generation field in recent years. The thermoelectric efficiency of a material is characterized by the dimensionless figure of merit, ZT = α2T/ρκ, where T, α, ρ, and κ are the absolute temperature, Seebeck coefficient, electrical resistivity, and thermal conductivity, respectively.
CoSb3 has been identified as a candidate for good thermoelectric materials because it has a large Seebeck coefficient and good electrical conductivity [1, 2]. The thermal conductivity of CoSb3, however, is still high for being an efficient thermoelectric material [3, 4]. To lower the thermal conductivity and further to increase ZT value, great effort has been made in recent years. Filling the Sb-icosahedron voids by rare earth or other metallic atoms was usually used [5–7], which can optimize the electrical transport properties and significantly depresses the lattice thermal conductivity due to the rattling of these atoms. In addition, doping with Fe, Ni, Te and Pd atoms can also reduce the thermal conductivity due to the phonons scattering by the introduced point defects [8, 9].
Nanostructuring of thermoelectric materials is also one of the effective approaches to achieve lower thermal conductivity. The reason is that large amount of grain boundaries introduced by nanostructuring can remarkably limit the mean free path of phonons and enhance phonon scattering [10, 11]. Many methods have been used to prepare nanosized CoSb3 compound. Mi et al. [12] synthesized CoSb3 by a solvothermal method in ethanol solution at 250 °C for a long reaction time of 72 h. There has been reported that nanostructured CoSb3 compounds were prepared using an ultrasonic spray pyrolysis (USP) method, in which a high temperature (800 °C) was required [13]. To reduce the reaction time and temperature, Yang et al. [11] prepared nanostructured CoSb3 powders by a modified polyol process at 180 °C for 15 min. The application of microwave heating in the synthesis of materials is a fast growing research area owing to its advantages such as rapid volumetric heating, higher reaction rate, selectivity, and shorter reaction time compared with conventional heating methods [14, 15]. In order to improve the heating speed resulted from microwave energy, the iron liquids are usually used as good microwave absorbefacient for their high polarizing character. But up to now, there have been no reports on the preparation of CoSb3 materials by a microwave heating method. In this study, nano CoSb3 particles of about 10 nm were obtained under conditions of microwave radiation assisted with iron liquid (BmimBr) for a short time of 5 min.
2 Experimental
The analytical pure SbCl3 and CoCl2·6H2O with different molar ratio were loaded into a beaker with 20 ml of glycol and vigorous magnetic stirred for 30 min, then sufficient reductant NaBH4 were added. The reaction lasted for 10 min and the color of the solution turned dark within only a few minutes. Iron liquid (BmimBr) was added and stirred for 5 min after the precipitates were deposited completely. Then the beaker containing the mixture was put into a microwave oven and heated. Microwave-assisted reactions with different time (5, 10, 15 and 20 min, respectively) were conducted in an 800 W microwave oven, with a 2.45 GHz working frequence. After cooling to room temperature naturally, the precipitates were centrifugally washed with distilled water and ethanol several times, and dried under vacuum at 70 °C for 6 h. All the experiments were carried out in a fume hood under normal atmosphere.
For the thermoelectric properties measurement, two bulk samples were used for comparison. They are both synthesized by cold isostatic pressing (CIP) method, and the sintering process was carried out in a GSL-1300X tubular furnace at 923 K for 3 h in Ar atmosphere. The difference is one sample (named as CS-1) synthesized from the powders prepared by mechanical alloying methods [16] and the other (named as CS-2) synthesized from the powders prepared in this study.
The samples were analyzed by X-ray diffraction (XRD, using Cu Kα radiation, Bruker D8, Germany) in the range of 2θ = 20–90°. The microstructures were characterized by field- emission scanning electron microscopy (FESEM, LEO-1550) and Tecnai20 transmission electron microscope (TEM). The compositions were analyzed with an energy-dispersive X-ray spectroscopy (EDX). The Seebeck coefficient α, and the electrical resistivity ρ, were simultaneously measured by the temperature differential and four-point probe methods (Ulvac-Riko ZEM 3) in a temperature range from 300 to 850 K. The thermal diffusivity D was measured by the laser flash method under Argon atmosphere using the thermal constant analyzer (NETZSCH LFA427), and the specific heat C p was measured from 300 to 850 K in argon using a Shimadzu DSC-50. The densities d of the samples were measured using the Archimedes method. The thermal conductivity k of the samples was calculated from the thermal diffusivity, D, the specific heat, C p , and the density, d, in the equation k = D × C p × d.
3 Results and discussion
Though the theoretical atom ratio of Sb/Co is 3:1 according to the molecular formula of CoSb3, it is difficult to obtain pure CoSb3 compound if we mix SbCl3 and CoCl2·6H2O in according with its stoichiometry ratio exactly, because SbCl3 powders will volatilize easily even under the condition of room temperature. So we judge that the SbCl3 powders must be excessive. In order to find the optimal content of SbCl3 for synthesizing pure CoSb3 compound, we prepared several powder samples with different Sb/Co atom ratio of 3:1, 3.5:1, 4:1 and 5:1 respectively. Because NaHB4 can also react with glycol slowly, therefore sufficient NaHB4 (the molar ratio of NaHB4/Co is 60:1) were used as reducing agent.
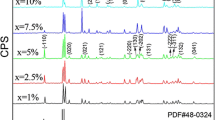
Figure 1 shows the XRD patterns of different powder samples synthesized by microwave radiation for 5 min. CoSb2 is the main phase and no peaks of CoSb3 were detected in the sample with Sb/Co atom ratio 3:1 and 3.5:1. It indicates that the content of Sb3+ in the mixed solution is reduced for their volatilization and thus leads to a decrease in Sb/Co ratio than 3:1. So there has no sufficient Sb which could react with Co to form CoSb3. When Sb/Co ratio increases to 4:1, the patterns of CoSb3 phase are visible and the peaks of CoSb2 become weaker. From the patterns of sample with a Sb/Co ratio of 5:1, we can see that all the peaks can be indexed to the skutterudite-type cubic CoSb3 phase of space group Im3 and no other impurities appear. The peaks of XRD patterns are very low and wide due to their low crystallization.
We hold the Sb/Co atom ratio with 5:1 as a constant and studied the influence of microwave radiation time (5, 10, 15 and 20 min, respectively) on the crystallization of CoSb3. The XRD patterns of powder samples synthesized under different microwave radiation time are shown in Fig. 2. As can be seen, all the diffraction peaks correspond well to CoSb3 phase. The samples synthesized in short time are not well crystallized as inferred from the wide and low XRD peaks. With increasing radiation time before 15 min, the XRD peaks became sharper and narrower, showing that these atoms were in better order and the crystalline degree was improved. However, the peaks become weaker when the radiation time was prolonged to 20 min. Generally, with increasing radiation time, more heat energy induced from microwave radiation will lead to more volatilization of a great deal of solvent because the boiling point of glycol is as low as 197.5 °C. Therefore, the synthesized CoSb3 powders will be exposed in air and oxidated quickly, which reduce the content of CoSb3 and thus weaken its diffraction peaks. At the same time, more heat energy also makes dehydration of glycol molecular and form viscous mixture, which will bring much trouble in subsequent wash process. Our study indicates that the appropriate microwave radiation time for synthesizing nano CoSb3 particles is not exceeding 15 min.
FESEM images of CoSb3 powders synthesized under different microwave radiation times are shown in Fig. 3. It reveals that the powder samples have a uniform microstructure and we cannot estimate the size of CoSb3 particles. The EDX analysis of the nano-particles (from the circle area in Fig. 3c) is shown in Fig. 3d. It shows that the prepared sample consists of Co and Sb only and no peaks for other elements were detected, indicating the pure phase of the CoSb3 samples. The average composition for this sample obtained by EDX is 25.71Co-74.29Sb (in at.%), very close to the theoretical ratio with Sb/Co = 3:1.
In order to know the size of CoSb3 particles, we carried out a TEM measurement. Figure 4 shows the typical TEM images of CoSb3 powders synthesized by microwave radiation for 5 min. The spherical shape of the particles suggests an isotropic crystal growth of the CoSb3 grains due to its cubic lattice structure. The average particle size is about 10 nm. The morphology and size is similar to the results previously reported by Li et al. [17]. It should be pointed out that although the prepared conditions are different between our work and other reported work [4], the same reaction mechanism was widely accepted.
In the beginning of the reaction process, namely before microwave reaction, the strong reducing agent NaHB4 rapidly and completely reduce the Co2+ and Sb3+ ions to Co and Sb atoms, respectively. The reaction formula can be written as:
When the solvent including Co and Sb atoms was put under the condition of microwave radiation, the active Co and Sb atoms will react with each other and CoSb3 is formed spontaneously. The reaction formula can be written as:
Earlier reports [11, 12, 17] indicated that the CoSb and CoSb2 as intermediate phases will appear before the formation of final CoSb3 phase. While in our present work, the appearance CoSb2 should attribute to the absence of Sb3+ induced from the volatilization of SbCl3 and we could not think the CoSb2 as an intermediate phase. A single phase CoSb3 was obtained and no other phase such as CoSb and CoSb2 appeared when the Sb/Co atom ratio is increased to 5:1. With the assistance of microwave radiation, we obtained the pure CoSb3 phase only in 5 min, and it is much shorter than solvothermal synthesis of CoSb3 [3] (250 °C for 72 h) and also polyol process synthesis of CoSb3 [11] (180 °C for 15 min). Such short reaction time must attribute to the sufficient energy offered by microwave radiation.
Figure 5a shows the FESEM image of the fracture surface of bulk sample CS-1. It reveals that the sintered sample has a uniform microstructure with average grain size of about 1.5 μm. Figure 5b is the FESEM image of fracture surface of bulk sample CS-2. Comparing with the powder sample shown in Fig. 5, the grain size grew up evidently after sintering process and the average grain size is about 200 nm.
Figure 6a shows the temperature dependence of Seebeck coefficient (α) for the two samples. For the sample CS-1, the α is negative at beginning and the maximum absolute value of 425.79 μV/K appears at 350 K, while it changes to positive at 600 K. The reason is that the hole mobility is much larger than the electron mobility in the temperature range higher than 600 K [18], at which intrinsic excitation commences. It has been reported that the intrinsic CoSb3 shows the p-type conductivity [19, 20]. In this study, the low purity of Co (99.5 %) may be the reason for the appearance of n-type conductivity initially. The sample CS-2 exhibits n-type conduction, as it has negative Seebeck coefficient in the measurement range from 300 to 850 K. The result is similar with the sample synthesized by solvothermal method reported by Mi et al. [12]. For an intrinsic n-type semiconductor, the calculation formula for the Seebeck coefficient can be expressed as follows:
where k 0 is Boltzman constant, E C the bottom of conduction band, and E f is the Fermi energy. The Fermi level shifts toward the midgap line with temperature increase, which leads to the increase of energy difference between the Fermi level and the conduction band and to the increase of absolute Seebeck coefficient. The maximum absolute value of 269.26 μV/K is reached at 600 K. After that, the absolute Seebeck coefficient starts to decrease at higher temperature. This is mainly ascribed to the occurrence of an increasing number of thermally excited minority carriers at higher temperature, which tends to decrease the Seebeck coefficient. The different sample preparation methods are responsible for the difference in the conduction type.
Figure 6b shows the temperature dependence of electrical resistivity (ρ) for the two samples. As can be seen, ρ of the sample CS-1 shows a very high value of 1,610 μΩ m at 323 K and it decreases considerably with increasing temperature, indicating a semiconductor behavior. For the sample CS-2, we can see that the electrical resistivity has little temperature difference and it shows relative low value of 150.25 μΩ m at room temperature. In general, when grain size increases, the electrical resistivity will decrease because scattering electrons from grain boundary is reduced. The reason about the decrease in sample CS-2 may be due to its high carrier concentrations induced from microwave-assistant synthesized powders, which originate from the impurity ions existing in chemical precursors CoCl2 and SbCl3. The similar study was reported by Mi et al. [3]. However, the ρ values in our study are still higher than the previous report [3, 21], which is due to the relative high porosity existing in our prepared samples, namely the sample synthesized by CIP methods in our study has relative lower density than that synthesized by hot pressing (HP) or spark plasma sintering (SPS) methods.
The temperature dependence of thermal conductivities (κ) for the two samples is shown in Fig. 6c. It is clear that thermal conductivity of the two samples both decrease first and then increases with increasing temperature. Obviously, CS-2 has much lower κ values than that of sample CS-1 in all test range and the lowest value is 5.71 W/m k, nearly 37 % lower than that of sample CS-1. The main reason is that sample CS-2 with nanostructure has more grain boundaries than sample CS-1, which could significantly scatters phonons and decreases the thermal conductivity.
The calculated dimensionless figure of merit ZT values are shown in Fig. 6d. The ZT value of sample CS-1 is quite low in the whole measurement range and the maximum is only about 0.01 at 450 K. The reason is mainly ascribed to its transition in Seebeck coefficient, higher electrical resistivity and thermal conductivity. For sample CS-2, at the beginning, the ZT value increases with increasing temperature and the maximum value is found to be 0.11 at 650 K. Comparing with sample CS-1, the increase in ZT value for sample CS-2 is mainly due to its low electrical resistivity and thermal conductivity. Although the ZT value we obtained is still not high, we believe that the figure of merit can be expected to improve if the sample is synthesized by HP or SPS methods.
4 Conclusion
In summary, nano CoSb3 particles of about 10 nm were obtained with the assistance of microwave radiation for a short time of 5 min. It is difficult to obtain pure CoSb3 compound if we mix SbCl3 and CoCl2·6H2O in according with its stoichiometry ratio exactly and the content of SbCl3 should be excessive. In contrast, we synthesized two bulk samples including nanostructure and microstructure. The results show that the former possesses lower resistivity and thermal conductivity than the latter. The low thermal conductivity is due to the stronger scattering of phonons for the high grain-boundary density induced from its small grain size. The maximum ZT value was found to be 0.11 at 650 K in nanostructured sample, which is about 10 times higher than that of microstruetured sample. Our study implies that nanostructuring of thermoelectric materials is an effective approach to enhancing thermoelectric properties.
References
D. Mandrus, A. Migliori, T.W. Darling, M.F. Hundley, E.J. Peterson, J.D. Thompson, Phys. Rev. B. 50, 4926 (1995)
E. Alleno, L. Chen, C. Chubilleau, B. Lenoir, O. Rouleau, M.F. Trichet, B. Villeroy, J. Electron. Mater. 39, 1966 (2010)
J.L. Mi, T.J. Zhu, X.B. Zhao, J. Ma, J. Appl. Phys. 101, 054314 (2007)
L. Kumari, W. Li, J.Y. Huang, P.P. Provencio, Nanoscale Res. Lett. 5, 1698 (2010)
Y.Z. Pei, S.Q. Bai, X.Y. Zhao, W. Zhang, L.D. Chen, Solid State Sci. 10, 1422 (2008)
S.Q. Bai, X.Y. Huang, L.D. Chen, W. Zhang, X.Y. Zhao, Y.F. Zhou, Appl. Phys. A 100, 1109 (2010)
B. Huang, M. Kaviany, Acta Mater. 58, 4516 (2010)
X. Zhang, Q.M. Lu, J.X. Zhang, Q. Wei, D.M. Liu, Y.Q. Liu, J. Alloys Compd. 457, 368 (2008)
M. Chitroub, F. Besse, H. Scherrer, J. Alloys Compd. 467, 31 (2009)
M.S. Toprak, C. Stiewe, D. Platzek, S. Williams, L. Bertini, E. Müller, C. Gatti, Y. Zhang, M. Rowe, M. Muhammed, Adv. Funct. Mater. 14, 1189 (2004)
L. Yang, H.H. Hng, H. Cheng, T. Sun, J. Ma, Mater. Lett. 62, 2483 (2008)
J.L. Mi, X.B. Zhao, T.J. Zhu, J.P. Tu, G.S. Cao, J. Alloys Compd. 417, 269 (2006)
K.T. Wojciechowski, J. Morgiel, in Proceedings of the 22nd International Conference on Thermoelectrics. La Grande Motte, France, August, pp. 97, 2003
Y. Jiang, Y.-J. Zhu, G.-F. Cheng, Cryst. Growth Des. 6, 2174 (2006)
L. Guo, G. Ji, X. Chang, M. Zheng, Y. Shi, Y. Zheng, Nanotechnology. 21, 035606 (2010)
Y. Zhu, H. Shen, L. Zuo, H. Guan, Solid State Commun. 151, 1388 (2011)
J.Q. Li, X.W. Feng, W.A. Sun, W.Q. Ao, F.S. Liu, Y. Du, Mater. Chem. Phys. 112, 57 (2008)
W.-S. Liu, B.-P. Zhang, J.-F. Li, H.-L. Zhang, L.-D. Zhao, J. Appl. Phys. 102, 103717 (2007)
I.-H. Kim, K.-H. Park, S.-C. Ur, J. Alloys Compd. 442, 351 (2007)
Ø. Prytz, O.M. Løvvik, J. Taftø, Phys. Rev. B. 74, 245109 (2006)
S. Katsuyama, M. Watanabe, M. Kuroki, T. Maehata, M. Ito, J. Appl. Phys. 93, 2758 (2003)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, Y., Shen, H. & Guan, H. Microwave-assisted synthesis and thermoelelectric properties of CoSb3 compounds. J Mater Sci: Mater Electron 23, 2210–2215 (2012). https://doi.org/10.1007/s10854-012-0754-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-0754-1