Abstract
Thin films of Lead sulphide (PbS) were grown on soda lime glass substrate by Successive Ionic Layer Adsorption and Reaction method from acidic, neutral and alkaline cationic precursor reaction bath by keeping the pH of the anionic precursor invariant. The structural and morphological aspects of the as prepared samples were investigated using XRD and SEM results. The as-prepared samples were polycrystalline with nanometer sized grains and identified as galena type cubic structure. The values of average crystallite size were found to be in the range 22–30 nm. The SEM micrographs show variations in morphology. Optical studies revealed the existence of both direct and indirect band gap with values in the range of 1.65–1.98 and 0.61–0.90 eV respectively. The room temperature conductivity of the PbS thin films were in the range 1.19 × 10−8–5.92 × 10−8 Ω cm−1. The optical band gap energy has inverse relation with grain size and electrical conductivity is closely related to structural parameters like grain size, crystallinity and micro strain. The estimated lattice parameter, grain size, optical band gaps, solid state and electrical properties were correlated with pH of the cationic solution. In this work, we establish that the pH of the cationic precursor media has colossal effect on the structural, morphological, optoelectronic, solid-state and electrical properties of PbS thin films.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of binary metal chalcogenides of IV–VI semiconductors in nanocrystalline form has experienced enormous development in recent years owing to their interesting size dependent optical and electrical properties. In particular, during the last decades, the research on the growth of nano particle PbS films has increased, for their technological applications in photo resistance, diode lasers, decorative coatings, and optoelectronic devices [1–4].
Polycrystalline PbS thin films are presently attracting common interest mainly due to their potential application in infrared detectors [5–9]. A lot of reports have exposed that PbS films deposited on glass substrates possess excellent solar control characteristics comparable to other metallic films. The possibility of using chemically deposited thin films of PbS as solar control coatings are also discussed [10–12]. Nanocrystalline PbS films have been prepared by various techniques including chemical bath deposition (CBD) technique. It is a well known method for preparing semiconductor layers and has been used mainly for metal sulphides and selenides. In the conventional CBD the substrate is immersed in a reaction bath containing precursors and complexing agents together, which may result in the precipitation of waste compounds. In the present work we have utilized a simple modified CBD method at room temperature named Successive Ionic Layer Adsorption and Reaction (SILAR). The technique has been first employed to develop zinc sulphide (ZnS), zinc cadmium sulphide (ZnCdS) and cadmium sulphide (CdS) by Nicolau et al. [13, 14]. This method offers the negligible waste generations since the cationic and anionic precursors are kept in different reaction baths. Thin film of solid materials can be synthesized by means of self limiting chemical reactions from bath containing ionic species of the reactants. Thus SILAR can be considered as the liquid phase analogue of Atomic layer epitaxy. The method is based on the sequential reactions at the substrate surface. Each reaction is followed by rinsing which enables heterogeneous reaction between the solid phase and the solvated ions in the solution. Accordingly, a thin film can be grown layer-by-layer. The fundamental feature of SILAR is that it is convenient, simple, inexpensive and eco friendly method [15, 16]. This technique has the ability to control the layer thickness and to deposit crystalline materials at room temperature in a variety of shapes and over large area. Preparative parameters such as concentration of Pb2+ and S2− ions, and pH can be optimized to obtain good quality PbS thin films on soda lime glass substrate.
The aim of this paper is to investigate the influence of the acidic, neutral and alkaline cationic precursor bath on the structural, morphological, optoelectronic, solid-state and electrical properties of the PbS thin films during SILAR growth. The characterization of the films was done using X-ray diffractometer (XRD), scanning electron microscope (SEM), UV–Vis-NIR spectrophotometer and Keithley Source Measurement Unit. The dependence of crystallinity, lattice parameters, grain size, optical band gap, refractive index, extinction coefficient and electrical conductivity on the cationic precursor pH has been highlighted in this work.
2 Experimental details
PbS thin films were grown on soda lime glass substrates by the SILAR method at room temperature. More specifics of SILAR method were accessible in literature [17]. The adsorption, reaction and rinsing times were optimized to get homogeneous thin film structures.
All the reagents used were of analytical grade (Merck). Lead nitrate (0.2 M) complexed with triethanolamine (TEA) in acidic, neutral and alkaline medium with pH 5.4, 7 and 8 respectively was used as cationic precursors and thioacetamide (0.4 M) with pH 4.7 as anionic precursor. In this work pH of the cationic reaction bath was controlled by varying the concentration of TEA alone. The formation of PbS film depends on the rate of release of Pb2+ and S2− ions from the bound state. As an efficient complexant, TEA forms [Pb(TEA)]2+ complex and thereby lowering the concentration of free Pb2+ ions below the level required for the precipitation of solid phase Pb(OH)2. The immersion and rinsing times in precursors and distilled water were optimized as 10 and 20 s respectively. The cycles of operation were continued to 100 for the preparation of all the samples. Highly adherent, grayish black PbS thin films were obtained for analysis. The synthesis of these films were repeated a number of times to confirm the reproducibility. As-grown samples in acidic, neutral and alkaline reaction baths were named as APbS, NPbS and BPbS respectively.
In the present study, gravimetric weight difference method was used to determine the film thickness. The samples were further characterized for structural, morphological, optical and electrical properties. Structural studies were carried out using XRD (Bruker AXS-8 advance) with CuKα radiation of wavelength 1.5406 Å as the source. The morphological studies have been carried out by SEM (JEOL Model JSM6490). The optical characterization was done by taking absorption, transmission and reflection spectra of the samples using a Hitachi-U-3410 UV–Vis-NIR spectrophotometer. Electrical conductivity was measured by two probe method using Keithley Source Measurement Unit by fabricating silver electrodes (Model SMU Keithley 2400).
3 Results and discussion
3.1 Structural properties
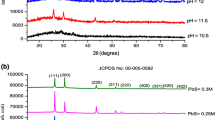
The X-ray diffraction pattern of PbS thin films synthesized from acidic, neutral and basic cationic reaction baths are shown in Fig. 1. The plots indicate that the as-prepared samples are polycrystalline and XRD peaks match with face centered cubic structure of PbS (JCPDS NO. 5-592). In acidic medium the preferred film orientation is found to be (200) while in neutral medium it has been changed to (111) direction. In alkaline medium the main orientation of the PbS thin film remains as (111) direction with improved intensity. Thus increase in pH of the cationic precursor promotes the three dimensional growth of the film with an enrichment in crystallinity.
The crystallite size determined using Debye–Scherrer formula is found to be in the range 22–30 nm. Thus the change in nature of the cationic reaction bath from acidic to alkaline favours growth of crystallites and ultimately leads to improvement in the degree of crystallinity of the films. Deviation of the calculated lattice parameter ‘a’ from the strain free bulk sample (a 0 = 5.936 nm) indicates that the as prepared films are under strain. The micro strain (ε) of the samples is calculated using equation [18],
The microstrain developed in the film decreases with increase in pH of cationic precursor. This may be explained as, the increase in grain size with increase in pH may increase the surface area of each grain and there by a reduction in force per unit area between grains and consequently paved the way for strain relaxation. Improved crystallinity as noticed in alkaline medium confirms that crystallites improve in relaxed medium.
3.2 Morphological properties
The surface morphology of the PbS thin films is depicted in Fig. 2. The SEM image of as-grown film from acidic bath (Fig. 2a) displays uniform coarse structure with small grains and well-defined grain boundaries, which are typical for PbS thin films deposited by SILAR technique [19]. Figure 2b shows the morphology of the as- grown sample at pH 7, which also exhibits granular structure, but with enhanced grain size. There is notable difference in the morphology of film prepared from alkaline bath. Alkalinity causes non-uniform arrangement of grains of enlarged size, results in cluster formation due to aggregation of smaller nano crystallites as shown in Fig. 2c. The grain boundaries become less defined in these images. As in XRD here also the largest grain size is achieved for the sample prepared in alkaline medium. Bigger grain size in surface morphology as compared to XRD implies that the film composed of agglomerates of smaller crystallites.
3.3 Optical properties
The optical absorption spectra of PbS thin film samples are shown in Fig. 3. The spectra indicate a gradually increasing absorbance from NIR to visible region. The increased absorption near band edge is due to the transition of electrons from valence band to conduction band. Maximum absorption in the visible-near infrared region of the spectrum, indicates the nanocrystalline nature of as-grown PbS thin films. Bulk PbS has an absorption onset at 3,020 nm, corresponds to a small band gap of 0.41 eV at 298 K [20]. The strong blue shift in the absorption threshold from the bulk is due to strong quantum confinement. With increase in pH, absorption edge is found to be shifted towards higher wavelength region. The PbS thin films grown from alkaline bath, having higher degree of crystallinity and crystallite size, higher thickness, less micro strain exhibit maximum optical absorption.
The optical band gap is calculated from the analysis of the spectral absorption in the vicinity of the fundamental absorption edge. The direct and indirect band gap of the samples is estimated using Tauc relation [21].
where ‘A’ is the parameter which depends on the transition probability and the exponent ‘n’ characterizes the nature of band transition between the valence band and the conduction band and n equals 2 for a direct band-gap semiconductor and n equals ½ for indirect band gap semiconductor.
The absorption coefficient ‘α’ can be deduced from the absorption spectra using the relation [22].
where ‘t’ is the thickness of the as deposited PbS thin film. In this work we have obtained the film thickness in the range 492–800 nm by gravimetric method [23]. Sample prepared from alkaline bath exhibits maximum film thickness. For direct transition in the fundamental absorption, (ahν)2 has linear dependence on the photon energy hν. The intercept on energy axis gives the direct band gap energy. The linear dependence corresponding to indirect transition is obtained from the intercept of (ahν)1/2 against hν plot on energy axis. We have obtained both direct and indirect band gaps (Fig. 4) which comes out to be 1.65–1.98 and 0.61–0.90 eV respectively. Both direct and indirect band gaps decrease with increase in metal source pH. These results are in concurrence with enhancement in crystallinity and grain size as evidenced from XRD and SEM results also. Thus we are able to tune optical band gap by changing the pH of the cationic precursor alone.
Optical transmission and reflection spectra of PbS thin films are shown in Fig. 5a, b respectively. Transmission spectra of all the films exhibit a sharp optical transmission cut-off near UV–VIS region corresponding to the band gap of PbS, which is an indication of good crystallinity of the film. In UV region the samples are non transparent, indicating maximum absorption in this region. The general behavior of the spectra is the increase in transmittance throughout the NIR region. PbS thin films prepared from alkaline bath show low transmissions (25 %) in IR region, whereas films prepared from acidic bath exhibits optical transmittance of about 35 %. Furthermore, it is clearly seen from the spectra that the absorption edge shifts towards higher wavelength on increasing the pH as expected from the structural and morphological properties.
In UV region, reflectance increases with increase in wavelength and becomes almost steady in visible region. For APbS, reflectance shows a slight decrease in IR region and for other two samples it increases with wavelength. Maximum reflectance in NIR region is around 60 % (for NPbS) and minimum for BPbS (40 %). The PbS thin film having low transmittance in UV region coupled with an appreciable reflectance in NIR region (NPbS) is a suitable material for solar control coatings [24].
3.4 Solid state properties
Solid state properties of the material can be illustrated by refractive index (n) and extinction coefficient (k). Refractive index for all the samples increases with increase in wavelength within the short wavelength range and becomes steady in the wavelength range 400–550 nm. The values are in the range of 3.8–5.4 at 500 nm for different medium as shown in Fig. 6a. As the grains grow bigger, the grain boundaries would become less defined due to the size distribution of the grains and thereby decreasing film density. These results are compatible with the SEM images of BPbS. This decrease in film density leads to decrease in refractive index as observed for BPbS with maximum grain size. Thus refractive index of the film has inverse relationship with grain size.
Density (ρ) of PbS thin film is calculated from the measured values of refractive index using Lorentz-Lorenz equation [25]. The density of all the PbS thin films attain steady values in visible region of spectrum and decreases with increase in pH as shown in Fig. 6b. The extinction coefficient represents the spectral dependence of absorption coefficient. In bulk material it depends only on absorption of light by atoms. But in thin films it is related to surface roughness, poly crystallinity, crystallite size etc. These factors change the attenuation of light in the film and apparently modify the values from the bulk. The variation of k with wavelength is shown Fig. 6c. It is found to be maximum in the lower wavelength region and shows the same trend as absorption spectra. In NIR region k is found to be almost a constant.
3.5 Electrical characterization
The electrical conductivities (K) of the as-deposited PbS thin films are determined by two-probe method using silver electrodes. Figure 7 shows the typical I–V characteristic of the PbS thin films prepared at different cationic pH. The measured conductivity of the PbS thin films are found to be in the range 1.19 × 10−8–5.92 × 10−8 Ω cm−1. The electrical conductivity and micro strain are found to be indirectly related in all samples. Thus BPbS, which is less strained, shows maximum electrical conductivity. These results are in accordance with the structural, morphological and solid state results. The increase in grain size resulted in the decrease in film density and hence enhancement in electrical conductivity.
The increase in conductivity with increase in pH can also be explained in terms of structural changes occurring in this thin film. The important factor, which is responsible for the physical properties of thin film is its structure. The film is composed of randomly oriented grains with grain boundaries. Increase in pH of the cationic precursor affects the structure significantly causing a considerable increase in the mean size of the grain and a decrease in the grain boundary. This decrease is due to the resettlement of the similarly oriented smaller crystallites into bigger crystallites. Because of these structural changes the inter grain boundary area decreases i.e. there is a decrease in the scattering of electrons. Consequently, the carrier concentration also increases with the increase in pH. This in turn increases the conductivity of given sample. Thus the significant feature of this work is not only the possibility of tuning the optical band gap with metal ion pH but the conductivity and carrier concentration also get customized accordingly.
The numerical values of structural, optoelectronic, solid state and electrical parameters with cationic precursor pH are shown in the Table 1. The correlation between the above mentioned properties of PbS thin films versus the pH of metal source media are depicted in Fig. 8. Enhancement in crystallinity favours grain growth and there by decrease the density of the as-deposited PbS thin films. Since denser medium is having higher refractive index, it decreases with increase in pH of the cationic precursor media. The optical band gap energy has inverse relation with grain size and it decreases as we shift from acidic to alkaline media Thus less strained sample, BPbS, with maximum conductivity, grain size, minimum optical band gap and lowest refractive index is found to be most useful for optoelectronic applications.
4 Conclusion
We have successfully utilized the SILAR method for the synthesis of PbS thin films using different cationic pH media. The optimization of various preparative parameters resulted in uniform and highly adherent films. The dependence of the cationic media pH on various physical properties of the samples has been investigated and compared. The XRD studies showed cubic structure with the particle size in nanometer regime. SEM morphology was in compatible with the structural analysis. The optical studies exposed the existence of both direct and indirect band gap and the alteration of the crystallite size enabled the tuning of the absorption edge for direct and indirect transitions with values in the range of 1.65–1.98 and 0.61–0.90 eV respectively. Further, enlarged grains in higher pH range reduced the film density and in turn refractive index of the as-deposited samples. It is also seen that the electrical conductivity and micro strain are inversely related to each other. The electrical conductivity has been increased to five times on shifting cationic pH from acidic to alkaline range. The alkaline medium which offered largest grain size, highest crystallinity, lowest microstrain, smallest band gap and highest electrical conductivity proved to be the most favorable medium for lead sulphide thin film growth. These tunable band gap materials can be applied to various optoelectronic devices. Thus in this work we established the flexibility of SILAR deposition technique to synthesize high quality PbS thin films by a cost effective approach.
References
J.J. Valenzuela, R. Ramirez, A. Mendoza, M. Sotelo, Thin Solid Films 441, 104 (2003)
A.M. Salim, M.O. Abou-Helal, Mater. Chem. Phys. 80, 740 (2003)
S. Kumara, T.P. Sharmaa, M. Zulfequarb, M. Husain, Phys. B 325, 8 (2003)
A. Osherov, J.P. Makai, J. Balazs, Z.J. Horvath, N. Gutman, A. Sa’ar, Y. Golan, J. Phys. Condens. Matter. 22, 262002 (2010)
H. Moreno-García, M.T.S. Nair, P.K. Nair, Thin Solid Films 519, 2287 (2011)
S. Seghier, N. Kamoun, R. Brini, A.B. Amara, Mater. Chem. Phys. 97, 71 (2006)
E.M. Larramendi, O. Calzadilla, A. Gonzalez-Arias, E. Hernandez, J. Ruiz-Garcia, Thin Solid Films 389, 301 (2001)
A.P. Gaiduk, P.I. Gaiduk, A.N. Larsen, Thin Solid Films 516, 3791 (2008)
J.A. Amusan, Res. J. Appl. Sci. 3, 1 (2008)
K.D. Dobsona, G. Hodesa, Y. Mastaib, Sol. Energy Mater. Sol. Cells 80, 283 (2003)
P.K. Nair, V.M. Garcia, A.B. Hrenandez, M.T.S. Nair, J. Phys. D Appl. Phys. 24, 1466 (1991)
P.K. Nair, M.T.S. Nair, J. Phys. D Appl. Phys. 23, 150 (1990)
Y.F. Nicolau, J.C. Menard, J. Cryst. Growth 92, 128 (1988)
Y.F. Nicolau, M. Dupuy, M. Brunel, J. Electrochem. Soc. 137, 2915 (1990)
J. Puis’o, S. Tamulevicius, G. Laukaitis, S. Lindroos, M. Leskela, V. Snitka, Thin Solid Films 403, 457 (2002)
T. Kanniainen, S. Lindroos, R. Resch, M. Leskela, G. Friedbacher, M. Grassrebauer, Mater. Res. Bull. 35, 1045 (2000)
T. Kanniainen, S. Lindroos, J. Ihanus, M. Leskela, J. Mater. Chem. 6, 161 (1996)
N. Choudhury, B.K. Sarma, Indian J. Pure Appl. Phys. 46, 261 (2008)
J. Puis’o, S. Lindroos, S. Tamulevicius, M. Leskela, V. Snitka, Thin Solid Films 428, 223 (2003)
B. Zhang, G. Li, J. Zhang, Y. Zhang, L. Zhang, Nanotechnology 14, 443 (2003)
J. Tauc, Amorphous and Liquid Semiconductor (Plenum Press, New York, 1974), p. 159
F.N. Dultsev, L.L. Vasilieva, S.M. Maroshina, L.D. Pokrovsky, Thin Solid Films 510, 255 (2006)
R. Das, Rajeshkumar, Mater. Res. Bull. 47, 239 (2012)
P.K. Nair, M. Ocampo, A. Fernadez, M.T.S. Nair, Sol. Energy Mater. 20, 235 (1990)
J.H. Leea, J.H. Euna, S.Y. Parka, S.G. Kimb, H.J. Kima, Thin Solid Films 435, 95 (2003)
Acknowledgments
The author K. C. P thanks UGC for financial assistance and also wishes to acknowledge SAIF-STIC, CUSAT, Kochi for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Remadevi, T.L., Preetha, K.C. Comparative studies on structural, optoelectronic and electrical properties of SILAR grown PbS thin films from acidic, neutral and alkaline cationic reaction bath. J Mater Sci: Mater Electron 23, 2017–2023 (2012). https://doi.org/10.1007/s10854-012-0696-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-012-0696-7