Abstract
This study aimed to evaluate the effects of the combined application of dimethylaminohexadecyl methacrylate (DMAHDM) and 10-methacryloyloxydecyl dihydrogen phosphate (MDP) on dentin bonding and its antimicrobial properties. DMAHDM was incorporated into the adhesives at 5% by mass, where two commercial adhesives were used: (1) MDP-free adhesive (single bond 2); (2) MDP-containing adhesive (single bond universal, SBU). The microtensile bond strength (μTBS) and nanoleakage were analyzed on the combination. The effects of DMAHDM on the interaction between MDP and hydroxyapatite and the degree of conversion were assessed by Fourier transform infrared spectroscopy (FTIR). Antimicrobial properties were examined by live/dead staining assay, colony-forming units (CFU), and metabolic activity, and cytotoxicity was measured using cell counting kit-8 (CCK-8) assay. 5% DMAHDM or MDP-containing groups showed increased immediate μTBS compared with the control group. Nanoleakage showed that 5% DMAHDM or MDP-containing groups presented little amount of silver penetration, while the control group exhibited extensive silver uptake along the bonding interface after water storage aging. FTIR showed that the interaction between MDP and hydroxyapatite was not affected by DMAHDM, besides DMAHDM did not hamper the polymerization of the bonding agents. The adhesives added with DMAHDM inhibited biofilm formation and reduced the biofilm CFU and metabolic activity of Candida albicans and Streptococcus mutans (P < 0.05). No cytotoxic or slight cytotoxic effect was detected for the experimental materials used (MDP and 5% DMAHDM). The addition of DMAHDM along with MDP can obtain improved dentin bonding with increased antimicrobial performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dental caries is a biofilm-dependent oral disease with a high prevalence, usually leading to tooth defects, affecting the masticatory function and impairing the quality of life [1]. Composite resins along with adhesives are commonly used in dentistry for tooth cavity restorations due to their esthetics and direct-filling ability [2], while secondary caries is claimed as a major long-term complication of dental restorations [3], typically occurring in primary carious lesions, as well as along the bonding interface between tooth and composite resins [4]. The main challenge for restorations with composite resins is to provide durable and effective bonding to tooth hard tissues [5], meanwhile preventing secondary caries caused by the bacteria present in dental plaque [6, 7]. The adhesives containing antibacterial agents combat the residual bacteria in the tooth cavity, with caries-inhibiting abilities, which are quite needed in clinical treatment.
Microorganisms adhesion on the surface of restorations produces a series of metabolites and creates an acidic environment to demineralize the teeth, leading to secondary caries over time [8, 9], besides the material properties of the restorations also influence the accumulation of biofilm [10]. Dental biofilm throughout the bonding interface plays a critical role in caries formation and serves as a source of infection [11,12,13]. Consequently, composite resins may be more susceptible to marginal discoloration and loss of retention caused by bacterial acid production [14, 15]. Therefore, efforts have been made to explore new bonding agents with longer-lasting antimicrobial activities and meanwhile maintain the bonding efficiency to suppress biofilm formation and secondary caries.
Currently, the resin–dentin bonding interface relies primarily on the interlocking between the infiltrated adhesive monomers and the exposed collagen networks [16]. Various types of monomers have been employed in different adhesive systems, with phosphoric acid ester and carboxylic acid monomers [17]. Among them, 10-methacryloyloxydecyl-dihydrogen phosphate (MDP), a phosphate ester monomer, can chemically react with hydroxyapatite to form the MDP-Ca salt structure, which is a key factor to improve the stability of the resin–dentin bonding interface [18]. Commercial MDP-based adhesive systems, such as single bond universal (3 M ESPE, USA) and Clearfil Protect Bond (Kuraray Medical Inc, Japan), have been reported that they can form an acid–base-resistant zone, which exhibit hydrolytically stable, leading to the promotion of restoration durability [19, 20]. Quaternary ammonium methacrylates (QAMs) are antimicrobials with a broad spectrum of activities including bacteria, fungi, etc. [21], and have been incorporated into resins, root canal sealers, and bonding agents, showing promising antimicrobial activities and low toxicity [22,23,24]. Dimethylaminohexadecyl methacrylate (DMAHDM) belongs to QAMs with a "contact killing" antibacterial mechanism, which displays strong antibiofilm potency [25]. However, the effects of the addition of DMAHDM into MDP-containing adhesive on dentin bonding and antimicrobial properties have not been reported.
The objective of this study was to evaluate the potential efficacies of interaction between DMAHDM and MDP on dentin bonding and antimicrobial performance by detecting the dentin bond durability, antimicrobial effects, and biocompatibility. It was hypothesized that: (1) Incorporating DMAHDM into adhesive would not compromise the dentin bonding; (2) DMAHDM alone would inhibit biofilm formation; (3) DMAHDM + MDP adhesive would achieve dual effects on dentin bonding performance and antimicrobial effects.
Materials and methods
Experimental design
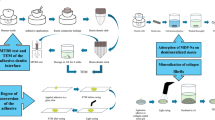
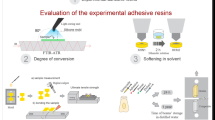
The present study incorporated DMAHDM into two commercial adhesives: MDP-free/MDP-containing adhesive. For dentin bonding performance, this study involved the analysis of two factors: 1. different dentin bonding agents (six groups); 2. storage time (24 h and 6 months). The main response variables were bond strength to dentin measured using a microtensile bond strength test (µTBS) and nanoleakage observed by scanning electron microscopy (SEM). For antimicrobial properties, this study involved the analysis of one factor: different dentin bonding agents. The main response variable was biofilm formation, detected by live/dead staining assay, colony-forming units (CFU), and metabolic activity. Besides the impact of DMAHDM on the interaction between MDP and hydroxyapatite, and the polymerization of the bonding agents measured by degree of conversion (DC) were estimated using Fourier transform infrared spectroscopy (FTIR). The biocompatibility of DMAHDM was measured through cytotoxicity.
Fabrication of DMAHDM
DMAHDM was synthesized according to a modified Menschutkin reaction, in which a tertiary amine group was reacted with an organo-halide [26]. In brief, 10 mmol of 2-(dimethylamino) ethyl methacrylate (DMAEMA, Aladdin, Shanghai, China) and 10 mmol of 1-bromohexadecane (BHD, Aladdin, Shanghai, China) were combined with 3 g of ethanol in a 20-mL vial. The vial was stirred at 70 °C for 24 h. After the reaction was completed, the solvent was removed via evaporation, and DMAHDM was acquired as a clear, colorless, and viscous liquid [27]. To determine whether DMAHDM has been synthesized, the reaction products can be analyzed by Fourier transform infrared spectroscopy (FTIR; Nicolet 6700, Thermo Fisher Scientific, Waltham, MA, USA) [28].
Teeth specimen preparation
Freshly extracted non-carious human third molars were collected under a protocol approved by the Ethical Committee Department, Affiliated Hospital of Stomatology, Nanjing Medical University, China. The teeth were cleaned and then stored in a 0.9% saline solution. The roots of teeth were removed via a low-speed saw (Isomet 1000, Buehler, Lake Bluff, IL, USA) under plentiful water irrigation. The occlusal one-third of the tooth crown was removed perpendicularly to the long axis of the tooth to expose the mid-coronal dentin, and a flat dentin disk with a thickness of approximately 3 mm was obtained from each tooth. The exposed dentin surface was wet-polished using 400-grit silicon-carbide (SiC) abrasive papers to acquire a uniform smear layer [29].
Dentin bonding procedure
Two commercial adhesives were selected in this study: (1) MDP-free adhesive (single bond 2), which served as a comparative control; (2) MDP-containing adhesive (single bond universal). The compositions of the two adhesives are listed in Table 1.
DMAHDM was added into two commercial adhesives at a mass fraction of DMAHDM/(adhesive + DMAHDM) = 5%, and the 5% was selected according to a previous study [30]. MDP-containing experimental primer used was prepared following the formula as previously described [31]: MDP (DM Healthcare Products, USA), 10 wt%; ethanol, 88.8 wt%; camphorquinone (CQ, Aladdin, Shanghai, China), 0.3 wt%; and 4-dimethylamino-benzoic acid ethyl ester (4EDMAB, Aladdin, Shanghai, China), 0.9 wt%.
The extracted teeth were randomly divided into 6 groups with 10 teeth in each group. The dentin bonding agents for each group are detailed in Table 2. The surfaces of the dentin specimens were etched with 35% phosphoric acid gel (Gluma Etch 35 Gel, Heraeus Kulzer, Hanau, Germany) for 15 s to remove the smear layer and rinsed with distilled water. A primer was applied with a brush-tipped applicator, and the solvent was removed gently with a stream of air for 5 s. The corresponding adhesives were used on the dentin surfaces of each group and light-cured for 20 s with a curing unit (Elipar™ S10, 3 M ESPE, St. Paul, MN, USA). The composite resin (Filtek Z250, 3 M ESPE, St. Paul, MN, USA) was built up on the adhesive-treated dentin surface in two 2-mm-thick increments, and each increment was light-cured for 40 s. The bonded specimens were then stored in distilled water at 37 °C for 24 h and 6 months, respectively.
Microtensile bond strength
Each bonded specimen was vertically sectioned into 0.9 mm × 0.9 mm resin–dentin beams after storage in distilled water at 37 °C for 24 h and 6 months. The three longest sticks from the beams of each tooth were selected at each period. Each beam was stressed to failure at a cross-head speed of 1 mm/min using a universal testing machine (Instron 3365 ElectroPuls, Instron, Boston, MA, USA). The load-at-failure (N) divided by the cross-sectional area (mm2) at the site of failure yielded the microtensile bond strength (μTBS) [32], and the μTBS was expressed in MPa. The μTBS of each group for 24 h and 6 months of water storage was recorded and mean, and standard deviation values were calculated.
After μTBS testing, the two ends of a fractured stick were observed under a stereoscopic microscope to determine the failure modes, which were classified as adhesive failure (failure along the adhesive interface), cohesive failure (failure in the composite or in dentin), or mixed failure (failure in the adhesive joint together with failure in the composite or in dentin).
Nanoleakage
Three middle slabs from each group were used for nanoleakage observation using scanning electron microscopy (SEM; TESCAN MAIA3, Kohoutovice, Czech Republic). Two layers of nail varnish were applied on both surfaces, leaving a blank 1-mm window along the bonded interface uncovered. The slabs were immersed in ammoniacal silver nitrate solution under light protection for 24 h, which was prepared as described by Tay et al. [33]. The slabs were rinsed with distilled water and immersed in a photo-developing solution for 8 h under a fluorescent light. The specimens were wet polished sequentially using 600-, 1200-, 2000-, and 3000-grit SiC abrasive papers, which were followed by cleaning ultrasonically for 5 min. The polished slabs were air-dried, sputter-coated with Au, and examined by SEM using back-scattered electron imaging mode.
Fourier transform infrared spectroscopy
The potential interaction between MDP and hydroxyapatite (HA) affected by the incorporation of DMAHDM was characterized chemically using FTIR. HA powder was treated with MDP experimental primer, 5% DMAHDM, and MDP + 5% DMAHDM, which were referred to as M-HA, D-HA, and M + D-HA, respectively. HA powder without any treatment was analyzed as the control (referred to as HA). The treated HA powders (referred to as M-HA, D-HA, and M + D-HA, respectively) were washed, centrifuged, and air-dried at ambient temperature overnight. The infrared spectra from the above three treated powders as well as the HA group were collected in the 3200–500 cm−1 region.
Degree of conversion
The dentin bonding agents for each group were prepared according to Table 2. The MDP-free adhesive (single bond 2) served as the control. One drop of each adhesive was spread on the top plate of FTIR and light-cured for 20 s at a standardized distance of 5 mm. The absorption spectra of uncured (0 s) and cured (20 s) adhesives were obtained by FTIR in the range of 1800 − 1500 cm−1. The degree of conversion (DC) was calculated by changes in C = C intensity according to the following equation:
where Abs(1638) indicated the absorption peak of aliphatic C=C at 1638 cm−1, while Abs(1608) indicated the aromatic C=C at 1608 cm−1. The average of three readings of each adhesive was obtained for statistical analysis.
Bacterial strains and culture
The standard strain Candida albicans SC5314 (C. albicans, ATCC® MYA-2876™) was purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA), and Streptococcus mutans (S. mutans UA159, ATCC® 700610) was purchased from Shanghai Bioresource Collection Center (SHBCC, Shanghai, China). Stock cultures were stored at -80 °C until use. C. albicans were cultured overnight at 30 °C and 200 rpm with shaking in Yeast Extract Peptone Dextrose (YPD) medium (2% peptone, 1% yeast extract, and 2% glucose). S. mutans were incubated aerobically at 37 °C with 5% CO2 in brain heart infusion (BHI; OXOID, Thermo Fisher Scientific, Waltham, MA, USA) medium. C. albicans was prepared in a concentration of 1 × 106 cells/mL and S. mutans in 1 × 107 cells/mL. Then, C. albicans and S. mutans were suspended, respectively, in the RPMI 1640 (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and BHI culture medium for further usage.
Induction of biofilm on resins
The cover of a sterile 96-well plate (Thermo Fisher Scientific, Waltham, MA, USA), which had 96 individual circular rims to lock with wells of the plate, was used as molds for fabricating resin disks following a previous study [34]. DMAHDM-incorporated adhesive or control adhesive was spread onto the bottom of each dent of the 96-well plate, air-dried, and light-cured for 20 s. For MDP and MDP + 5% DMAHDM group, the experimental primer containing MDP was spread first on the bottom of the dents and left for 20 s. Then, the adhesive was applied, air-dried, and light-cured for 20 s. Subsequently, the composite resin was applied onto the adhesive layer of each group to the height flush with the rim of the dent, to obtain a disk of approximately 8 mm in diameter and 0.5 mm in thickness. The cured disks were immersed in distilled water and magnetically stirred with a bar for 1 h to remove any uncured monomers [35]. The disks were exposed to ultraviolet light on each side for disinfection.
A one-mL bacteria suspension of C. albicans or S. mutans was added to each well of 24-well plates with a resin disk and incubated at 37 °C in 5% CO2 for 24 h. Then, the resin disks were transferred to the new 24-well plates with fresh medium and incubated for another 24 h. This constituted a 2-day culture, which was previously shown to form relatively mature biofilms on resins [36, 37].
Live/dead staining assay
Resin disks with 2-day biofilms were gently rinsed with phosphate-buffered saline (PBS) and stained using the LIVE/DEAD® BacLight™ Bacterial Viability Kit (Molecular Probes, Eugene, OR, USA). Live bacteria were stained with Syto 9 to produce green fluorescence, and bacteria with compromised membranes were stained with propidium iodide to produce red fluorescence. The stained disks were observed using a confocal laser scanning microscope (CLSM; Stellaris STED, Leica, Wetzlar, Germany). Three fields of view were photographed at random locations on each disk, and three specimens per group were evaluated. The area of green staining was measured using the ImageJ software (NIH, Frederick, MD, USA), and the area fraction of live bacteria = green staining area/total area of the image.
Colony-forming unit (CFU) counts
Colony-forming unit (CFU) counts were used to quantitatively analyze the total number of viable bacteria existing on each resin disk. Resin disks covered with 2-day biofilms were washed with PBS to remove unattached bacteria. Then, the disks were transferred into tubes containing 1 ml PBS, and the biofilms were harvested by sonication for 5 min, followed by vortexing for 30 s using a vortex mixer (MX-S, DLAB, Beijing, China), as described in previous studies [38]. The microbial suspensions (C. albicans and S. mutans) obtained were serially diluted, spread onto YPD and BHI agar plates, respectively, and incubated at 37 °C in 5% CO2 for 24 h. The number of colonies that grew was counted and used, along with the dilution factor, to calculate the CFUs on each disk. Six replicates of each group were tested for CFUs analysis, and the assays were repeated at least thrice.
XTT assay of metabolic activity
The disks with adherent biofilm grown for 2 days were washed with PBS and transferred to a new 24-well plate. A 100 μL of the XTT (2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide) solution [XTT (0.5 mg/mL in PBS), menadione (0.1 mM in acetone)] (APExBIO Technology LLC, Houston, TX, USA) was added to each well for the assessment of metabolic activity [39]. After incubation at 37 °C for 2 h, 80 μL of the solution from each well was collected and transferred to a new 96-well plate. The absorbance was measured via a microplate reader (SpectraMax M2, Molecular Devices, CA, USA). A higher absorbance value indicates a greater biofilm metabolic activity on the disks [40]. This experiment was performed in quadruplicate and repeated thrice.
Cytotoxicity tests
The cytotoxicity tests followed the International Organization for Standardization (ISO) 10,993–5 (2009) in which L929 mouse fibroblasts were used [41]. Resin disks were prepared for cytotoxicity tests as described above, and the disks without any treatment were used as the positive control (referred to as Z250 resin). The recommended area to volume of medium ratio was fixed at 3 cm2 /ml in accordance with ISO 10993–12 (2021) [42]. The specimens (n = 12/group) were eluted with fresh RPMI 1640 at 37 °C for 24 h. The elution obtained was then filtered through 0.22-µm filters and stored at -80 °C for downstream experiments.
L929 mouse fibroblasts were incubated at 37 °C in 5% CO2 and seeded at a density of 2 × 104 cells per well in 96-well culture plates. The cells were allowed to attach for 24 h, prior to exposure to the elution of each group. The medium was discarded and 100 µL of the elution was added into each well for further incubation (4 wells at 24, 72, and 120 h each). Untreated cultures without any material served as a negative control (referred to as NC), which was replaced with RPMI 1640 containing 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, Waltham, MA, USA). Following incubation, cell morphological changes were observed by an inverted microscope (DMIL LED, Leica, Wetzlar, Germany).
Cell viability was assessed by cell counting kit-8 (CCK-8) assay as previously described [43]. After incubating for 24, 72, and 120 h, cells were then incubated for 2 h in 100 μL fresh medium with 10 μL CCK-8 reagent (APExBIO Technology LLC, Houston, TX, USA), and optical density (OD) values were measured with a microplate reader at 450 nm wavelength. Cytotoxicity was evaluated based on the relative growth rate (RGR) of the cells, and the RGR was calculated by the following formula [44]: RGR (%) = OD of the experimental group/OD of the negative control group × 100%.
Statistical analysis
Statistical analysis was carried out using SPSS 22.0 statistical software (IBM SPSS Inc., Chicago, IL, USA). After validating the equal variance assumption of data, the μTBS results were analyzed using two-way ANOVA followed by Tukey post hoc test to examine the effects of different dentin bonding agents (i.e., six groups) and storage time (i.e., 24 h or 6 months) and the interaction of these two factors on dentin bonding strength. The results including DC, live/dead staining assay, CFU, metabolic activity, and cytotoxicity tests were submitted to one-way ANOVA followed by Tukey post hoc test. The significance level was set at 0.05 for all analyses.
Results
Fabrication of DMAHDM
The spectrum of DMAHDM in the range of 3200–1000 cm−1 is plotted in Fig. 1A. Characteristic IR bands at 2925 cm−1 (−CH2), 1719 cm−1 (C=O), 1635 cm−1 (C=C), and 1463 cm−1 (− CH2) were indicated in the spectrum of the DMAHDM [45], which corresponded to the chemical structure of DMAHDM (Fig. 1B).
μTBS
The μTBS results from the six groups after 24 h and 6 months of water storage are summarized in Table 3. In this study, no pre-test failure occurred. Two-way ANOVA revealed that the different dentin bonding agents and storage time had significant effects on μTBS (P < 0.01). The addition of DMAHDM and/or MDP attained higher bond strength values, irrespective of the water storage time. All groups manifested inspiringly initial μTBS, while the control group was the lowest (35.65 ± 3.14 MPa), and no significant differences were found among the other five groups. However, the μTBS of all the groups were weakened by being submerged for 6 months, where the control group yielded the lowest μTBS (28.36 ± 2.69 MPa). The percentage of the failure mode of the de-bonded dentin specimens of each group is shown in Table 3, where mixed failure was the predominant failure mode in all groups.
Nanoleakage
SEM images revealed the infusion of tracer (silver) within the hybrid layer, which presented as water tree-like patterns [29]. The representative SEM images of silver infiltration that were randomly distributed along the resin–dentin interfaces after 24 h and 6 months of water storage are shown in Fig. 2 and 3, respectively. The control group showed relatively continuous silver penetration indicating the presence of interfacial gaps after 24 h of water storage (Fig. 2). The control group also showed the extensive nanoleakage that the silver deposits occurred through the entire length of the hybrid layer, while less deposition can be detected in other five groups after 6 months of water storage (Fig. 3), which was consistent with μTBS results.
Representative back-scattered SEM images of silver deposition within the hybrid layer after 6 months of water storage. The presence of silver deposition can be observed along the resin–dentin bonding interface consistently. a: Control; b: MDP; c: 5% DMAHDM; d: MDP + 5% DMAHDM; e: SBU; f: 5% DMAHDM + SBU. Scale bar, 20 μm
FTIR
The FTIR spectra of hydroxyapatite (HA) submitted to different treatments in the range of 3200–500 cm−1 are presented in Fig. 4. The characteristic peak C = O stretching vibration of the methacryloxy carbonyl group at 1719 cm−1 [46] could be distinguished in M-HA and M + D-HA, but not in HA and D-HA, indicating the immobilization of MDP onto the HA and that the chemical interaction between MDP and HA would not be affected by DMAHDM treatment (Fig. 4b, d).
FTIR spectra of hydroxyapatite with different treatments in the range of 3200–500 cm−1. a HA; b M-HA; c D-HA; d M + D-HA. The spectrum of hydroxyapatite (HA) is depicted in Fig. 4 a as a control. The black arrows revealed the characteristic peak of the C = O stretching vibration of methacryloxy carbonyl (1719 cm−1)
Degree of conversion
The DC results of the dentin bonding agents for six groups are presented in Fig. 5. There was no significant difference in DC among these six groups (P > 0.05), which indicated that DMAHDM and its combined application with MDP did not significantly affect the polymerization of adhesives used in this study (single bond 2 and single bond universal).
Live/dead staining assay
The typical live/dead staining images of 2-day biofilms grown on resin disks are shown in Fig. 6. Live bacteria were stained green, and compromised bacteria were stained red. When live and dead bacteria were in close proximity or on top of each other, the staining showed yellow or orange colors. The biofilms on the adhesives containing 5% DMAHDM (5% DMAHDM, MDP + 5% DMAHDM, and 5% DMAHDM + SBU) were composed of primarily red C. albicans or S. mutans, while other groups (Control, MDP, and SBU) were completely covered by green C. albicans or S. mutans biofilms (Fig. 6A and C), showing that DMAHDM showed strong microorganism inhibition abilities. The live microorganism area fraction in biofilms of C. albicans and S. mutans are plotted in Fig. 6B, D, respectively. The fractions of the adhesives containing 5% DMAHDM were significantly lower than those of the control, MDP, and SBU groups (P < 0.05), which was in accordance with the CFU results presented in the next section.
Representative live/dead staining images of 2-day single-species biofilms adherent on composite resin disks and live bacteria fraction in biofilm (green staining) (mean ± SD; n = 3): A and B: C. albicans; C and D: S. mutans; a: Control; b: MDP; c: 5% DMAHDM; d: MDP + 5% DMAHDM; e: SBU; f: 5% DMAHDM + SBU. The live bacteria were stained green, and the dead bacteria were stained red. The control group was covered with biofilms consisting of mostly live bacteria. The adhesives with 5% DMAHDM consisted of primarily dead bacteria. Different letters indicate significant differences (P < 0.05). Scale bar, 100 μm
CFU counts
The CFU counts of 48-h biofilms on composite resins are shown in Fig. 7. C. albicans were less than 106 CFU/disk, and S. mutans were close to 1011 CFU/disk. The control, MDP, and SBU groups showed similar CFU values (P > 0.05), while 5% DMAHDM, MDP + 5% DMAHDM, and 5% DMAHDM + SBU groups showed lower CFU counts than the groups above (P < 0.05), regardless of C. albicans or S. mutans collected from the resins. DMAHDM greatly decreased the biofilms CFU of C. albicans or S. mutans, and the incorporation of DMAHDM added a stronger antimicrobial potency against the C. albicans or S. mutans biofilms.
Colony-forming unit (CFU) of 2-day single-species biofilms on composite resins. A and B: C. albicans; C and D: S. mutans. a: Control; b: MDP; c: 5% DMAHDM; d: MDP + 5% DMAHDM; e: SBU; f: 5% DMAHDM + SBU. The adhesives added with DMAHDM resulted in much lower biofilm CFU, compared with the other three groups without DMAHDM, regardless of C. albicans or S. mutans. Values with dissimilar letters are significantly different from each other (P < 0.05)
XTT assay of metabolic activity
The biofilm metabolic activity of C. albicans and S. mutans is plotted in Fig. 8. The XTT assay showed that groups with the addition of DMAHDM to adhesives presented significantly lower biofilm-forming ability than the others without DMAHDM (P < 0.05), regardless of the biofilms formed from C. albicans or S. mutans. The metabolic activity of MDP-containing commercial adhesives (SBU group) resembled that of the groups pretreated with MDP-containing experimental primer (MDP group) (P > 0.05).
Effects of DMAHDM on biofilm formation in C. albicans a and S. mutans b. Biofilm viability was quantified by the XTT assay. A decrease in biofilm metabolic activity was observed with the incorporation of DMAHDM. Data are shown as mean ± SD. Values with dissimilar letters are significantly different from each other (P < 0.05)
Cytotoxicity tests
No or slight cytotoxicity was detected in the experimental materials (MDP and 5% DMAHDM). The OD values were lower in the MDP, 5% DMAHDM, and MDP + 5% DMAHDM group than that in other groups after 24, 72, and 120 h of culture (Table 4), which were in agreement with the results of relative cell proliferation (Fig. 9). According to the standard of the cytotoxicity grade (CG) in Table 5, after 24 and 72 h of culture, the CG of MDP, 5% DMAHDM and MDP + 5% DMAHDM group were Grade 2 (RGR > 50%), and after 120 h, all groups (except the MDP + 5% DMAHDM group) were Grade 1 (RGR > 75%) (Table 6).
Cell morphology observation showed that the L929 cell was basically normal, and the L929 cells grew adhering to the wall for all the groups (Fig. 10). The density of cells in the MDP, 5% DMAHDM, and MDP + 5% DMAHDM group was less than that in the other groups after 120 h of culture.
Discussion
The oral biofilm accumulation in adjacent tooth–restoration margins brings out detrimental responses which is the main reason for restoration failures [47]. Therefore, it is highly desirable to develop dental materials that were capable of suppressing cariogenic species. If an antibacterial adhesive can inhibit the invasion and colonization of oral microorganisms along the bonding interface, it will provide persistent antimicrobial efficacy to the tooth–restoration interface. The present study determined the effects of the combined application of DMAHDM and MDP on dentin bonding and antimicrobial properties. The hypotheses were proven here that the incorporation of DMAHDM into adhesives exhibited higher dentin bond strength and could effectively inhibit biofilm formation compared with the control group. DMAHDM + MDP adhesive reduced biofilm CFU and metabolic activity, which possessed antibiofilm functions, as well as improved dentin bonding performance.
The antimicrobial activities of QAMs are related to the alkyl chain length [48]. DMAHDM, with an alkyl chain length of 16, has a positively charged quaternary amine N + , which can attract negatively bacteria cells and disturb the electric balance [49]. Previous studies have suggested that DMAHDM was incorporated into root canal sealers, composite resins, and sealants, showing potent antimicrobial activities [50,51,52]. The 5% DMAHDM possesses an excellent biofilm inhibition potential among those dental materials as mentioned above and would not bring out obvious changes in physical and chemical properties [53]. The combined application of DMAHDM and adhesive on antimicrobial properties was evaluated here, which showed that the adhesives containing 5% DMAHDM had substantial amounts of compromised microorganisms, significantly inhibiting the growth of C. albicans and S. mutans biofilms, while the control group was mainly composed of live biofilm according to the live/dead staining results. Besides incorporating 5% DMAHDM into adhesives remarkably reduced the CFU counts and metabolic activity of biofilms on the composite resins. These findings indicated that 5% DMAHDM applied can prevent the adhesion to the tooth surface of these two tested strains and demonstrated the antibiofilm effects against C. albicans and S. mutans.
Furthermore, the combined application of DMAHDM and MDP on dentin bonding was evaluated. Restorative materials including adhesives are vulnerable to hydrolysis in a warm and humid oral environment [54]. Previous studies have employed diverse methods to simulate degradation conditions in vitro, such as storing in water [55] or in artificial saliva [56], and some accelerated aging methods including thermal cycling [57], etc. During long-term water storage, the retained water in the adhesive interface may detriment the bonding durability [58]. Under thermal cycling conditions, the differences in the thermal expansion between the dentin and adhesives might result in cracks at the resin/dentin interface [59]. However, no established standardized methods can fully reproduce the oral environment. It has been reported that the bonding strength could present similar trends to some extent under different aging conditions: thermal cycling and storage in water at 37 °C [60]. In the present study, the μTBS was evaluated after 6 months of water storage. Besides phosphoric acid used prior to adhesives was effective in removing the smear layer and dissolving the mineral, which allowed penetration of the adhesives into the tooth surfaces sufficiently and achieved the mechanical bond [61]. The addition of MDP or DMAHDM into adhesives did not negatively affect the μTBS after 24 h and 6 months of water storage compared with the control group, and the μTBS results showed encouragingly immediate resin–dentin bonds (around 35–45 MPa). Although the decreases of bond strength still occurred over time, fortunately, the adhesives containing MDP or DMAHDM showed less reduction in μTBS after 6 months of water storage, while the control group exhibited lower μTBS values. The methacrylate group in DMAHDM allows it to be copolymerized and then immobilized in the adhesive monomers so that the DMAHDM would not leach out and be lost, displaying durable bonding effects [62].
However, even if the dentin surface was thoroughly air-dried, the water remaining in the hybrid layer was difficult to be fully eliminated, which may enhance the water absorption within the hydrophilic adhesive layer, leading to rapid deterioration along the resin–dentin bonding interface [63]. Furthermore, collagenolytic enzymes such as endogenous matrix metalloproteinases (MMPs) and cysteine cathepsins may progressively degrade the hybrid layer, eventually leading to interfacial gaps between the teeth and restorations [64]. The interfacial gaps lead to a decrease in bonding strength and provide an available area for the microorganisms to adhere to form the biofilms. In the present study, SEM images showed that the adhesives containing MDP or DMAHDM had less nanoleakage, especially after long-time (6 months) water storage, while nanoleakage was observed through the entire thickness of the bonding interface in the control group, suggesting that MDP or DMAHDM has a higher resistance to nanoleakage. The nanoleakage or the interfacial gaps are with an appearance of the reticular and spotted patterns and striking silver deposition under SEM observation [65]. The QAMs with long-lasting anti-MMPs effects may inhibit the host-derived collagenolytic enzymes from demineralizing the dentin matrix [66], providing evidence for yielding much greater μTBS and less nanoleakage of the DMAHDM-containing adhesives during 6 months of water storage aging. Nevertheless, further studies are needed to clarify the inhibition mechanisms of DMAHDM on collagenase within the hybrid layer.
MDP is a common functional monomer that can chemically bond to HA to form stable calcium salts, thus achieving long-term bonding durability at the MDP/HA interface [67]. Some previous studies have indicated that MDP-containing adhesives can contribute to the sealing of restoration margins [68]. In the present study, absorbance peaks of the characteristic functional group in MDP can be still detected from FTIR even after the treatment with DMAHDM, which showed that the corporation of DMAHDM had no adverse effects on the interaction between MDP and HA. FTIR results also confirmed the bonding performance of the combined application of MDP, and DMAHDM was not significantly different from those of MDP applied alone. Moreover, DMAHDM and its combined application with MDP did not significantly hamper the polymerization of adhesives, which can also promote the application of DMAHDM in future clinical practice.
C. albicans and S. mutans are the most important microorganisms in dental antimicrobial tests [69]. S. mutans is considered to be the primary cariogenic bacteria, which can colonize the oral cavity and form biofilms, ultimately leading to the demineralization of tooth hard tissue [70]. C. albicans is the most common opportunistic pathogen and is frequently found in oral biofilms together with S. mutans [71]. Although oral biofilms are formed by a variety of microorganisms residing in the oral cavity [72], C. albicans and S. mutans were employed to form biofilms alone in this study, which was conducive to acquiring a better understanding of the antimicrobial effect of biofilms formed from the single species on adhesives containing DMAHDM and/or MDP [73].
Cytotoxicity test was a simplified method to assess how specific cell types were affected by the tested materials concerning biocompatibility, although cell experiments in vivo may be more accurate to simulate the conditions in vitro [73]. In this study, the measured parameters are the OD values of L929 cells treated with the elutions of samples from each group using a CCK-8 assay. The OD values of each group increased, with the increase of culture days, which is consistent with the trend of the relative cell proliferation curve. A slight cytotoxic effect was detected for MDP, 5% DMAHDM, and MDP + 5% DMAHDM groups after the treatment with elution for 24 h and 72 h, and this effect appeared to be more apparent for the combined applied group (MDP + 5% DMAHDM) at 120 h, while the cytotoxicity of other groups was not observed. A reason for the increased cytotoxicity may be the potential interaction between DMAHDM and MDP, which requires further studies. Campos et al. indicated that DMAHDM was incorporated into the resin materials, showing acceptable cytotoxicity, which corroborated with the present outcomes, despite the different methods used to evaluate cytotoxicity [73].
Based on these results, the addition of DMAHDM along with MDP can obtain improved dentin bonding with increased antimicrobial performance. It is prospective to develop novel dental adhesives, which not only can prevent the colonization of microorganisms but also improve the bonding performance. Further studies are needed to investigate the antimicrobial effects of DMAHDM on multi-species biofilms that are clinically relevant, especially anaerobic bacteria should also be tested to establish a subgingival root caries model.
Conclusions
The study concluded that DMAHDM at a concentration of 5% together with MDP can provide potent and stable dentin bonding with less nanoleakage after long-term water storage aging. The DMAHDM-containing adhesive can substantially inhibit the biofilm growth and reduce the CFU and metabolic activity of C. albicans and S. mutans. Therefore, the combined application of MDP and DMAHDM is highly looking forward to protecting the bonding interface from degradation and reducing the lesion caused by biofilms, whereas further clinically relevant studies are still needed.
Data availability
The data that support the findings of this study are available from the corresponding authors on reasonable request.
References
Mathur VP, Dhillon JK (2018) Dental caries: a disease which needs attention. Indian J Pediatr 85:202–206. https://doi.org/10.1007/s12098-017-2381-6
Imazato S, Ma S, Chen JH, Xu HH (2014) Therapeutic polymers for dental adhesives: loading resins with bio-active components. Dent Mater 30:97–104. https://doi.org/10.1016/j.dental.2013.06.003
Askar H, Krois J, Göstemeyer G, Schwendicke F (2021) Secondary caries risk of different adhesive strategies and restorative materials in permanent teeth: systematic review and network meta-analysis. J Dent 104:103541
Askar H, Krois J, Göstemeyer G, Bottenberg P, Zero D, Banerjee A, Schwendicke F (2020) Secondary caries: what is it, and how it can be controlled, detected, and managed? Clin Oral Investig 24:1869–1876. https://doi.org/10.1007/s00784-020-03268-7
Liu Y, Tjäderhane L, Breschi L, Mazzoni A, Li N, Mao J, Pashley DH, Tay FR (2011) Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J Dent Res 90:953–968. https://doi.org/10.1177/0022034510391799
Breschi L, Maravic T, Cunha SR, Comba A, Cadenaro M, Tjäderhane L, Pashley DH, Tay FR, Mazzoni A (2018) Dentin bonding systems: from dentin collagen structure to bond preservation and clinical applications. Dent Mater 34:78–96. https://doi.org/10.1016/j.dental.2017.11.005
Liang J, Liu F, Zou J, Xu HHK, Han Q, Wang Z, Li B, Yang B, Ren B, Li M, Peng X, Li J, Zhang S, Zhou X, Cheng L (2020) pH-responsive antibacterial resin adhesives for secondary caries inhibition. J Dent Res 99:1368–1376. https://doi.org/10.1177/0022034520936639
Liu Y, Yang J, Yang Y, Li M, Xu HHK, Weir MD, Zhou X, Liang K, Li J (2022) Evaluation of the ability of adhesives with antibacterial and remineralization functions to prevent secondary caries in vivo. Clin Oral Investig 26:3637–3650. https://doi.org/10.1007/s00784-021-04334-4
Jung JH, Kim DH, Yoo KH, Yoon SY, Kim Y, Bae MK, Chung J, Ko CC, Kwon YH, Kim YI (2019) Dentin sealing and antibacterial effects of silver-doped bioactive glass/mesoporous silica nanocomposite: an in vitro study. Clin Oral Investig 23:253–266. https://doi.org/10.1007/s00784-018-2432-z
Mazurek-Popczyk J, Nowicki A, Arkusz K, Pałka Ł, Zimoch-Korzycka A, Baldy-Chudzik K (2022) Evaluation of biofilm formation on acrylic resins used to fabricate dental temporary restorations with the use of 3D printing technology. BMC Oral Health 22:442. https://doi.org/10.1186/s12903-022-02488-5
Ferracane JL (2017) Models of caries formation around dental composite restorations. J Dent Res 96:364–371. https://doi.org/10.1177/0022034516683395
Fan M, Li M, Yang Y, Weir MD, Liu Y, Zhou X, Liang K, Li J, Xu HHK (2022) Dual-functional adhesive containing amorphous calcium phosphate nanoparticles and dimethylaminohexadecyl methacrylate promoted enamel remineralization in a biofilm-challenged environment. Dent Mater 38:1518–1531. https://doi.org/10.1016/j.dental.2022.07.003
Tao S, Su Z, Xiang Z, Xu HHK, Weir MD, Fan M, Yu Z, Zhou X, Liang K, Li J (2020) Nano-calcium phosphate and dimethylaminohexadecyl methacrylate adhesive for dentin remineralization in a biofilm-challenged environment. Dent Mater 36:e316–e328. https://doi.org/10.1016/j.dental.2020.08.001
Chen H, Zhang B, Weir MD, Homayounfar N, Fay GG, Martinho F, Lei L, Bai Y, Hu T, Xu HHK (2020) S. mutans gene-modification and antibacterial resin composite as dual strategy to suppress biofilm acid production and inhibit caries. J Dent 93:103278. https://doi.org/10.1016/j.jdent.2020.103278
Josic U, Maravic T, Mazzitelli C, Radovic I, Jacimovic J, Del Bianco F, Florenzano F, Breschi L, Mazzoni A (2021) Is clinical behavior of composite restorations placed in non-carious cervical lesions influenced by the application mode of universal adhesives? A systematic review and meta-analysis. Dent Mater 37:e503–e521. https://doi.org/10.1016/j.dental.2021.08.017
Brackett MG, Li N, Brackett WW, Sword RJ, Qi YP, Niu LN, Pucci CR, Dib A, Pashley DH, Tay FR (2011) The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J Dent 39:238–248. https://doi.org/10.1016/j.jdent.2010.12.009
Fehrenbach J, Isolan CP, Münchow EA (2021) Is the presence of 10-MDP associated to higher bonding performance for self-etching adhesive systems? A meta-analysis of in vitro studies. Dent Mater 37:1463–1485. https://doi.org/10.1016/j.dental.2021.08.014
Yoshihara K, Nagaoka N, Yoshida Y, Van Meerbeek B, Hayakawa S (2019) Atomic level observation and structural analysis of phosphoric-acid ester interaction at dentin. Acta Biomater 97:544–556. https://doi.org/10.1016/j.actbio.2019.08.029
Ochiai Y, Inoue G, Nikaido T, Ikeda M, Tagami J (2019) Evaluation of experimental calcium-containing primer in adhesive system on micro-tensile bond strength and acid resistance. Dent Mater J 38:565–572. https://doi.org/10.4012/dmj.2018-266
Elkaffas AA, Hamama HHH, Mahmoud SH (2018) Do universal adhesives promote bonding to dentin? A systematic review and meta-analysis. Restor Dent Endod 43:29. https://doi.org/10.5395/rde.2018.43.e29
Elena P, Miri K (2018) Formation of contact active antimicrobial surfaces by covalent grafting of quaternary ammonium compounds. Colloids Surf B Biointerfaces 169:195–205. https://doi.org/10.1016/j.colsurfb.2018.04.065
Makvandi P, Jamaledin R, Jabbari M, Nikfarjam N, Borzacchiello A (2018) Antibacterial quaternary ammonium compounds in dental materials: a systematic review. Dent Mater 34:851–867. https://doi.org/10.1016/j.dental.2018.03.014
Assad-Loss TF, Vignoli JF, Garcia IM, Portela MB, Schneider LFJ, Collares FM, Cavalcante LMA, Tostes MA (2021) Physicochemical properties and biological effects of quaternary ammonium methacrylates in an experimental adhesive resin for bonding orthodontic brackets. J Appl Oral Sci 29:e20201031-98. https://doi.org/10.1590/1678-7757-2020-1031
Zhang K, Baras B, Lynch CD, Weir MD, Melo MAS, Li Y, Reynolds MA, Bai Y, Wang L, Wang S, Xu HHK (2018) Developing a new generation of therapeutic dental polymers to inhibit oral biofilms and protect teeth. Materials (Basel) 11:1747. https://doi.org/10.3390/ma11091747
Baras BH, Melo MAS, Sun J, Oates TW, Weir MD, Xie X, Bai Y, Xu HHK (2019) Novel endodontic sealer with dual strategies of dimethylaminohexadecyl methacrylate and nanoparticles of silver to inhibit root canal biofilms. Dent Mater 35:1117–1129. https://doi.org/10.1016/j.dental.2019.05.014
Cheng L, Weir MD, Zhang K, Arola DD, Zhou X, Xu HH (2013) Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J Dent 41:345–355. https://doi.org/10.1016/j.jdent.2013.01.004
Li Y, Hu X, Ruan J, Arola DD, Ji C, Weir MD, Oates TW, Chang X, Zhang K, Xu HHK (2019) Bonding durability, antibacterial activity and biofilm pH of novel adhesive containing antibacterial monomer and nanoparticles of amorphous calcium phosphate. J Dent 81:91–101. https://doi.org/10.1016/j.jdent.2018.12.013
Wu J, Zhou H, Weir MD, Melo MA, Levine ED, Xu HH (2015) Effect of dimethylaminohexadecyl methacrylate mass fraction on fracture toughness and antibacterial properties of CaP nanocomposite. J Dent 43:1539–1546. https://doi.org/10.1016/j.jdent.2015.09.004
Chen C, Niu LN, Xie H, Zhang ZY, Zhou LQ, Jiao K, Chen JH, Pashley DH, Tay FR (2015) Bonding of universal adhesives to dentine–Old wine in new bottles? J Dent 43:525–536. https://doi.org/10.1016/j.jdent.2015.03.004
Li F, Weir MD, Chen J, Xu HH (2014) Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent Mater 30:433–441. https://doi.org/10.1016/j.dental.2014.01.002
Yang J, Shen J, Wu X, He F, Xie H, Chen C (2020) Effects of nano-zirconia fillers conditioned with phosphate ester monomers on the conversion and mechanical properties of Bis-GMA- and UDMA-based resin composites. J Dent 94:103306. https://doi.org/10.1016/j.jdent.2020.103306
Li F, Liu XY, Zhang L, Kang JJ, Chen JH (2012) Ethanol-wet bonding technique may enhance the bonding performance of contemporary etch-and-rinse dental adhesives. J Adhes Dent 14:113–120. https://doi.org/10.3290/j.jad.a21853
Tay FR, Pashley DH, Yoshiyama M (2002) Two modes of nanoleakage expression in single-step adhesives. J Dent Res 81:472–476. https://doi.org/10.1177/154405910208100708
Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S (2009) Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J Dent 37:289–296. https://doi.org/10.1016/j.jdent.2008.12.004
Wang L, Melo MA, Weir MD, Xie X, Reynolds MA, Xu HH (2016) Novel bioactive nanocomposite for Class-V restorations to inhibit periodontitis-related pathogens. Dent Mater 32:e351–e361. https://doi.org/10.1016/j.dental.2016.09.023
Cheng L, Exterkate RA, Zhou X, Li J, ten Cate JM (2011) Effect of Galla chinensis on growth and metabolism of microcosm biofilms. Caries Res 45:87–92. https://doi.org/10.1159/000324084
Xie X, Wang L, Xing D, Zhang K, Weir MD, Liu H, Bai Y, Xu HHK (2017) Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent Mater 33:553–563. https://doi.org/10.1016/j.dental.2017.03.002
Zhang K, Melo MA, Cheng L, Weir MD, Bai Y, Xu HH (2012) Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent Mater 28:842–852. https://doi.org/10.1016/j.dental.2012.04.027
Karayazgan B, Atay A, Saracli MA, Gunay Y (2010) Evaluation of Candida albicans formation on feldspathic porcelain subjected to four surface treatment methods. Dent Mater J 29:147–153. https://doi.org/10.4012/dmj.2009-016
Martins CHG, Pires RH, Cunha AO, Pereira CAM, Singulani JL, Abrão F, Moraes T, Mendes-Giannini MJS (2016) Candida/Candida biofilms. First description of dual-species Candida albicans/C. rugosa biofilm. Fungal Biol 120:530–537. https://doi.org/10.1016/j.funbio.2016.01.013
International Organization for Standardization: ISO 10993–5 (2009) Biological evaluation of medical devices - part 5: tests for in vitro cytotoxicity.
International Organization for Standardization: ISO 10993–12 (2021) Biological evaluation of medical devices - Part 12: sample preparation and reference materials.
He GN, Bao NR, Wang S, Xi M, Zhang TH, Chen FS (2021) Ketamine induces ferroptosis of liver cancer cells by targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther 15:3965–3978. https://doi.org/10.2147/DDDT.S332847
Wu X, Dai S, Chen Y, He F, Xie H, Chen C (2019) Reinforcement of dental resin composite via zirconium hydroxide coating and phosphate ester monomer conditioning of nano-zirconia fillers. J Mech Behav Biomed Mater 94:32–41. https://doi.org/10.1016/j.jmbbm.2019.03.002
Liu Q, Wu B, Yu Q, Wang Y (2019) Immobilization of quaternary ammonium based antibacterial monomer onto dentin substrate by non-thermal atmospheric plasma. Dent Mater J 38:821–829. https://doi.org/10.4012/dmj.2018-267
Feitosa VP, Ogliari FA, Van Meerbeek B, Watson TF, Yoshihara K, Ogliari AO, Sinhoreti MA, Correr AB, Cama G, Sauro S (2014) Can the hydrophilicity of functional monomers affect chemical interaction. J Dent Res 93:201–206. https://doi.org/10.1177/0022034513514587
Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H (2007) Economic impact of regulating the use of amalgam restorations. Public Health Rep 122:657–663. https://doi.org/10.1177/003335490712200513
Li F, Weir MD, Xu HH (2013) Effects of quaternary ammonium chain length on antibacterial bonding agents. J Dent Res 92:932–938. https://doi.org/10.1177/0022034513502053
Maia AC, Mangabeira A, Vieira R, Neves AA, Lopes RT, Pires TM, Viana GM, Cabral LM, Cavalcante LM, Portela MB (2019) Experimental composites containing quaternary ammonium methacrylates reduce demineralization at enamel-restoration margins after cariogenic challenge. Dent Mater 35:e175–e183. https://doi.org/10.1016/j.dental.2019.05.021
Baras BH, Wang S, Melo MAS, Tay F, Fouad AF, Arola DD, Weir MD, Xu HHK (2019) Novel bioactive root canal sealer with antibiofilm and remineralization properties. J Dent 83:67–76. https://doi.org/10.1016/j.jdent.2019.02.006
Zhang N, Ma J, Melo MA, Weir MD, Bai Y, Xu HH (2015) Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J Dent 43:225–234. https://doi.org/10.1016/j.jdent.2014.11.008
Ibrahim MS, Ibrahim AS, Balhaddad AA, Weir MD, Lin NJ, Tay FR, Oates TW, Xu HHK, Melo MAS (2019) A novel dental sealant containing dimethylaminohexadecyl methacrylate suppresses the cariogenic pathogenicity of Streptococcus mutans Biofilms. Int J Mol Sci 20:3491. https://doi.org/10.3390/ijms20143491
Vidal ML, Rego GF, Viana GM, Cabral LM, Souza JPB, Silikas N, Schneider LF, Cavalcante LM (2018) Physical and chemical properties of model composites containing quaternary ammonium methacrylates. Dent Mater 34:143–151. https://doi.org/10.1016/j.dental.2017.09.020
Santerre JP, Shajii L, Leung BW (2001) Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med 12:136–151. https://doi.org/10.1177/10454411010120020401
Kalagi S, Feitosa SA, Münchow EA, Martins VM, Karczewski AE, Cook NB, Diefenderfer K, Eckert GJ, Geraldeli S, Bottino MC (2020) Chlorhexidine-modified nanotubes and their effects on the polymerization and bonding performance of a dental adhesive. Dent Mater 36:687–697. https://doi.org/10.1016/j.dental.2020.03.007
Stape THS, Uctasli M, Cibelik HS, Tjäderhane L, Tezvergil-Mutluay A (2021) Dry bonding to dentin: broadening the moisture spectrum and increasing wettability of etch-and-rinse adhesives. Dent Mater 37:1676–1687. https://doi.org/10.1016/j.dental.2021.08.021
Sai K, Shimamura Y, Takamizawa T, Tsujimoto A, Imai A, Endo H, Barkmeier WW, Latta MA, Miyazaki M (2016) Influence of degradation conditions on dentin bonding durability of three universal adhesives. J Dent 54:56–61. https://doi.org/10.1016/j.jdent.2016.09.004
Moszner N, Salz U, Zimmermann J (2005) Chemical aspects of self-etching enamel-dentin adhesives: a systematic review. Dent Mater 21:895–910. https://doi.org/10.1016/j.dental.2005.05.001
Suzuki S, Takamiazawa T, Imai A, Tsujimoto A, Sai K, Takimoto M, Barkmeier WW, Latta MA, Miyazaki M (2018) Bond durability of universal adhesive to bovine enamel using self-etch mode. Clin Oral Investig 22:1113–1122. https://doi.org/10.1007/s00784-017-2196-x
Kawazu M, Takamizawa T, Hirokane E, Tsujimoto A, Tamura T, Barkmeier WW, Latta MA, Miyazaki M (2020) Comparison of dentin bond durability of a universal adhesive and two etch-and-rinse adhesive systems. Clin Oral Investig 24:2889–2897. https://doi.org/10.1007/s00784-019-03153-y
Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A (2011) State of the art etch-and-rinse adhesives. Dent Mater 27:1–16. https://doi.org/10.1016/j.dental.2010.10.016
Zhang K, Cheng L, Wu EJ, Weir MD, Bai Y, Xu HH (2013) Effect of water-ageing on dentin bond strength and anti-biofilm activity of bonding agent containing antibacterial monomer dimethylaminododecyl methacrylate. J Dent 41:504–513. https://doi.org/10.1016/j.jdent.2013.03.011
Hiraishi N, Breschi L, Prati C, Ferrari M, Tagami J, King NM (2007) Technique sensitivity associated with air-drying of HEMA-free, single-bottle, one-step self-etch adhesives. Dent Mater 23:498–505. https://doi.org/10.1016/j.dental.2006.03.007
Shokati B, Tam LE, Santerre JP, Finer Y (2010) Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. J Biomed Mater Res B Appl Biomater 94:230–237. https://doi.org/10.1002/jbm.b.31645
Cavalheiro A, Cruz J, Sousa B, Silva A, Coito C, Lopes M, Vargas M (2021) Dentin adhesives application deviations: effects on permeability and nanoleakage. Dent Mater J 40:1160–1168. https://doi.org/10.4012/dmj.2020-404
Li F, Majd H, Weir MD, Arola DD, Xu HH (2015) Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent Mater 31:284–292. https://doi.org/10.1016/j.dental.2014.12.011
Yoshihara K, Yoshida Y, Nagaoka N, Fukegawa D, Hayakawa S, Mine A, Nakamura M, Minagi S, Osaka A, Suzuki K (2010) Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta Biomater 6:3573–3582. https://doi.org/10.1016/j.actbio.2010.03.024
Matsui N, Takagaki T, Sadr A, Ikeda M, Ichinose S, Nikaido T, Tagami J (2015) The role of MDP in a bonding resin of a two-step self-etching adhesive system. Dent Mater J 34:227–233. https://doi.org/10.4012/dmj.2014-205
Rego GF, Vidal ML, Viana GM, Cabral LM, Schneider LFJ, Portela MB, Cavalcante LM (2017) Antibiofilm properties of model composites containing quaternary ammonium methacrylates after surface texture modification. Dent Mater 33:1149–1156. https://doi.org/10.1016/j.dental.2017.07.010
Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A (2014) The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 33:499–515. https://doi.org/10.1007/s10096-013-1993-7
Pereira D, Seneviratne CJ, Koga-Ito CY, Samaranayake LP (2018) Is the oral fungal pathogen Candida albicans a cariogen? Oral Dis 24:518–526. https://doi.org/10.1111/odi.12691
Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J (2011) Adherence and biofilm formation of non-Candida albicans Candida species. Trends Microbiol 19:241–247. https://doi.org/10.1016/j.tim.2011.02.003
Campos KPL, Viana GM, Cabral LM, Portela MB, Hirata Junior R, Cavalcante LM, Lourenço EJV, Telles DM (2020) Self-cured resin modified by quaternary ammonium methacrylates and chlorhexidine: cytotoxicity, antimicrobial, physical, and mechanical properties. Dent Mater 36:68–75. https://doi.org/10.1016/j.dental.2019.10.007
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81970945 and No. 81371156).
Funding
This work was supported by the National Natural Science Foundation of China (No. 81970945 and No. 81371156).
Author information
Authors and Affiliations
Contributions
JS helped in methodology, writing—original draft, visualization. MM contributed to software and data curation. YH was involved in validation and investigation. HM helped in software and formal analysis. XW performed writing—review & editing and supervision.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This study involving extracted non-carious human third molars has been approved by the Ethical Committee Department, Affiliated Hospital of Stomatology, Nanjing Medical University, China.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, J., Ma, M., Huang, Y. et al. Evaluation of the effects of combined application of dimethylaminohexadecyl methacrylate and MDP on dentin bonding and antimicrobial properties. J Mater Sci 58, 12685–12705 (2023). https://doi.org/10.1007/s10853-023-08820-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08820-w