Abstract
Y (yttrium)-substituted M-type hexaferrite of composition BaYxFe12−xO19 (x ranges from 0.1 to 0.8 with step of 0.1) was synthesized through ceramic process. Pure phase formation of M-type hexaferrite was confirmed by XRD patterns for prepared sample when x < 0.4. The second phase (Y3Fe5O12) was detected when the substitution content exceeded 0.4. As the substitution dosage of yttrium increased, unconspicuous transform were observed in the microstructure of all samples BaFe12−xYxO19, such as compactness, size of grain. The bulk densities (ρ) of the samples decreased first and then increased with the increase of X. It means that the compactness of the sample receded first and then enhanced with rise of Y3+ substitution contents. Moreover, with increase of Y3+ amount, Y3+ substituted the Fe3+ in the octahedral position of m-type barium ferrite, the saturation magnetization (Ms) of the sample decreases rapidly and obtained a minimum of 55.63 emu/g, and then increased gradually attributed to the appearance of the second phase (Y3Fe5O12). Appropriate values of Ms (56.57 emu/g) and Hc (1794.17 Oe) were obtained simultaneously when x = 0.6. These two characters made the material suitable for the applications infield of magnetic recording.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
M-type barium ferrite has been receiving continuous attention, and the researchers did a lot of research on it, since its discovery in the 1950s [1]. M-type barium ferrite (BaFe12O19) is an excellent permanent magnetic material with high coercive force and residual magnetization, good temperature stability and corrosion resistance [2]. M-type barium ferrite has been proposedfor applications in permanent magnets, self-biased microwave and millimeter wave devices (isolators, phase shifters and circulators) [3, 4]. However, with the development of electronic information technology, the traditional materials like M-type barium ferrite was also faced with the application in new field, such as magnetic recording. The magnetic head material in the field of magnetic recording require materials with high saturation magnetization and low coercive force, but pure m-type barium ferrite is difficult to meet the requirements. The M-type barium ferrite has high saturation magnetization, so the reduction of coercive force on the premise of ensuring the saturation magnetization is a direct method to make it more applied in the field of magnetic recording. Quantities investigations have concentrated on reducing the coercive force of M-type barium ferrite [5, 6]. Ion substitution is an effective technique to adjust the properties of M-type barium ferrite [7, 8]. Ion substitution is mainly though substituting ions to adjusting the crystal structure or magnetic structure of M-type barium ferrite, simultaneously the magnetic properties, electrical properties and microwave properties changed. Similarly, the density, grain size and porosity of doped M-type barium ferrite samples also have an important effect on the properties. So far, in the method of ion substituted the M type barium ferrite, the main method is to replace Fe3+ or Ba2+ with other ions, it mainly includes two methods of co-substitution [5, 9, 10] and single-ion substitution. The co-substitution method means the Fe3+ will substituted by a divalent ion and a tetravalent ion. It has been proved in the past that the properties of M-type barium ferrite can be obviously changed by replacing the Fe3+ with trivalent nonmagnetic ions (Al3+, Sc3+, Ga3+, etc.) [11,12,13,14,15,16,17].
In this paper, the substitution of Fe3+ with Y3+ in M-type barium ferrite has been studied. Yttrium is a rare earth element in the periodic table 39, and Y3+ is a nonmagnetic ion. The ionic radius of Y3+ (0.9 Å) is larger than that of Fe3+ (0.64 Å). Therefore, the single ion (Y3+) substitution of M-type barium ferrite not only changed the magnetic structure of M-type barium ferrite, but also caused obvious distortion of lattice structure. Of course, because the radius of Y3+ is larger than that of Fe3+, it is not easy for Y3+ to occupy the tetrahedral and hexahedral positions of M-type barium ferrite are occupied. Excessive yttrium ions which cannot enter the lattice of M-type barium ferrite, form the second phase (Y3Fe5O12) with iron ions and oxygen atoms. The saturation magnetization, coercive force and bulk densities of the samples had obvious regular changed. This paper was explored the influence of magnetic ion Yttrium Ion (Y3+) substitution on the magnetic properties of M type barium ferrite, including the influence on magnetic structure, and coercive force.
2 Experiment
M-type barium ferrite substituted with Y for Fe, BaFe12−xYxO19 were synthesized using analytical grade BaCO3, Fe2O3, Y2O3 via solid-state reactions. The X value was varied as X = 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8. To weigh the powdered raw material according to the chemical formula. The powders were mixed using a ball mill for 12 h in a steel vial with zirconia balls and deionized water as the milling media. The mixed powder was dried and then calcined at 1300 °C for 12 h in air. The calcined powder was milled again for 12 h in deionized water. After drying, the powder was granulated by adding 8 wt% of polyvinyl alcohol (PVA) as a binder and pressed into 2- to 3-mm thick plates or rings. The compacted samples were sintered at 1150 °C for 12 h.
The phase compositions of the samples determined using the X-ray diffractometer (XRD, DX-2700, Haoyuan Co) with Cu Kα radiation. The bulk density of samples was measured by Archimedes method. The microstructures of the samples were presented using a scanning electron microscope (SEM, JEOL, JSM-6490). The basic magnetic properties were characterized using a vibrating sample magnetometer (VSM, MODEL, BHL-525).
3 Results and discussion
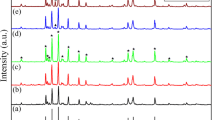
Figure 1 showed X-ray diffraction patterns for BaFe12−xYxO19 solid solutions with x ranging from 0.1 to 0.8. All the diffraction peaks were indexed to be the M-type barium ferrite phase (space group P63/mmc, JCPDS file number 43-002), demonstrating a single phase structure with X = 0.1 to 0.3. The heterogeneity peak of second phase (Y3Fe5O12) has been observed at X = 0.4. The XRD patterns of the BaFe12−xYxO19 (X = 0.1, 0.2, 0.3) matches well with the standard card of M-type barium ferrite at room temperature (300 K). It showed that when the substitution amount of Y3+ is small (X = 0.1, 0.2, 0.3), the Y3+ enters the lattice of M-type barium ferrite.
The second phase (Y3Fe5O12) was found in all samples (X = 0.4–0.8), and the diffraction peak of the second phase became more and more obvious with increased of Y3+ substitution contents(X = 0.4 to 0.8). The limiting substitutions amount that yttrium ions can enter into a lattice of M-type barium ferrite may be between x = 0.3 and 0.4 without producing the second phase (Y3Fe5O12).
XRD analysis and Rietveld refinement were performed on all the samples to estimate the microstructure parameters of the system. Figure 2 describes the experimental X-ray diffraction data of the sample (X = 0.8) and its corresponding Rietveld refinement mode. Table 1 shows the finishing parameters and lattice constants of each sample.
It was seen that with the increase of X, the unit-cell parameters a increases first, then decreases slowly, unit-cell parameters c increases steadily. This is due to the influence of the ionic radius. Y3+ is a nonmagnetic ion, the ionic radius of Y3+ (0.9 Å) is larger than that of Fe3+ (0.64 Å). When the substitution amount is too large(X ≧ 0.4), some Y3+ unable to enter the crystal lattice of M-type barium ferrite, thus the second phase (Y3Fe5O12) was produced and the unit-cell parameters a was reduced to a certain extent.
The bulk densities of all samples were measured by Archimedes and shown in Table 2.It can be clearly seen from the figure that with the increase of the substitution contents of yttrium ions (Y3+), the bulk densities of the samples first decreases and then increases.
The reason for the decrease of BaFe12−xYxO19 (X = 0.1 to 0.3) bulk densities is that the small amount of large yttrium ions (Y3+) substituted iron ions (Fe3+), which increases the volume of the crystal lattice. With the rise of the substitution amount of yttrium ions (X = 0.4 to 0.8), the second phase (Y3Fe5O12) appeared in samples. Since the second phases (Y3Fe5O12) has a denser structure than M-type barium ferrites, the bulk densities of the samples had limited reduced as shown in Fig. 3.
The SEM results were shown in Fig. 4. Hexagonal grain structure was obviously found in the SEM image of BaYxFe12−xO19. From the SEM images, the density of the samples did not change significantly with the increase of the substitution contents of yttrium ions (Y3+). This is consistent with the tiny change in bulk density. In addition, with the increase of substitution contents of yttrium ions (Y3+), the grain size of the sample was increased. Moreover, the growth of grain size has a positive effect on reducing the coercive force of m-type barium ferrite as shown in Fig. 4.
Figure 5 shows the variation of the specific magnetization of the BaYxFe12−xO19 with the external magnetic field. Saturation magnetization (Ms) and coercive force (Hc) extracted from the loops are given in Fig. 6 as functions of the x value. The maximum saturation magnetization of the sample had obtained from the Fig. 6 at room temperature (300 K) was 67.19 emu/g at x = 0.1. Figure 5 showed that before the point of X = 0.4, the saturation magnetization decreases monotonously with the increase of substitution amount of Y3+. This is also consistent with the results of other nonmagnetic ions substitution [12, 16]. But, after X = 0.4, due to the formation of the second phase(Y3Fe5O12), non-magnetic yttrium ions no longer occupy the iron position of M-type barium ferrite, so the total magnetic moment of the sample is recovered the saturation magnetization of the sample increases slowly.
The factors affecting the magnetic properties of ferrite include chemical composition, ion distribution in crystallographic sites and bulk densities [10]. In M-type barium ferrite, each unit cell contains two molecules (BaFe12O19), and there are ten oxygen ion dense layers, of which two dense layers each contain one barium ion occupying oxygen potential. In M-type barium ferrite the magnetic Fe3+ ions are located in positions which have three octahedral site(Fe1-2a, Fe4-4f2 and Fe5-12 k), one tetrahedral(Fe3-4f1) and one bipyramidal (Fe2-2b) oxygen environment [19]. The Fe3+ at 2a, 12 k and 2b sites have upward spin configurations, while those at 4f1 and 4f2 have downward spin configurations. The iron ions in M-type barium ferrite are all trivalent iron ions, and their ion magnetic moment is 5 µB. M-type barium ferrite, given that the molar ratio of Fe3+ ions in different sites is Fe(12 k):Fe(2a):Fe(2b):Fe(4f1):Fe(4f2) = 6:1:1:2:2 [18, 19], the total magnetic moment (m) of M-type barium ferrite can be computed as: \(m=(6+1+1 - 2 - 2) \times 5\upmu B=20\upmu B\). When the Y3+ substituted the Fe3+, the total magnetic moment and saturation magnetization of M-type barium ferrite will be affected by the different ionic magnetic moment of Y3+ and Fe3+.
According to Ligand theory, ions site occupancy depends on d-configuration of electrons and the nature of other participating ions. Ions with d1, d2, d3 and d4 electrons prefer tetrahedral site, ions with d6, d7, d8 and d9 electrons prefer octahedral site, and ions with d0, d5 and d10 have no preference [4]. Y3+ have the electronic structure of 4p6, so they tend to be octahedral in substitution. Fe3+ located at octahedral position in M-type barium ferrite and with upward spin (2a, 12 k) and downward spin(4f2) configurations. According to the change curve of magnetization with external magnetic field, saturation magnetization decreases rapidly from 67.1872 emu/g to 56.7128 emu/g with X ranging from 0.1 to 0.4, indicating that Y3+ should be inclined to substituted 2b (↑), 2a (↑) and 12 k (↑) sites. From the point of view of ionic electronegativity, Ions of high electronegativity have higher tendencies for octahedral sites. Yttrium Ion has a higher electronegativity (1.22) and tends to occupy octahedral position when replacing iron ion. Its seem that for Y substitution Y3+ ions first preferentially occupy the 12 k(↑) and 2a (↑). But, because of the ionic radius, when the Y3+ substitution reached to higher level, a second phase (Y3Fe5O12) was produced.
As described in Stoner–wohlfarth’s theory, the coercive force (HC) of ferrite can be determined by saturation magnetization (MS) and magnetocrystalline anisotropy constant (K1) by the following equation [20]:
Ms and Hc are determined from VSM measurements. According to the law of approaching saturation, the magnetocrystalline anisotropy constant is calculated:
Where parameter a represents the crystal local inhomogeneous stress and inhomogeneity of doped region, vP is the high field paramagnetic susceptibility, and parameter b originates from the resistance of magnetocrystalline anisotropy on domain wall rotation [21], which can be expressed for the hexagonal crystal structure exhibiting uniaxial anisotropy as:
K1 can be calculated by determining the value of parameter b. In the case of high H field region, the parameters a and \(\chi _{p}^{{}}\) are small enough to be negligible, MH exhibited a linear tendency as a function of H2, which makes it possible to calculate the value of parameter b. Calculated K1 versus increased Y substitution contents was present in Table 3.
Coercive force represents the additional magnetic field required to make M equal to 0 in the reverse magnetization process. The irreversible magnetization is the cause of the hysteresis in the magnetization process. The reverse magnetization process, like the magnetization process, can also occur reversible and irreversible magnetization process. Therefore, the fundamental reason for the formation of hysteresis in the reverse magnetization process is the irreversible magnetization process caused by stress bullying, impurities and magnetic anisotropy in the ferromagnetic material. Yttrium ions (Y3+) substituted the iron ions (Fe3+) in M-type barium ferrite and changed the Magnetic crystal anisotropy and causes the coercive force changed. It can be found from Table 2 that the variation trend of coercive force is consistent with the variation rule of the anisotropic constant of magnetic crystals. When the grain size of the sample increases, the number of grain boundaries in the sample is reduced, the internal stress is fully released, and the coercive force is also reduced. Therefore, when the substitution amount of yttrium ions exceeded x = 0.4, the second phase (Y3Fe5O12) was generated in samples, which increases the number of grain boundaries to a certain extent, increases the stress in the sample and increases the coercive force.
4 Conclusions
In this work, we have investigated the effects of Y substitution on the structure and magnetic properties of M-type barium ferrite. The new m-type barium ferrite (BaFe12−xYxO19) was synthesized by solid state reaction and sintering temperature of 1150 °C.It is found that single ion yttrium can adjust the magnetic anisotropic field of M-type barium ferrite and reduce the saturation magnetization intensity and coercive force of M-type barium ferrite (T = 300 k). Due to the large ionic radius of Y3+, some yttrium ions cannot enter the lattice point of m-type barium ferrite lattice when x reached to a highvalue, thus forming the second phase (Y3Fe5O12). According to the experimental results, it is suggested that the substitution content of Y3+ in the synthesis process should be between X = 0.3 and 0.4. The appearance of the second phase will cause the saturation magnetization strength and coercive force of the original sharply decreasing samples to rise slightly. By adjusting the substitution content of Y3+, the ratio of high saturation magnetization intensity and low coercive force can be found and applied in magnetic head materials.
References
V. Dixit, C.N. Nandadasa, S.G. Kim, S. Kim, J. Park, Y.K. Hong, L.S.I. Liyanage, A. Moitra, Site occupancy and magnetic properties of Al-substituted M-type strontium hexaferrite. J. Appl. Phys. 117(24), 243904 (2015)
R. Sun, X. Li, A. Xia, S. Su, C. Jin, Hexagonal SrFe12O19 ferrite with high saturation magnetization. Ceram. Int. 44(12), 13551–13555 (2018)
J. Singh, C. Singh, D. Kaur, S.B. Narang, R. Jotania, R. Joshi, Corrigendum to ‘Investigation on structural and microwave absorption property of Co2+ and Y3+ substituted M-type Ba-Sr hexagonal ferrites prepared by a ceramic method. J. Alloys Compd. 695, 792–798. https://doi.org/10.1016/j.jallcom.2016.09.251) (2017)
R.C. Pullar, Hexagonal ferrites: a review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater Sci. 57(7), 1191–1334 (2012)
J. Li, H. Zhang, Y. Liu, Q. Li, G. Ma, H. Yang, Structural and magnetic properties of M–Ti (M=Ni or Zn) co-substituted M-type barium ferrite by a novel sintering process. J. Mater. Sci. Mater. Electron. 26(2), 1060–1065 (2014)
J. Chen, Y. Wang, Y. Liu, H. Wang, Q. Yin, Q. Liu, C. Wu, Y. Chen, Investigation of oriented Co3+ doped M-type hexaferrite Sr0.5Ba0.5Fe12–xCoxO19 for microwave application. J. Mater. Sci. Mater. Electron. 29(17), 14371–14377 (2018)
Q. Fang, H. Cheng, K. Huang, J. Wang, R. Li, Y. Jiao, Doping effect on crystal structure and magnetic properties of chromium-substituted strontium hexaferrite nanoparticles. J. Magn. Magn. Mater. 294(3), 281–286 (2005)
I. Sadiq, M. ud D. Rana, S. Naseem, Synthesis and characterization of rare earth elements substituted X-type hexagonal ferrites, 2. Amsterdam: Elsevier, 2015
J. Li, H. Zhang, Y. Liu, Q. Li, T. Zhou, H. Yang, Phase formation, magnetic properties and Raman spectra of Co–Ti co-substitution M-type barium ferrites. Appl. Phys. A Mater. Sci. Process. 119(2), 525–532 (2015)
Y. Liu, Q. Liu, C. Wu, Y. Wang, J. Li, L. Gao, H. Zhang, Investigation on Zn-Sn co-substituted M-type hexaferrite for microwave applications. J. Magn. Magn. Mater. 444, 421–425 (2017)
I. Ali, M. Ahmad, M.U. Islam, M.S. Awan, Substitution effects of La3 + ions on the structural and magnetic properties of Co2Y hexaferrites synthesized by sol-gel autocombustion method. J. Sol-Gel. Sci. Technol. 68(1), 141–149 (2013)
A.V. Trukhanov, V.G. Kostishyn, L.V. Panina, S.H. Jabarov, V.V. Korovushkin, S.V. Trukhanov, E.L. Trukhanova, Magnetic properties and Mössbauer study of gallium doped M-type barium hexaferrites. Ceram. Int. 43(15), 12822–12827 (2017)
A.V. Trukhanov, S.V. Trukhanov, V.G. Kostishin, L.V. Panina, M.M. Salem, I.S. Kazakevich, V.A. Turchenko, V.V. Kochervinskii, D.A. Krivchenya, Multiferroic properties and structural features of M-type Al-substituted barium hexaferrites. Phys. Solid State 59(4), 737–745 (2017)
P. Behera, S. Ravi, Influence of Al substitution on structural, dielectric and magnetic properties of M-type barium hexaferrite. J. Supercond. Nov. Magn. 30(6), 1453–1461 (2017)
X. Wu, W. Chen, W. Wu, Y. Ning, S. Chen, Synthesis of hexagonal Co3+-substituted Sr-ferrites via ball-milling assisted ceramic process and their magnetic properties. J. Mater. Sci. Mater. Electron. 28(24), 18815–18824 (2017)
D. Shekhawat, S. Verma, P. Sharma, Effect of La on magnetic properties of BaFe12O19. Trans. Indian Ceram. Soc. 76(4), 247–251 (2017)
A.G. Chesnokov, E.P.N. Den, Influence of diamagnetic cations Sc3+ on the magnetoelastic energy of M-type hexaferrites. Phys. Solid State 43(9), 1728–1730 (2001)
H. Sözeri, H. Deligöz, H. Kavas, A. Baykal, Magnetic, dielectric and microwave properties of M-Ti substituted barium hexaferrites (M=Mn2+, Co2+, Cu2+, Ni2+, Zn2+). Ceram. Int. 40(6), 8645–8657 (2014)
J. Li, H. Zhang, Y. Liu, Q. Li, G. Ma, H. Yang, The transformation behavior of M-type barium ferrites due to Co–Ti substitution. J. Mater. Sci. Mater. Electron. 26(7), 4668–4674 (2015)
Z.F. Zi, Q.C. Liu, J.M. Dai, Y.P. Sun, Effects of Ce-Co substitution on the magnetic properties of M-type barium hexaferrites. Solid State Commun. 152(10), 894–897 (2012)
J.H. You, H.J. Kim, S.I. Yoo, Preparation of strontium W-type hexaferrites in a low oxygen pressure and their magnetic properties. J. Alloys Compd. 695, 3011–3017 (2017)
Funding
This study was funded by National natural fund of China (51872041).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Wang, Y., Liu, Y. et al. Microstructure and magnetic properties of Y substituted M-type hexaferrite BaYxFe12−xO19. J Mater Sci: Mater Electron 30, 5911–5916 (2019). https://doi.org/10.1007/s10854-019-00889-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-00889-x