Abstract
Objectives
The purpose of this study was to examine the enamel bond durability of universal adhesives in the self-etch mode under 2-year water storage and thermal cycling conditions.
Materials and methods
Three commercially available universal adhesives and a gold standard two-step self-etch adhesive were used. Ten specimens of bovine enamel were prepared per test group, and shear bond strength (SBS) was measured to determine the bonding durability after thermal cycling (TC) or long-term water storage (WS). The bonded specimens were divided into three groups: (1) specimens subjected to TC, where the bonded specimens were stored in 37 °C distilled water for 24 h before being subjected to 3000, 10,000, 20,000 or 30,000 TC; (2) specimens stored in 37 °C distilled water for 3 months, 6 months, 1 year or 2 year; and (3) specimens stored in 37 °C distilled water for 24 h, serving as a baseline.
Results
The two-step self-etch adhesive showed significantly higher SBS than the universal adhesives tested, regardless of the type of degradation method. All universal adhesives showed no significant enamel SBS reductions in TC and WS, when compared to baseline and the other degradation conditions.
Conclusions
Compared to the bond strengths obtained with the two-step self-etch adhesive, significantly lower bond strengths were obtained with universal adhesives. However, the enamel bond durability of universal adhesives was relatively stable under both degradation conditions tested.
Clinical relevance
The present data indicate that the enamel bond durability of universal adhesives in the self-etch mode might be sufficient for clinical use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Improvements in resin-based materials and adhesive technologies over the years have contributed to the ongoing development in the field of adhesive dentistry [1, 2]. Direct restorations in particular have benefited from the use of resin composites, and the aesthetically pleasing results, simple treatment procedures and preservation of tooth structures have led to it becoming a widely used technique [3]. However, biological and biomechanical degradations invariably result in such restorations deteriorating over time [4,5,6,7,8]. It is therefore clinically important that the degradation over time of these restorations in intraoral environmental situations is fully understood.
Although long-term clinical trials of resin composite restorations might provide information about the bonding performance of the adhesive materials used or the durability of the resin composite itself, such trials would be very time consuming, expensive and difficult to achieve without significant biases [9, 10]. Although it is difficult for all aspects of an intraoral environmental condition to be simulated simultaneously, in vitro simulated testing is thought to be an effective way of acquiring results and diminishing the biases caused by case selection [9, 11]. In addition, simulated oral condition tests can be designed so that the mechanisms governing specific aspects of the degradation processes happening in clinical situations can be precisely investigated [9]; several simulation tests have been developed in order for certain degradation processes to be clarified, such as those of biofilm attack, hydrolytic degradation, enzymatic degradation by matrix metalloproteinases, thermal shock and fatigue stress [4,5,6,7,8, 12].
Long-term water storage (WS) and thermal cycling (TC) tests combined with bond strength tests have been common methods for testing the in vitro degradation of restored teeth, because the results before and after the degradation processes can be standardized and easily compared to previous reports [9]. The degradation mechanism that occurs near an adhesive during WS tests is thought to be mainly related to the hydrolytic degradation of the resinous materials [11,12,13,14]. TC tests, however, can accelerate degradation near the adhesive layers due to thermal stress; this is because of both the discrepancies between the thermal expansion rates of the substrates and the hydrolytic degradation caused by the water bath [9, 12, 15]. Although no specific correspondence has been found between an intraoral period and a water storage period or the number of thermal cycles used, it is possible that the results from these tests can help predict the long-term bonding durability of resin composite restorations in vivo.
The most recent advancement in the adhesive technology is the introduction of the universal adhesive, which is distinguished by both its suitability for different types of adherent substrates and for use in total-etch, selective-etch and self-etch modes with mineralized tooth tissue [16,17,18,19,20,21]. The versatility of this adhesive allows clinicians to tailor their approach to the prevailing cavity conditions; variables such as size, depth, location and proportion of the enamel and/or dentin can be considered and optimized for. Several studies have investigated the influence of biomechanical factors on the bonding effectiveness of different type of self-etch adhesive through shear fatigue testing, and although the bonding effectiveness of universal adhesives have not been found to be superior to two-step self-etch adhesives, their bonding effectiveness has been found equal to or greater than that of conventional single-step self-etch adhesives [7, 18, 19]. In addition, universal adhesives in the total-etch mode have been found to exhibit greater enamel bonding performances without affecting dentin bonding [18, 19]. However, from a biodegradation perspective, there is limited information on the durability of the enamel bonds of universal adhesives.
The purpose of this study was to determine the enamel bond durability of universal adhesives in the self-etch mode using different simulated degradation tests. The null hypothesis to be tested was that different degradation processes are not influenced by the type of adhesive (i.e. universal or two-step).
Materials and methods
Study materials
Table 1 shows the materials used in this study. Three universal adhesives were used: Scotchbond Universal (SU) (3M ESPE, St Paul, MN, USA), G-Premio Bond (GP) (GC Corp., Tokyo, Japan) and All Bond Universal (AB) (Bisco, Schaumburg, IL, USA). A conventional two-step self-etch adhesive, Clearfil SE Bond (SE) (Kuraray Noritake Dental, Tokyo, Japan), was used for comparison. A resin composite, Clearfil AP-X (Kuraray Noritake Dental, Tokyo), was used for the bonding to the enamel.
Specimen preparation
Specimen preparation was performed according to the International Organization for Standardization (ISO) 29022 specification [22]. Mandibular bovine incisors extracted from cattle that were between 2 and 3 years old were used instead of human teeth, and they were frozen and stored for up to 6 months before being used. The apical root structure of each tooth was removed at the cemento–enamel junction (CEJ) by a slow-speed saw that used a diamond-impregnated disc (Isomet Low Speed Saw) (Buehler, Lake Bluff, IL, USA). Samples of pulp tissue were then removed, and the labial surfaces were ground with wet 240-grit silicon carbide (SiC) paper (Fuji Star Type DDC) (Sankyo Rikagaku Co. Ltd., Saitama, Japan) in order to create flat enamel surfaces. Each tooth was then mounted in a self-curing acrylic resin (Tray Resin II, Shofu Inc., Kyoto, Japan) so that the flattened enamel area could be exposed to the adhesive. The bonding enamel sites were ground flat using a water coolant and a sequence of SiC papers ending with 320-grit (Fuji Star Type DDC) before the surfaces were dried with oil-free compressed air.
Storage condition and shear bond strength tests
Table 1 shows the experimental protocols for the bonding procedures. Ten specimens were used in each test group in order to determine their shear bond strength (SBS) to enamel without the use of phosphoric acid pre-etching (i.e. the self-etch mode). For each prepared surface, the adhesives were applied in accordance with their manufacturer’s instructions. Following the adhesions, the specimens were secured by an Ultradent Bonding Jig (Ultradent Products Inc., South Jordan, UT, USA) and a plastic mould, whose internal diameter and height were 2.4 and 2.0 mm, respectively, was set in place. A condenser was used to insert a resin composite into the mould-enclosed assembly on the enamel surfaces used for the SBS tests before the surfaces were irradiated for 30 s by a visible-light curing unit (Optilux 501, sds Kerr, Danbury, CT, USA) that was set at a light irradiance average of 600 mW/cm2.
The bonded assemblies were then subjected either to thermal cycling (TC group) or storage in distilled water at 37 °C for long periods of time (WS group). For the TC groups, the bonded specimens were stored in distilled water at 37 °C for 24 h before being treated with 3000, 10,000, 20,000 or 30,000 thermal cycles between 5 and 60 °C with dwell times of 30 s. The bonded specimens of the WS groups were stored in distilled water at 37 °C for 3 months, 6 months, 1 year or 2 year before the SBS tests. The storage water, which did not contain antibiotics, was changed every week during the course of the experiment. Baseline specimens were stored in distilled water at 37 °C for 24 h before the SBS tests (baseline group).
The SBS was measured using an Ultradent Bonding Assembly (Ultradent Products Inc.), as described by ISO 29022 [22]. The bonded specimens were loaded to failure at 1.0 mm/min using a universal testing machine (Type 5500R, Instron Corp., Canton, MA, USA). The SBS values were calculated from the peak load at failure divided by the bonded surface area. After testing, the bonded tooth surfaces and resin composite cylinders were observed through an optical microscope (SZH-131, Olympus Ltd., Tokyo, Japan) at a magnification of × 10 to determine the failure mode. Based on the percentage of the substrate area (adhesive–resin composite–enamel) observed on the de-bonded cylinders and bonded tooth sites, the types of bond failure were recorded as being either (1) adhesive failure, (2) cohesive failure in the composite, (3) cohesive failure in the enamel or (4) mixed failure—partially adhesive and partially cohesive.
SEM observations
After the SBS tests, the restorative–enamel interfaces and the representative fracture sites were observed by a field-emission scanning electron microscope (ERA-8800FE, Elionix Ltd., Tokyo, Japan). Prior to the ultrastructure observations of the restorative–enamel interfaces, the bonded specimens (stored in 37 °C distilled water for 24 h) were embedded in an epoxy resin (Epon 812, Nisshin EM Co., Tokyo, Japan) and then longitudinally sectioned by the Isomet Low Speed Saw. The sectioned surfaces were then polished to a high gloss with abrasive discs (Fuji Star Type DDC) followed with diamond paste having 0.25 μm particles (DP-Paste, Struers, Ballerup, Denmark). The fracture sites from each storage condition were prepared directly for SEM. All of the SEM specimens were dehydrated in ascending grades of tert-butyl alcohol (50% for 20 min, 75% for 20 min, 95% for 20 min and 100% for 2 h) and then transferred to a critical-point dryer (Model ID-3, Elionix Ltd.) for 30 min. The restorative–enamel interfaces were then subjected to argon-ion beam etching (EIS-200ER, Elionix Ltd.) for 40 s with the ion beam (accelerating voltage 1.0 kV, ion current density 0.4 mA/cm2) directed perpendicular to the polished surfaces. Finally, all of the SEM specimens were coated with a thin film of gold (Quick Coater, Type SC-701, Sanyu Denchi Inc., Tokyo, Japan). Observations were performed under operating voltage of 10 kV.
Statistical analysis
A two-way analysis of variance (ANOVA) and a subsequent Tukey’s honest significant difference (HSD) test (α = 0.05) were used to analyse the SBS data. Two factors were considered: the degradation period (the number of thermal cycles or the water storage period) and the adhesive system used.
According to a report by Gale et al. [23], approximately 10,000 thermal cycles are equivalent to 1 year in intraoral conditions. Therefore, in order to determine whether the influence of the type of degradation method used interacted with the influence of the degradation period and adhesive system, a three-way ANOVA was also performed on all of the SBS data; the factors of it included the degradation method, the degradation period ((a) 3000 thermal cycles/3 months of water storage, (b) 10,000 thermal cycles/1 year of water storage and (c) 20,000 thermal cycles/2 years of water storage) and the adhesive system. The statistical analysis was performed using statistical software (Sigma Plot ver. 11.0; SPSS Inc., Chicago, IL, USA).
Results
Thermal cycling tests
The results for the enamel SBS under TC conditions are shown in Table 2. The two-way ANOVA revealed that the number of thermal cycles and the adhesive system significantly influenced the enamel SBS values (p < 0.001), and the interaction between the two factors was significant (p < 0.05).
For the baseline group, although the two-step self-etch adhesive showed a significantly higher SBS value (p < 0.05) than the other adhesives, no significant differences were found between the SBS values of the universal adhesives. Regarding the results in the TC groups, although some adhesives did not show any significant differences, all of the adhesives showed higher SBS values early on in the TC (for the 3000 and 10,000 thermal cycles) than the baseline group, and their SBS values tended to fall as the number of thermal cycles increased; that is, most of the adhesives showed significantly higher SBS values at 3000 thermal cycles than the baseline values, and the SU and SE adhesives showed significantly lower SBS values at 30,000 cycles than at 3000 cycles. By defining the baseline enamel SBS value for each tested adhesive as being 100%, we found that the SBS values ranged from 97.5 to 126.6% (Table 2). By observing the SBS values under various amounts of TC for each adhesive, we found that although the bond strengths initially increased with the number of thermal cycles, they decreased after a certain point.
Long-term water storage tests
The results for the enamel SBS under WS conditions are shown in Table 3. The two-way ANOVA revealed that the water storage period and adhesive system used significantly influenced the enamel SBS values (p < 0.001), but the interaction between the two factors was not significant (p = 0.076).
In the WS groups, SE was found to result in a significantly higher SBS value (p < 0.05) than the other adhesives over all of the storage periods, and there was no significant difference in enamel SBS value among any water storage periods. In contrast, all of the universal adhesives that were tested showed significantly higher SBS values at 3 months than the baseline. In addition, all of the universal adhesives were found to have stable SBS values, in that there were no significant differences between the 6-month, 1-year and 2-year groups. By defining the baseline enamel SBS value as being 100% for each of the tested adhesives, we found that the SBS values for all of the adhesives ranged from 104.3 to 130.8% (Table 3). We found that, for most of the adhesives, the SBS values in enamel initially increased over time before flattening out to a plateau.
Three-way ANOVA
The three-way ANOVA for the enamel SBS values (Table 4) was performed to determine whether the degradation method used interacted with the influence of the degradation periods and adhesive systems. We found that all three of the factors significantly influenced the SBS values (p < 0.05), but none of the interactions were significant (p > 0.05) apart from the interaction between the degradation method and the adhesive system. Figure 1 shows the relationship between the TC and WS degradation methods when the baseline enamel SBS value was defined as being 100% for each tested adhesive and 10,000 thermal cycles were assumed to be equivalent to a water storage period of 1 year. All of the tested adhesives were found to show enamel SBS percentages above the baseline percentages for all of the degradation periods, regardless of the degradation method used; however, the trend in the change over time depended on both the adhesive and degradation method used. In general, the enamel SBS values under TC were much more likely to vary than under the WS condition in this study (Fig. 1).
Influence of the degradation method on enamel SBS values (where 10,000 thermal cycles is assumed to be equivalent to 1 year of water storage). SU TC, Scotchbond Universal with thermal cycling; SU WS, Scotchbond Universal with water storage; GP TC, G-Premio Bond with thermal cycling; GP WS, G-Premio Bond with water storage; AB TC, All Bond Universal with thermal cycling; AB WS, All Bond Universal with water storage; SE TC, Clearfil SE Bond with thermal cycling; SE WS, Clearfil SE Bond with water storage
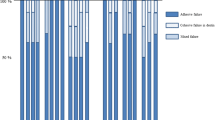
Failure mode analysis of de-bonded specimens
The frequencies of the different failure modes after the SBS tests are shown in Figs. 2 and 3. For all of the groups, the predominant failure mode was that of adhesive failure, regardless of the type of degradation condition used. In particular, all of the de-bonded specimens exhibited an adhesive failure mode at 1 and 2 years of water storage.
SEM observations
SEM images of representative resin–enamel interfaces are shown in Fig. 4. The thickness of the adhesive layer was material dependent, and the two-step self-etch SE adhesive was found to have formed a thicker adhesive layer than those of the universal adhesives (approximately 40 μm (Fig. 4d)); due to this thicker layer, the SE samples are shown at a different magnification. Conversely, SU and GP were found to have formed adhesive layers of approximately 10 μm (Fig. 4a, b), whereas AB’s layer was 4–5 μm (Fig. 4c). Although AB did not contain any inorganic fillers and its adhesive layer was observed to be homogeneous (Fig. 4c), the adhesive layers of SU, GP and SE were all found to contain nano-sized fillers (Fig. 4a, b, d).
Representative SEM images of the resin side of the de-bonded specimens after the SBS test are shown in Fig. 5. After the SBS test, at lower magnifications, all of the groups were found to predominantly have adhesive failures at the resin–enamel interface; however, at higher magnifications, cracks, cleavages and cohesive failures could be observed more clearly in the enamel. These features were particularly apparent after degradation, regardless of the type of adhesive observed.
Representative SEM micrographs of the de-bonded resin sides of bonds after the SBS testing of SU and SE. a De-bonded failure site of SU for 24 h of water storage at magnifications of (a) × 40 and (b) × 1000. b De-bonded failure site of SU for 30,000 thermal cycles at magnifications of (a) × 40 and (b) × 1000. c De-bonded failure site of SU for 2-year water storage at magnifications of (a) × 40 and (b) × 1000. d De-bonded failure site of SE for 24-h water storage at magnifications of (a) × 40 and (b) × 1000. e De-bonded failure site of SE for 30,000 thermal cycles at magnifications of (a) × 40 and (b) × 1000. f De-bonded failure site of SE for 2-year water storage at magnifications of (a) × 40 and (b) × 1000. The visible materials are indicated with the following abbreviations—Ad, adhesive; En, enamel; RC, resin composite. The arrows indicate the enamel
Discussion
Bovine teeth were used in this study. This study required the preparation of a very large number of samples at the same time, and it is difficult to obtain that many human teeth at once. The advantage of using bovine teeth instead of human teeth is that they are easy to obtain in large quantities in good condition and have a less variable composition than human teeth. Moreover, bovine teeth have large flat surfaces and have not had prior caries challenges that might affect the test results. In addition, it has been reported that results from bovine teeth are very similar to these from human teeth. Therefore, bovine superficial enamel was used as a substitute for human enamel in this study, as in previous studies [24, 25].
In this study, the changes over time of the degradation under the different degradation conditions were investigated for three universal adhesives and a two-step self-etch adhesive. The statistical analysis assumed that 10,000 thermal cycles was equivalent to 1 year of water storage, and it revealed that the degradation method, type of adhesive used and degradation period significantly influenced the SBS values (Table 4). Therefore, the null hypothesis that the influence of the different degradation methods would not depend on the type of adhesive used was rejected. The two-step self-etch adhesive, SE, was found to show significantly higher SBS values than all of the universal adhesives that were tested, regardless of the degradation method and degradation period used; this result agreed with previous studies [7, 8, 19].
Although the value of the bond strength is generally important, the differences in it between different conditions can make it hard to compare the changes in degradation over time in these conditions. Therefore, in order to make the trends in bond strength clearer, we defined the 24 h SBS value as being 100% for each adhesive tested, and we recorded the percentages at different degradation periods. When observing the changes over time, we found that the SE adhesive showed similar trends under different degradation conditions, in that the SBS values increased early on in the degradation processes before decreasing afterwards. However, the universal adhesives were found to show different trends over time for the two degradation methods. This disparity may be due to the complex compositions of these adhesives; such complexity is necessary for etching, priming and bonding to simultaneously occur [26, 27]. Moreover, the compositions of the universal adhesives were highly diverse; for example, they varied in whether they contained 2-hydroxyethyl methacrylate (HEMA) and inorganic nano-fillers or not, their pH values and the types of solvent and resin monomers used. As a result, these differences influenced the characteristics of the adhesive layers that formed in addition to the length of time they stayed adhered to the enamel. These might be the reasons as to why the universal adhesives varied more significantly for the different degradation methods than the SE adhesive.
With regard to the influence of the degradation methods on the enamel SBS values, the values were not found to be significantly lower than the baseline values for any of the adhesives in either the TC or WS condition for any of the degradation periods. However, most of the adhesives varied less over time during WS than during TC. Unlike the mechanisms of dentin bond degradation, enamel bond degradation in WS is thought to mainly rely on the hydrolysis of resin components, which is due to water absorption over time [8, 28,29,30]. However, in the TC test, the main factor behind the deterioration was thermal stress, which was caused by discrepancies in the thermal expansion rates in the vicinity of the adhesive layer [9, 15, 31]. Defects or cracks created by thermal stress have been found to possibly induce percolation and the breakdown of poorly polymerized oligomers in an adhesive layer [15, 31]. The present study, however, indicates that thermal stress might induce much greater damage in the vicinity of an adhesive layer than hydrolytic degradation, at least in the case of enamel bonds. Although the hydrolytic degradation of an adhesive layer due to the absorption of water may not per se be a critical factor in TC when the dwelling time is calculated, water absorption may nevertheless change the mechanical properties of the adhesive layer and potentially result in accelerated bond degradation. Therefore, although there was no clear difference between the HEMA-containing and HEMA-free universal adhesives in the WS condition, the SBS values of the HEMA-containing SU were found to decrease much more than the HEMA-free GP adhesive in the TC conditions.
The most interesting result obtained by this study was the inter-relationship between the two common degradation methods investigated. By assuming that 10,000 thermal cycles are equivalent to 1 year of water storage (Fig. 1), it is possible to consider how these degradation methods relate to the longevity of bonds in the real world. Previous studies into simulated degradation methods have indicated that the geometry, size and storage environment of bonded specimens influence the degradation of the bonds [9]. In addition, the time at which water is changed and the additives in the water influence the degradation process in the WS method [9, 32, 33]. Early on in the degradation process for WS (3000 thermal cycles/3 months of water storage in the present study), the SBS values were found to be adhesive dependent; however, most of the adhesives exhibited similar trends, in that the SBS values increased above that of the baseline SBS values, this particular trend was remarkable in the universal adhesives. This phenomenon has been observed our previous studies [8, 12, 34]. It can be speculated that the mechanical properties of the adhesive layer of the universal adhesives might improve after 24 h due to the post-curing effects within these adhesives, and the functional monomers increase their chemical bonding to the substrate [8, 34]. For enamel bonds, although the changes over time were adhesive and degradation method dependent, each adhesive was found to have had enamel SBS values that were higher than the baseline SBS value until 20,000 thermal cycles/2 years of water storage. In addition, the morphological appearances of the failure sites observed through the SEM observations after both degradation methods showed no clear differences. Therefore, for the enamel bonds, it may be desirable for the degradation period of both TC and WS conditions to be extended beyond the point where the bonded specimens are fabricated, according to ISO standard 29022 [22].
The ultimate aim of in vitro degradation tests is to provide useful predictions of in vivo bond durability. To this end, it is important that in vitro tests are developed that match clinical results. The differences between the two popular in vitro degradation tests investigated in this study suggest that more work is needed on this problem. Arguably, the most pressing need is for a standard against which clinical and laboratory results can be compared, and as such, this is an important topic for future research.
Conclusion
This laboratory study showed that the degradation method, type of adhesive and degradation period significantly influence enamel SBS. In all the degradation conditions, a two-step self-etch adhesive showed significantly higher enamel SBS values than all the tested universal adhesives. We found that the changes in time of the SBS values of the universal adhesives were both adhesive and degradation method dependent. However, the enamel bond durability of the universal adhesives was relatively stable under both the thermal cycling and water storage degradation conditions. These results suggest that there ought to be no concerns about the durability of enamel bonds formed by universal adhesives in the self-etch mode. We also found that when considering degradation testing, it might be necessary for the degradation periods to be extended in some situations.
References
Ferracane JL (2011) Resin composite-state of the art. Dent Mater 27:29–38. https://doi.org/10.1016/j.dental.2010.10.020
Ruggeberg FA (2011) State of the art: dental photocuring—a review. Dent Mater 27:39–52. https://doi.org/10.1016/j.dental.2010.10.021
Tyas MJ, Anusavice KJ, Frencken JE, Mount GJ (2000) Minimal intervention dentistry—a review. FDI Commission Project 1–97. Int Dent J 50:501–512. https://doi.org/10.1111/j.1875-595X.2000.tb00540.x
Breschi L, Mazzoni A, Ruggeri A, Carencro M, Di Lenarda R, De Stefano DE (2008) Dental adhesion review: aging and stability of the bonded interface. Dent Mater 24:90–101. https://doi.org/10.1016/j.dental.2007.02.009
Carvalho RM, Manse AP, Geraldeli S, Tay FR, Pashley DH (2012) Durability of bonds and clinical success of adhesive restorations. Dent Mater 28:72–86. https://doi.org/10.1016/j.dental.2011.09.011
Tezvergil-Mutluy A, Pashley DH, Mutluray MM (2015) Long-term durability of dental adhesives. Curr Oral Health Rep 2:174–181. https://doi.org/10.1007/s40496-015-0070-y
Takamizawa T, Barkmeier WW, Tsujimoto A, Scheidel DD, Erickson RL, Latta MA, Miyazaki M (2015) Effect of phosphoric acid pre-etching on fatigue limits of self-etching adhesives. Oper Dent 40:379–395. doi: https://doi.org/10.2341/13–252-L
Takamizawa T, Barkmeier WW, Tsujimoto A, Scheidel DD, Watanabe H, Erickson RL, Latta MA, Miyazaki M (2015) Influence of water storage on fatigue strength of self-etch adhesives. J Dent 43:1416–1427. https://doi.org/10.1016/j.jdent.2015.10.018
De Munck J, Van Landuyt KL, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B (2005) A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res 84:118–132. https://doi.org/10.1177/154405910508400204
Peumans M, De Munck J, Mine A, Van Meerbeek B (2014) Clinical effectiveness of contemporary adhesives for the restorations of non-carious cervical lesions. A systematic review. Dent Mater 30:1089–1103. https://doi.org/10.1016/j.dental.2005.02.003
Amaral FLB, Colucci V, Palma-Dibb RG, Corona SAM (2007) Assessment of in vitro methods used to promote adhesive interface degradation: a critical review. J Esthet Restor Dent 19:340–354. https://doi.org/10.1111/j.1708-8240.2007.00134.x
Sai K, Shimamura Y, Takamizawa T, Tsujimoto A, Imai A, Endo H, Barkmeier WW, Latta MA, Miyazaki M (2016) Influence of degradation conditions on dentin bonding durability of three universal adhesives. J Dent 54:56–61. https://doi.org/10.1016/j.jdent.2016.09.004
Deng D, Yang H, Guo J, Chen X, Zhang W, Huang C (2014) Effects of different artificial ageing methods on the degradation of adhesive-dentine interfaces. J Dent 42:1577–1585. https://doi.org/10.1016/j.jdent.2014.09.010
Frassetto A, Breschi L, Turco G, Marchesi G, Di Lenarda R, Tay FR, Pashley DH, Cadenaro M (2016) Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—a literature review. Dent Mater 32:e41–e53. https://doi.org/10.1016/j.dental.2015.11.007
Miyazaki M, Sato M, Onose H (1998) Influence of thermal cycling on dentin bond strength of two-step bonding systems. Am J Dent 11:118–122
da Rosa WL, Piva E, da Silva AF (2015) Bond strength of universal adhesives: a systematic review and meta-analysis. J Dent 43:765–776. https://doi.org/10.1016/j.jdent.2015.04.003
Seabra B, Arantes-Oliveira S, Portugal J (2014) Influence of multimode universal adhesives and zirconia primer application techniques on zirconia repair. J Prosthet Dent 112:182–187. https://doi.org/10.1016/j.prosdent.2013.10.008
Takamizawa T, Barkmeier WW, Tsujimoto A, Berry TP, Watanabe H, Erickson RL, Latta MA, Miyazaki M (2016) Influence of different etching modes on bond strength and fatigue strength to dentin using universal adhesive systems. Dent Mater 32:e9–e21. https://doi.org/10.1016/j.dental.2015.11.005
Suzuki T, Takamizawa T, Barkmeier WW, Tsujimoto A, Endo H, Erickson RL, Latta MA, Miyazaki M (2016) Influence of etching mode on enamel bond durability of universal adhesive systems. Oper Dent 41:520–530. https://doi.org/10.2341/15-347-L
Siqueria F, Cardenas AM, Gutierrez MF, Malaquias P, Hass V, Reis A, Loguercio AD, Perdigão J (2016) Laboratory performance of universal adhesive systems for luting CAD/CAM restorative materials. J Adhes Dent 18:331–340. https://doi.org/10.3290/j.jad.a36519
Tsujimoto A, Barkmeier WW, Takamizawa T, Wilwerding T, Latta MA, Miyazaki M (2017) Influence of surface free energy characteristics on universal adhesives on boding to various substrates. Oper Dent 42:e59–e70. https://doi.org/10.2341/15–353-L.
ISO 29022: 2013 Dentistry-Adhesion-Notched-edge shear bond strength test. 1stedn. Geneva, Switzerland: International Organization for Standardization. ISO (2013) 1–12.
Gale MS, Darvell BW (1999) Thermal cycling procedures for laboratory testing of dental restorations. J Dent 27:89–99. https://doi.org/10.1016/S0300-5712(98)00037-2
Flower CS, Swartz ML, Moore BK, Rhodes BF (1992) Influence of selected variables on adhesion testing. Dent Mater 8:265–269
Schilke R, Bauss O, Lisson JA, Schuckar M, Geurtsen W (1999) Bovine dentin as a substitute for human dentin in shear bond strength measurements. Am J Dent 12:92–96
Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechts P, Van Meerbeek B (2007) Systematic review of the chemical composition of contemporary dental adhesives. Biomater 28:3757–3785. https://doi.org/10.1016/j.biomaterials2007.04.044
Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL (2011) State of the art of self-etch adhesives. Dent Mater 27:17–28. https://doi.org/10.1016/j.dental.2010.10.023
Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S (2004) Collagen degradation by host-derived enzymes during aging. J Dent Res 83:216–221. https://doi.org/10.1177/154405910408300306
De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdebakker G, Van Meerbeek B (2009) Inhibition of enzymatic degradation of adhesive-dentin interfaces. J Dent Res 88:1101–1106. https://doi.org/10.1177/0022034509346952
Garcia-Godoy F, Krӓmer N, Feilzer AJ, Frankenberger R (2010) Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dent Mater 26:1113–1118. https://doi.org/10.1016/j.dental.2010.07.012
Powers JM, Wataha JC (2013) Dental materials: properties and manipulation chapter 2 properties of materials 14–25, 10th ed. Missouri:Mosby, Elsevier Inc., USA.
Kitasako Y, Burrow MF, Nikaido T, Tagami J (2000) The influence of storage solution on dentin bond durability of resin cement. Dent Mater 16:1–6. https://doi.org/10.1016/S0109-5641(99)00061-5
Loguercio AD, Uceda-Gomez N, Carrilho MR, Reis A (2005) Influence of specimen size and regional variation on long-term resin-dentin bond strength. Dent Mater 21:224–231. https://doi.org/10.1016/j.dental.2004.03.012
Tsuchiya K, Takamizawa T, Barkmeier WW, Tsubota K, Tsujimoto A, Berry TP, Erickson RL, Latta MA, Miyazaki M (2016) Effect of functional monomer (MDP) on the enamel bond durability of single-step self-etch adhesives. Eur J Oral Sci 124:96–102. https://doi.org/10.1111/eos.12232
Funding
This work was supported in part by Grants-in-Aid for Scientific Research, 16K11565, 17K11716 and 17K17141, from the Japan Society for the Promotion of Science. This project was also supported in part by the Sato Fund and by a grant from the Dental Research Center of the Nihon University School of Dentistry, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study does not contain any studies with human participants and subjects or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Suzuki, S., Takamizawa, T., Imai, A. et al. Bond durability of universal adhesive to bovine enamel using self-etch mode. Clin Oral Invest 22, 1113–1122 (2018). https://doi.org/10.1007/s00784-017-2196-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2196-x