Abstract
M-type Hexaferrites B0.5Sr0.5Fe12−2x Ni x Zr x O19 were synthesized and investigated. The XRD patterns show single phase of the magnetoplumbite barium strontium ferrite and no other phases were present. The samples exhibit well defined crystallization; all of them are hexagonal platelet grains. As the substitution level increased from x = 0.2 to 0.8 mol%, the grains are agglomerated and the average diameter increased. This suggests that Ni–Zr substitution increases the grain size, as observed in the FE-SEM micrographs. The results of magnetic measurement revealed that Ms of barium strontium hexaferrite increased when the value of x increased from 0 to 0.4 mol% and then decreased with the increasing Ni–Zr content. The Hc decreases remarkably with increasing Ni and Zr ions content. Hard magnetic material is converted into soft magnetic material when the substitution level is increased from 0.2 to 0.8 mol%. In particular, Ba0.5Sr0.5Fe12−2x Ni x Zr x O19 with x = 0.2, 0.4, 0.6, 0.8 mol% has suitable magnetic characteristics with particle size small enough for high-density magnetic recording applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
M-type hexaferrites, due to its perfect chemical stability, good thermal durability, corrosion resistivity, unique electrical and magnetic properties, has extensive applications in permanent magnet and high density magnetic recording devices, telecommunication, magneto optical and microwave devices [1–4]. The properties of the ferrites are strongly influenced by their composition and synthesis method [5]. A variety of different cation substitutions are possible in BaFe12O19. Divalent transition metals such as Ni2+ and Zr4+ for Fe3+ are frequently used due to their similarity in ionic radii and electronic configurations. However, the magnetic properties of substituted hexaferrites are strongly dependent on the synthesis conditions as disproportionate charge distributions generally occur for multivalent cationic doping. The preparation techniques as well as the structural doping of Ni–Zr ions influence the magnetic properties of this compound. However, they provided little experimental information as to the material preparation or synthesis method. The conventional way of synthesizing hexaferrites involves solid state reaction route having high calcinations temperature (≥1,200 °C), which results in powders with large particle size, limited chemical homogeneity and low sinter ability [6].
Nowadays, the demand for the development of new magnetic materials has increased extraordinarily, owing to their applications in new emerging technologies. The magnetic properties of substituted hexaferrites, depend directly on the electronic configuration of the dopant cations and on their preference to occupy the different Fe sub lattices of the magnetoplumbite structure. In addition, it has been shown that when doped with Ni2+ and Co3+ ions, both the saturation magnetization (Ms) and the intrinsic coercivity (Hc) can be easily controlled [7]. The magnetic properties can also be controlled by Ni–Zr substitution. It is also possible that a combination of Ni2+ with Zr4+ can improve the magnetic properties like saturation magnetization and coercive field without affecting phase formation. In order to improve the properties of barium strontium hexaferrite (BSF), substitution of metals such as Co–Ru, Co–Zr on the Fe3+ site of hexaferrite have been investigated by several researchers [8, 9]. The work presented in this paper is hoped to show the magnetic properties of BSF when doped with increasing quantities of Ni–Zr.
2 Experimental details
The starting reagents Ba(NO3)2, Sr(NO3)2, Fe(NO3)3·9H2O, Ni(NO3)2·6H2O, ZrOCl2·8H2O, and d-Fructose were of analytical grade. Metal sources and d-Fructose in the molar ratio of 1:1 were dissolved in distilled water to form aqueous solutions using magnetic stirrer. The resultant solution was stirred for 2 h at room temperature, to get a gel. The gel was heated in hot air oven for 2 days to get precursor. The calcined powder was uniaxially pressed into pellets using a hydraulic press and polyvinyl alcohol (PVA) as a binder. The pressed pellets were sintered at 1,150 °C for 3 h. The Powder X-ray diffraction patterns were collected on a (PANalytical X’pert pro) CuKα radiation at 45 kV and 40 mA (λ = 0.15406 nm) in a wide range of 2θ (20° <2θ <80°). The microstructure observation of the specimens was performed in FEI Quanta FEG 200-High Resolution Scanning Electron Microscope (HR-SEM). Hysteresis loops were measured at room temperature using by Vibrating Sample Magnetometer (Lakeshore 7304).
3 Results and discussion
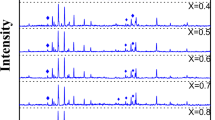
X-ray powder diffraction patterns of Ba0.5Sr0.5Fe12−2x Ni x Zr x O19 (x = 0.2, 0.4, 0.6 and 0.8 mol%) sintered at 1,150 °C are displayed in Fig. 1. Here, all samples show a single hexagonal magnetoplumbite phase (JCPDS No; 51-1879). The diffraction peak (114) is shifted slightly to a larger angle with the increase of doping content, which suggests that the doping ions have been placed on the crystal sites of Barium strontium hexaferrite [10]. The variation in relative intensities may be related to occupation of lattice sites by substituted ions.
From structural parameters (Table 1) characterized by lattice constants ‘a’ and ‘c’, it becomes evident that lattice constant ‘a’ reflects less variation, while lattice constant ‘c’ initially varies rapidly and then slows with the increased substitution. This is in agreement with the fact that all hexagonal types exhibit constant lattice parameter ‘a’ and variable parameter ‘c’ [11]. It indicates that change of easy magnetized c-axis is larger than a-axis with Ni2+ and Zr4+ ions substitution. This is attributed to large ionic radii of Ni2+ ion (0.69 Å) and Zr4+ ion (0.72 Å) than Fe3+ ion (0.645 Å) [12, 13]. The change in lattice constants also varies with the distance between magnetic ions resulting in change of exchange interaction and thus magnetic properties are altered with substitution.
The FE-SEM images of sintered Ni–Zr substituted samples x = 0.2, and 0.8 mol% are presented in Fig. 2. The grains of different size are attributed to large ionic radii of substituted Ni2+ and Zr4+ ions in comparison to Fe3+ ions. Thus grain size can be controlled with substitution. The samples exhibit relatively well defined, nearly hexagonal grains. The grain size in the micrograph seems to increase as the substitution level increased from x = 0.2 to 0.8 mol%.
Figure 3 shows the effect of Ni–Zr concentration on the saturation magnetization and coercivity for the samples sintered at 1,150 °C. It is clear that the saturation magnetization increases up to x = 0.4 mol% and then decreases on further increase of the dopant content, while the coercivity decreases continuously with the increase in Ni–Zr content. Variations can be explained on the basis of the Ni2+ and Zr4+ ions occupying different sites in the Fe3+ sub lattices based on the previous research reports [14, 15]. From this report, Zr4+ ions replace Fe3+ ions at the 2b site for small substitutions (x ~0.1 mol%) and at 4f1 for higher substitutions, while Ni2+ ions replace Fe3+ ions at the 4f2 site for x ~0.1 mol%, and at the 12k site for larger values of substitutions. When a nonmagnetic Zr4+ ion replaces a Fe3+ ion at the 4f1 site with spin down, then the total number of unpaired electrons with upward spin is increased, causing the saturation magnetization of the samples to increase. The Ni2+ ions replace Fe3+ at the 12k site with spin up and a magnetic moment of 2 μB, which is also less than that of Fe3+ (5 μB), but the total magnetic moment increases by 2 μB due to replacement of ferric ions by a nonmagnetic Zr4+ ion at the 4f1 site. The decrease in saturation magnetization above x = 0.4 mol% is due to the larger amount of nonmagnetic ions which are responsible for the weakening of the exchange interactions.
Saturation magnetization increases where as the coercivity decreases with substitutions in M-type hexaferrites. Recording media requires high coercivity above 600 Gauss and a saturation magnetization as high as possible [16]. For the materials we have synthesized, the coercivity decreases continuously from 2,121.66 to 177.337 Gauss. But the sample with x = 0.4 mol% has coercivity 807.11 Gauss and saturation magnetization increases from 54.08 to 64.43 Am2/kg with Zr–Ni, substitution. These results are in good agreement with those already reported for the Zn–Nb substituted strontium hexaferrite [15].
The changes in the coercivity are mainly related to the reduction of the magneto crystalline anisotropy of the magnetic phase, which decreases the coercivity [17]. In addition, the change in morphology and particle size may also affect the magnetic properties [18]. However, from the economical point of view, Ni and Zr bearing oxides are cheaper than rare earth oxides or salts.
4 Conclusion
The Ni–Zr substituted Ba-Sr hexaferrite is produced by sol–gel method. The crystal structure of barium strontium hexaferrite with substitutions still remains in hexagonal magnetoplumbite phase. The substitution of Ni2+ and Zr4+ ions causes grain coarsening and more expansion of crystal lattice towards c-axis than a-axis. The coercivity decreases due to micro structural and grain size variation while saturation magnetization is enhanced due to replacement of Fe3+ ions in spin down state. The saturation magnetization was found to increase at low doping content of x = 0.4 mol% while the coercivity decreased for all the doped samples. The transition from hard phase to soft phase results in reduction of hysteresis loss area per cycle. Changing the substitution rate ‘x’ mol%; the coercivity could be easily controlled without a significant reduction of Ms. Hc can be easily tuned at lower substitution level, while enhancing saturation magnetization at the same time. There is a large reduction in hysteresis and hard ferrite is converted to soft ferrite, making it useful for recording media.
References
A.A. Nourbakhsh, M. Noorbakhsh, M. Nourbakhsh, M. Shaygan, K.J.D. Mackenzie, J. Mater. Sci. Mater. Electron. 22, 1297 (2011)
J. Huang, H. Zhuang, W. Li, J. Mater. Sci. Lett. 22, 399 (2003)
I. Kucuk, H. Sozeri, H. Ozkan, J. Supercond. Nov. Magn. 24, 1333 (2011)
J. Qiu, M. Gu, H. Shen, J. Magn. Magn. Mater. 295, 263 (2005)
U. Ghazanfar, S.A. Siddiqi, G. Abbas, Mater. Sci. Eng. B 118, 132 (2005)
B.D. Cullity, C.D. Graham, Introduction to magnetic materials, 2nd edn. (Wiley, New Jersey, 2008)
Z. Yang, C.S. Wang, X.H. Li, X.H. Zeng, Mater. Sci. Eng. B 90, 142 (2002)
C. Singh, S. Bindra Narang, I.S. Hudiara, Y. Bai, J. Alloys. Compd. 464, 429 (2008)
C. Singh, S. Bindra Narang, I.S. Hudiara, Y. Bai, F. Tabatabaei, Mater. Res. Bull. 43, 176 (2008)
Q.Q. Fang, H.W. Bao, D.M. Fang, J.Z. Wang, X.G. Li, J. Magn. Magn. Mater. 278, 122 (2004)
M. Mozaffari, A. Arab, M.H. Yousefi, J. Amighian, J. Magn. Magn. Mater. 322, 2670 (2010)
T.M. Meaz, C.B. Koch, Hyp. Interact. 156/157, 341 (2004)
A. Gonzalez-Angeles, G. Mendoza-Suarez, A. Gruskova, J. Slama, J. Lipka, M. Papanova, Mater. Lett. 59, 1815 (2005)
M.V. Rane, D. Bahadur, A.K. Nigam, C.M. Srivastava, J. Magn. Magn. Mater. 2, 288 (1999)
M.J. Iqbal, M.N. Ashiq, P. Hernandez-Gomez, J.M. Munoz, Scripta Mater. 57, 1093 (2007)
Y. Li, R. Liu, Z. Zhang, C. Xiong, Mater. Chem. Phys. 64, 256 (2000)
M.J. Iqbal, M.N. Ashiq, P. Hernandez-Gomez, J.M. Munoz, Scripta Mater. 57, 1093 (2007)
M.M. Hessien, M.M. Rashad, K. El-Barawy, J. Magn. Magn. Mater. 320, 336 (2008)
Acknowledgments
The authors thank SRM University for providing the facilities available in Nanotechnology center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanagesan, S., Jesurani, S., Velmurugan, R. et al. Preparation and magnetic properties of Ni–Zr doped barium strontium hexaferrite. J Mater Sci: Mater Electron 23, 952–955 (2012). https://doi.org/10.1007/s10854-011-0526-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-011-0526-3