Abstract
Pure CoSb3 and 0.1 vol% nano-SiC-composited CoSb3 were prepared by mechanical alloying and subsequent fast microwave sintering. The electrical transport properties display totally different behaviors in microwave-annealed CoSb3 and CoSb3/SiC composites, implying their susceptibility to the preparation conditions or uncertainties. The unique microstructure including the inter-granular and intra-granular precipitates combined with high porosity of the microwave-synthesized CoSb3 and CoSb3/SiC composites lead to low thermal conductivity, which compensates the loss in electrical conductivity and results in comparable figure of merit ZT value with those reported for undoped CoSb3 from conventional method requiring high energy consumption and lengthy synthesis time. The results show that low compactness is not detrimental for the thermoelectric performance and microwave is a highly cost-effective technique for large-scale production of thermoelectric materials possessing nanostructure and low thermal conductivity. In fact, such synthesis route combining mechanical alloying and microwave annealing might be also suitable for other high performance thermoelectric materials. Actually, continuous fabrication can be readily realized in an upgraded tube microwave heating system for higher energy efficiency ratio.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thermoelectric (TE) materials have attracted extensively attention in the past decades because of their important applications in waste heat recovery and environmentally friendly refrigeration. The thermoelectric energy conversion efficiency is mainly governed by the dimensionless figure of merit, defined as ZT = α 2 σT/κ, where α is the Seebeck coefficient, σ is the electrical conductivity, T is the absolute temperature and κ is the total thermal conductivity which is composed of the carrier contribution κ c and the lattice contribution κ l . To optimize the ZT value, high α, high σ and low κ are required for high performance TE materials [1–3].
CoSb3-based skutterudite compounds have been identified as one of the most promising TE materials for intermediate temperature range [4, 5]. CoSb3 has good σ, large α, but very high κ, which leads to small ZT and inhibits its applications. Many efforts have been made to improve the TE performance of CoSb3, including doping by partial Co-site or Sb-site substitution, filling structure voids and nanostructuring, from which the κ l can be significantly reduced due to enhanced phonon scattering [6–13]. Incorporating nanoscale heterogeneity or nanoinclusions (such as Yb2O3, ZrO2 and WO3) into the CoSb3 matrix has been proved effective to enhance the ZT [14–16]. Actually, to find a suitable nanodispersion is the key to achieve successful compositing of TE materials, which should reduce the κ while maintain the σ as possible at the same time. Being a promising TE semiconductor with a wide band gap, SiC has large α and relatively high σ, which makes it possible to improve the TE performance by adding SiC nanoparticles into other TE materials [17–20]. Moreover, the amount of SiC nanodispersion should be optimized against different TE parent systems to balance the electrical and the thermal properties. For example, Li et al. reported the enhanced ZT value up to 1.33 in Bi0.3Sb1.7Te3 alloys by incorporating 0.4 vol% SiC nanoparticles [18]. However, Akao et al. observed remarkable degradation of the ZT in the Zn4Sb3 with 1–5 vol% addition of SiC whiskers [19]. Furthermore, the introduction of hard SiC nanoparticles can improve the mechanical performance of TE materials, which has already been demonstrated [18].
Preparation cost is another key factor to evaluate the feasibility of a promising TE material for applications. During the last decade, various techniques including solid state reaction, mechanical alloying (MA), co-precipitation, sol–gel and solvothermal methods have been used for the synthesis of CoSb3. The solid state reaction is a simple process, but it requires heating at high temperature (above 1273 K) with long soaking time (usually 100 h) to obtain pure CoSb3 because of very slow kinetics [13]. Yang et al. synthesized single phase CoSb3 easily by annealing the as-MAed elemental powders, and the annealing time could be as short as 1 h for the powders milled for over 10 h [21]. Moreover, in order to get dense bulks maintaining nanostructure, the precursor CoSb3 powders need to be rapid sintered normally by hot pressing (HP) or spark plasma sintering (SPS), which requires expensive equipments, leading to much high energy consumption but low yield [12–16].
Microwave heating is a volumetric method by converting electromagnetic energy into thermal energy. In advantage over conventional heating, microwave-assisted solid state reaction is a rapid, simple, economically feasible and environmental friendly method [22]. Recently, microwave heating has been applied to fast sintering of the TE materials [23]. Biswas et al. adapted microwave synthesis for In-filled CoSb3, reducing the initial calcinations time from 2 days to 2 min [24]. Ioannidou et al. obtained Fe-substituted CoSb3 using microwave heating within 14 min, while subsequent SPS was still employed to obtain final bulk products [25]. Generally, the microwave-sintered sample has lower density (less than 90%) compared with that (normally above 97%) after HP or SPS, which might increase the Seebeck coefficient and decrease the electrical conductivity due to stronger grain boundary scattering. On the other hand, the lower density can efficiently decrease the thermal conductivity by reducing the phonon transport channels and increasing the phonon scattering. Delaizir et al. carried out a comparative study of SPS, HP and microwave sintering techniques on p-type Bi2Te3 [26]. The highest ZT value was obtained for the ceramic processed by microwave, which originates from the very low thermal conductivity due to high porosity. In this work, bulk CoSb3 and CoSb3/SiC composites were fabricated by MA and microwave sintering with the total process being as short as several hours. This method can be an energy-efficient and economical solution to large-scale fabrication of CoSb3-based TE compounds.
2 Experiments
Commercial elemental powders of Co (99.9%), Sb (99.9%) and SiC (99%, 0.1 vol% about 0.047 mol% in addition for CoSb3/SiC composites) were weighted in the desired stoichiometric quantities and fully ground with an agate mortar and pestle. The mixtures of these powders were subjected to MA in a planetary ball milling using a hardened stainless steel vial and ball. The weight ratio of balls to powders was about 20:1 and the mill vial was filled with Ar gas to prevent the powders from oxidation during the milling process. The milling was performed at 500 rpm for 5 h. The MA-derived powders were tablet pressed and then sintered at 873, 923 and 973 K for 30 min, respectively, using a programmable tube microwave heating system (HY-ZG1512, 2.45 G, maximum output 1400 W, Huae Microwave) equipped with an in situ infrared temperature sensor (Raytek). Finally, the obtained pellets were polished first and cut into proper shapes for the subsequent measurements of TE properties.
The phase structure of the products was analyzed using powder X-ray diffraction (XRD) with Cu Kα radiation (Rigaku Miniflex 600). The microstructure and compositions were observed by scanning electron microscopy (SEM, ZEISS ULTRA 55) and energy dispersive spectroscopy (EDS, Bruker Quantax-XFlash 5030), respectively. The Seebeck coefficient and electrical conductivity were determined simultaneously on a commercial ZEM-3 system (ULVAC-RIKO) in the 300–700 K temperature range. The thermal diffusivity coefficient λ and the specific heat C P were measured utilizing a laser flash system (NETSCZ, LFA-457) and a differential scanning calorimeter (NETSCZ, DSC-204F1), respectively. The thermal conductivity κ was calculated from κ = C P λ d, d is the bulk density of the sample acquired by the Archimedes method.
3 Results and discussion
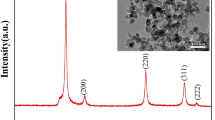
Figure 1a shows the XRD patterns of the Co, Sb, SiC mixture after the MA pretreatment and the final bulks after microwave heating to 873, 923 and 973 K, respectively. For the MAed mixture, all the peaks can be indexed to the Co and Sb, indicating no CoSb3 or CoSb2 comes into being. No diffraction peaks of SiC were detected owing to its low content. Yang et al. reported that single phase CoSb3 cannot be directly obtained by MA of the mixed raw elemental powders up to 50 h, with the CoSb3 phase appears after 20 h MA [21]. While Liu et al. obtained single phase CoSb3 powder by only 5 h MA [27]. Such noteworthy difference may come from different MA conditions, such as the time, the ratio of ball to powder, especially the speed of the mill. For example, Liu et al. utilized the rotating velocity of 1100 rpm, Yang et al. probably used that less than 300 rpm, while we used that of 500 rpm. For the microwave-sintered bulks, the location and strength of most diffraction peaks match with the standard card JCPDS: 88-2437 of CoSb3 skutterudite structure with space group Im3 without any SiC trace. This result indicates that the MAed mixture transforms into CoSb3 at 873 K, and has the best crystallization and purity after sintering at 973 K. Compared with the previously reported route of MA + annealing [21], the MA pretreatment time is shortened from 10 to 5 h, and the sintering time is reduced from 1 h to 30 min even at lower temperature by microwave. Most important, the final products in this route of MA + microwave sintering are bulks with no need for further fast sintering. Since the SiC particles were expected to work as nanodispersions in the CoSb3 matrix, it is necessary to characterize their morphology. Figure 1b presents the SEM images for the solo MAed SiC and the MAed mixture of Co, Sb, SiC for comparison. Having endured the repeated impacting, cold welding and interdiffusion during the MA treatment, both the SiC and the mixture have similar granularity distribution unevenly around several hundred nanometers. Amounts of finer SiC grains with diameters less than 50 nm in the assembly can also be observed.
Figure 2a gives the SEM image for the fractured surface of microwave-synthesized CoSb3. Porosity and various grain sizes in the range of 100–200 nm can be readily seen. The microstructure of CoSb3/SiC composites is almost identical as that of CoSb3 without obvious suppression on grain size observed. Due to the low content, the SiC particles can hardly be distinguished from the MAed raw mixture or the final sintered product using the SEM or even at its backscattered mode. Alternatively, the EDS, as shown in Fig. 2b, presents tiny trace at 1.7 keV of Si element, indicating the existence of SiC. In addition, a small peak around 6.4 keV corresponding to the Fe Kα line can be observed as well. Such subtle Fe contamination is probably introduced during the MA process using stainless steel vial and balls. It has been reported that the Fe substitution into CoSb3-based compound may significantly influence the TE performance especially drastically reduce the thermal conductivity, mostly leading to enhancement of the ZT value [28, 29]. From this point of view, the MA technique might have natural advantage to prepare CoSb3-based TE material. In earlier study, Delaizir et al. compared the microstructures of Bi2Te3 sintered by SPS, HP and microwave, respectively. They observed the intra-granular precipitates in all ceramics processed by SPS, HP and microwave, while some small particles at the surface of grain were only found in the sample after microwave treatment, which were then confirmed to originate from inter-granular precipitates using TEM observation [26]. As marked by the arrows in Fig. 2a, many small particles manifest at the surface of grains. These results suggest that such inter-granular precipitates are particular in microwave-synthesized sample, which may favor to decrease the thermal conductivity combined with high porosity.
Figure 3a gives the Seebeck coefficient α of the microwave-synthesized CoSb3 and CoSb3/SiC composites as a function of temperature. The positive α of CoSb3 indicates that the holes are the major carriers. The α value increases from 67 μV/K at 300 K to the maximum value 157 μV/K at 650 K and then decreases. This is mainly due to the appearance of an increasing number of thermally excited minority carriers at high temperatures [8]. For the microwave-synthesized CoSb3/SiC composites, the α is negative at room temperature, reaches the maximum absolute value of −331 μV at 350 K, then increases with temperature and changes to positive near 515 K. This behavior could be ascribed to bipolar effects arising from the positive α of intrinsic holes carriers compensating the negative α of extrinsic electron carriers. At high temperatures, the hole mobility is much larger than the electron mobility [5]. It has been reported that intrinsic CoSb3 shows p-type conductivity. Theoretical and computational studies reveal the direct and indirect band gaps in CoSb3, which would make its majority carriers very sensitive to impurities or small off-stoichiometry [10]. Previous experimental investigations have shown that various conditions, such as either tiny Sb or Co deficiency, substituting small amount of Ni in Co sites or Te in Sb sites and even low purity of Co (<99.95%) could bring about the n-type conduction [4, 5, 7, 10, 12, 30]. On the other hand, Sb rich, Fe substitution for Co and appropriate nanostructuring helps to maintain the p-type behavior [6, 8, 25, 28, 29]. It has been revealed that nanodispersion incorporated into a semiconducting matrix might increase the local density of states near the Fermi level and enhance the α value [18]. Because of the p-type semiconducting characteristic of SiC, the α value of the CoSb3/SiC composites is expected to be positive and increased. However, the opposite result is obtained in this study, which might originate from the uncertainties such as the Sb/Co ratio changed by the sublimation of Sb during the heat treatment. The detailed reason will be studied in the future work. Figure 3b displays the temperature dependence of electrical conductivity σ for the microwave-synthesized samples. With the temperature increases from 300 to 700 K, the σ of CoSb3 decreased from 0.17 × 105 to 0.10 × 105 S/m, while the σ of CoSb3/SiC composites increases sharply from 0.004 × 105 to 0.146 × 105 S/m. The σ behavior of CoSb3 agrees well with those reported for pure CoSb3, CoSb3/WO3 composites and In0.2Co4Sb12 where the decrease of σ as temperature increases is attributed to the decrease of carrier mobility [9, 10, 16, 24]. The smaller σ value of the microwave-synthesized CoSb3 (approximately 40%) may originate from the lower compactness. It should be mentioned that the microwave-synthesized CoSb3/SiC composites reveals completely different electrical conductivity behavior, indicating an activated type conducting nature. This behavior with comparable value is reminiscent of those reported by Khaliq et al. and Zhu et al. for pure CoSb3 [7, 12]. Based on the measured α and σ results, the temperature dependence of the power factor, PF = α 2 σ, is presented in Fig. 3c. The CoSb3 and CoSb3/SiC samples reach the maximum values 274 and 493 μW m− 1K− 2 at 600 and 650 K, respectively. The PF value of CoSb3 is comparable with that of the CoSb3 sample after SPS the nanoparticles [5]. The remarkable enhancement of the maximum PF value (nearly 80%) in CoSb3/SiC originates mainly from the higher σ value above 650 K.
In Fig. 4a, the total thermal conductivity κ of the microwave-synthesized samples are plotted vs. temperature. As can be seen, the κ of CoSb3 decreases from 3.24 W/mK first and then increases slowly with the temperature increasing, reaching the minimum value 2.17 W/mK at 600 K. Similar behavior has been reported in previous literatures, where the preceding decrease of κ could be attributed to the enhanced phonon scattering with the temperature increasing and the subsequent increase of κ may be predominated by intrinsic conduction [16]. For CoSb3/SiC composites, the κ decreases from 3.11 W/mK monotonically to 2.16 W/mK at 700 K. The minimum values around 2.1 W/mK for both the microwave-synthesized CoSb3 and CoSb3/SiC composites are much lower than those reported for pure CoSb3 and comparable to those of optimized nanostructured or nanocomposited CoSb3 compounds after SPS or HP [8, 16]. It is worthy mentioned that Ioannidou et al. fabricated Co1 − xFexSb3 by the route of microwave heating, ball milling and SPS, but got the minimum κ exceeding 4.0 W/mK [25]. Since the total thermal conductivity κ consists of the carrier contribution κ c and the lattice contribution κ l, the former can be estimated from Wiedemann–Franz law as κ c = L 0 σ T, where the Lorenz number L 0 = 2.44 × 10− 8 V2K− 2, σ is the electrical conductivity and T is the absolute temperature, the lattice thermal conductivity κ l can be obtained by subtracting κ c from κ. As shown in Fig. 4b, the κ l reaches the minimum value 1.99 W/mK at 600 K for CoSb3, 1.91 W/mK at 700 K for CoSb3/SiC composites, respectively. From the calculation, the κ l contributes more than 91% to the κ of CoSb3 and more than 88% to the κ of CoSb3/SiC composites. Generally, the grain boundaries, wide or point defects and impurities could reduce the κ l. Like those reported by Delaizir et al. for the microwave-sintered Bi2Te3 [26], the special microstructure including the inter-granular precipitates and high porosity (see Fig. 2a) would further decrease the κ l due to stronger phonon scattering. Then, one can conclude that the microwave sintering has natural superiority for economical and large-scale fabrication of low κ TE materials with nanostructure. Moreover, as proved previously, small amount of Fe contamination from the MA process in our case might also benefit the decrease of thermal conductivity κ [28, 29].
The temperature dependence of ZT values for the microwave-synthesized samples are given in Fig. 5. Both the CoSb3 and CoSb3/SiC composites reach their maximum ZT values at 650 K of 0.08 and 0.15, respectively. The ZT value 0.08 of CoSb3 is comparable to those of undoped samples prepared by conventional method [5, 12, 13]. This result is also close to the best ZT value of CoSb3 composited with CoSb3 nanoparticles reported by Yang et al. [6]. Ioannidou et al. got the maximum ZT of 0.06 after SPS the microwave-synthesized CoSb3 because of the high κ [25]. Introducing 0.1 vol% SiC nanodispersion into CoSb3 matrix in this study increases the maximum ZT significantly, which is mostly due to the improvement of electrical properties at high temperatures. It should be emphasized that the ZT values in this work are still very low in comparison with those of Co4Sb2.85Te0.15 (ZT 0.93) [5], nanostructured CoSb3 (ZT 0.35) [8], Sm0.6Co4Sb12 (ZT 0.80) [13] and CoSb3/2%WO3 (ZT 0.40) [16]. Considering that current study is a tentative exploring of microwave synthesis of CoSb3 and CoSb3/SiC composites, one can expect promising further enhancement of ZT value by optimizing the SiC compositing content, introducing appropriate substitution to improve the electrical properties of CoSb3 and filling the cages in the CoSb3 structure to decrease the κ l based on present results. In fact, the investigation on microwave-synthesized Te-doped CoSb3 and optimizing the nanocomposition is in progress.
4 Conclusion
In summary, pure and SiC-composited CoSb3 were fabricated using mechanical alloying and microwave anealing. The electrical properties including the p-type or n-type conducting were susceptible to the preparation conditions or uncertainties. The particular nanostructure and high porosity in the microwave-synthesized samples leads to low thermal conductivity, which compensates the loss of electrical conductivity and results in comparable ZT value to those prepared with conventional route with high energy consumption and low yield. These results demonstrate the feasibility of high efficient and economical synthesis of CoSb3-based thermoelectric compounds and promising ZT enhancement by use of microwave heating technique, which can also be utilized for scale fabricating other thermoelectrical materials.
References
A.J. Minnich, M.S. Dresselhaus, Z.F. Ren, G. Chen, Bulk nanostructured thermoelectric materials: current research and future prospects. Energy Environ. Sci. 2, 466 (2009)
E.S. Toberer, A.F. May, G.J. Snyder, Zintl chemistry for designing high efficiency thermoelectric materials. Chem. Mater. 22, 624 (2010)
M.G. Kanatzidis, Nanostructured thermoelectrics: the new paradigm?, Chem. Mater. 22, 648 (2010)
X.Y. Li, L.D. Chen, J.F. Fan, W.B. Zhang, T. Kawahara, T. Hirai, Thermoelectric properties of Te-doped CoSb3 by spark plasma sintering. J. Appl. Phys. 98, 083702 (2005)
W.S. Liu, B.P. Zhang, J.F. Li, H.L. Zhang, L.D. Zhao, Enhanced thermoelectric properties in CoSb3–xTex alloys prepared by mechanical alloying and spark plasma sintering. J. Appl. Phys. 102, 103717 (2007)
L. Yang, H.H. Hng, D. Li, Q.Y. Yan, J. Ma, T.J. Zhu, X.B. Zhao, H. Huang, Thermoelectric properties of p-type CoSb3 nanocomposites with dispersed CoSb3 nanoparticles. J. Appl. Phys. 106, 013705 (2009)
Y.G. Zhu, H.L. Shen, L.Y. Zuo, H. Guan, Thermoelectric properties and electronic structure of Te-doped CoSb3 compounds. Solid State Commun. 121, 1388 (2011)
P.F. Wen, P. Li, Q.J. Zhang, Z.W. Ruan, L.S. Liu, P.C. Zhai, Effects of annealing on microstructure and thermoelectric properties of nanostructured CoSb3. J. Electron. Mater. 42, 1443 (2013)
A. Khan, M. Saleemi, M. Johnsson, L. Han, N.V. Nong, M. Muhammed, M.S. Toprak, Fabrication, spark plasma consolidation, and thermoelectric evaluation of nanostructured CoSb3. J. Alloy. Compd. 612, 293 (2014)
A. Gharleghi, C.J. Liu, Rapid fabrication and transport properties of n-type Co4–xNixSb12 via modified polyol process synthesis combined with evacuated-and-encapsulated sintering. J. Alloy. Compd. 592, 277 (2014)
L. Deng, L.B. Wang, X.P. Jia, H.A. Ma, J.M. Qin, Y.C. Wan, Improvement of thermoelectric performance for Te-doped CoSb3 by higher synthesis pressure. J. Alloy. Compd. 602, 117 (2014)
J. Khaliq, Q.H. Jiang, J.Y. Yang, K. Simpson, H. Yan, M.J. Reece, Utilizing the phonon glass electron crystal concept to improve the thermoelectric properties of combined Yb-stuffed and Te-substituted CoSb3. Scripta. Mater. 63, 72–73 (2014)
Q. Zhang, C. Chen, Y.L. Kang, X.D. Li, L. Zhang, D.L. Yu, Y.J. Tian, B. Xu, Structural and thermoelectric characterizations of samarium filled CoSb3 skutterudites. Mater. Lett. 143, 41 (2015)
X.Y. Zhao, X. Shi, L.D. Chen, W.Q. Zhang, S.Q. Bai, Y.Z. Pei, X.Y. Li, T. Goto, Synthesis of YbyCo4Sb12/Yb2O3 composites and their thermoelectric properties. Appl. Phys. Lett. 89, 092121 (2006)
Z.M. He, C. Stiewe, D. Platzek, G. Karpinski, E. Muller, S.H. Li, M. Toprak, M. Muhammed, Effect of ceramic dispersion on thermoelectric properties of nano-ZrO2/CoSb3 composites. J. Appl. Phys. 101, 043707 (2007)
D.G. Zhao, M. Zuo, J.F. Leng, H.R. Geng, Synthesis and thermoelectric properties of CoSb3/WO3 thermoelectric composites. Intermetallics 40, 71 (2013)
R.D. Schmidt, E.D. Case, J.E. Ni, R.M. Trejo, E. Lara-Curzio, R.J. Korkosz, M.G. Kanatzidis, High-temperature elastic moduli of thermoelectric SnTe1 ± x-ySiC nanoparticulate composites. J. Mater. Sci. 48, 8244 (2013)
J.H. Li, Q. Tan, J.F. Li, D.W. Liu, F. Li, Z.Y. Li, M. Zou, K. Wang, BiSbTe-based nanocomposites with high ZT: the effect of SiC nanodispersion on thermoelectric properties. Adv. Funct. Mater. 23, 4317 (2013)
T. Akao, Y. Fujiwara, Y. Tarui, T. Onda, Z.C. Chen, Fabrication of Zn4Sb3 bulk thermoelectric materials reinforced with SiC whiskers. J. Electron. Mater. 43, 2047 (2014)
Y.L. Wang, J.Y. Zhang, Z.W. Shen, M.Q. Yang, X.Q. Liu, W. Wang, Preparation of Bi2Te3/Nano-SiCcomposite thermoelectric films by electrodeposition. J. Electron. Mater. 44, 2166 (2015)
J.Y. Yang, Y.H. Chen, J.Y. Peng, X.L. Song, W. Zhu, J.F. Su, R.G. Chen, Synthesis of CoSb3 skutterudite by mechanical alloying. J. Alloys Compd. 375, 229 (2004)
M. Oghbaei, O. Mirzaee, Microwave versus conventional sintering: a review of fundamentals, advantages and applications. J. Alloys Compd. 494, 175 (2010)
O. Kim-Hak, M. Soulier, P.D. Szkutnik, S. Saunier, J. Simon, D. Goeuriot, Microwave sintering and thermoelectric properties of p-type (Bi0.2Sb0.8)2Te3 powder. Powder Technol. 226, 231 (2012)
K. Biswas, S. Muir, M.A. Subramanian, Rapid microwave synthesis of indium filled skutterudites: an energy efficient route to high performance thermoelectric materials. Mater. Res. Bull. 46, 2288 (2011)
A.A. Ioannidou, M. Rull, M. Martin-Gonzallez, A. Moure, A. Jacquot, D. Niarchos, Microwave synthesis and characterization of the series Co1–xFexSb3 high temperature thermoelectric materials. J. Electron. Mater. 43, 2637 (2014)
G. Delaizir, G. Bernard-Granger, J. Monnier, R. Grodzki, O. Kim-Hak, P.D. Szkutnik, M. Soulier, S. Saunier, D. Goeuriot, O. Rouleau, J. Simon, C. Godart, C. Navone, A comparative study of spark plasma sintering (sps), hot isostatic pressing (hip) and microwaves sintering techniques on p-type Bi2Te3 thermoelectric properties. Mater. Res. Bull. 47, 1954 (2012)
K.G. Liu, J.X. Zhang, D. Xiang, The exploration for synthesizing CoSb3 powder by mechanical alloying. J. Mater. Processing Technol. 184, 257 (2007)
J.Y. Peng, J.Y. Yang, X.L. Song, Y.H. Chen, T.J. Zhang, Effect of Fe substitution on the thermoelectric transport properties of CoSb3-based skutterudite compound. J. Alloys Compd. 426, 7 (2006)
S.C. Ur, J.C. Kwon, I.H. Kim, Thermoelectric properties of Fe-doped CoSb3 prepared by mechanical alloying and vacuum hot pressing. J. Alloys Compd. 442, 358 (2007)
Y. Kawaharada, K. Kurosaki, M. Uno, S. Yamanaka, Thermoelectric properties of CoSb3. J. Alloys Compd. 315, 193 (2001)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (11404350, 11404348, 11304327, and 11234012), Zhejiang Provincial Science Foundation for Distinguished Young Scholars (LR16E020001) and Ningbo Science and Technology Innovation Team (2014B82004).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhu, H., Liang, S., Ouyang, T. et al. Thermoelectric properties of CoSb3 and CoSb3/SiC composites prepared by mechanical alloying and microwave sintering. J Mater Sci: Mater Electron 28, 10509–10515 (2017). https://doi.org/10.1007/s10854-017-6824-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-6824-7