Abstract
Sustainable betalain pigments extracted from beetroots were used as eco-friendly film coating over aluminium surface. The film coating was examined and verified by corrosive 1.0 M HCl solution. Characterization of chemical structure of the betalain pigments was done using spectroscopic attenuated total reflectance Fourier transform infrared (ATR-FTIR) and UV–Vis technique. Scanning electron microscope technique was used to demonstrate the eco-friendly film coating before and after been examined via corrosive 1.0 M HCl solution. Various operating conditions were studied to meet highest inhibition efficiency values such as betalain concentration, operating temperature and turbulent flow, and maximum inhibition efficiency = 98.4% was displayed. Multiple adsorption isotherms like Langmuir, Temkin, and Freundlich were used effectively to determine equilibrium constants and adsorption parameters. Dynamic investigations revealed a decline of rate of corrosion (RC) and rise of activation energy values (Ea) at higher betalain concentrations. Thermodynamic investigations revealed physisorption process, exothermic enthalpy, and low entropic values of betalain on aluminium surface. The noticeable specifications of low cost, abundance and sustainability, environmentally friendly, and high technical feasibility of betalain pigments adapt them to be the futuristic outstanding sustainable film coating for metal surfaces.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Recently, the concept of green, sustainable, and environmental friendly materials became a trend that captured the attention of several researchers [1]. Nowadays, one of the most fundamentals of sustainability is the growing interests of "referring back to nature" and to the green natural materials acquired from the extracts of various plant leaves, stems, vegetable skins, and roots for using them in artificial sectors [2]. Basically, one of these successful implementations inspired by nature is the use of such green materials as a protective layer on metal surfaces against severe corrosive conditions [3]. Most of the synthetic corrosion inhibitors are not only can cause toxicity to living organisms, but also can cause damage to the environment [4]. Furthermore, the low cost of the green inhibitors extracted from sustainable sources, their high inhibition efficiency values to protect the metal surface against severe corrosive conditions, and their environmental friendliness make them unusual alternatives for many toxic and expensive inhibitors [5]. Many studies have confirmed the high inhibition efficiency of promising green inhibitors for metal surfaces like; sustainable onion skin extract [6], Eucalyptus leaves extract [7], and beetroot extract [8]. On the other hand, betalain pigments are available in the tuberous part of beetroots (Beta vulgaris L.). They can be subdivided into two components: betacyanins responsible for red-violet pigments and betaxanthins responsible for orange-yellow colouring [9]. Betanin (betanidin 5-O-β-D-glucoside) is the most abundant betacyanins and is the major constituent of the red-violet pigments and approved for use as natural colourant in food products, cosmetics and pharmaceuticals [10, 11]. In fact, more than 200000 tonnes of beetroots rich in betalain pigments are produced in Western Europe per annum [12], most of which are peeled and its skin are wasted. In our study, beetroot disposable waste and sustainable source were used to extract betalain pigments, which then were used as potential green and environmentally friendly film coating for aluminium surface. The green coating was examined with severe corrosive 1.0 M HCl solution, and different optimization conditions were investigated to affirm its high inhibition efficiency. Moreover, various adsorption isotherms investigated equilibrium constants and adsorption parameters. Dynamic and thermodynamic parameters were also evaluated. Eventually, surface topology of the coated and uncoated aluminium surfaces was examined and evaluated using scanning electron microscope technique.
Experimental
Materials
Fresh beetroots was purchased from local market in Irbid city (Jordan) and well washed with distilled water, air-dried at ambient temperature. Ultra-gradient ethanol was purchased from Carlo Erba (France). Hydrochloric acid HCl (37%) was purchased from Carlo Erba (France). Deionized water was used in all experiments.
Extraction of betalain pigment
The fresh beetroots were thoroughly washed with deionized water, air-dried at ambient temperature, peeled and cut into small slices. The beetroot slices were soaked in 200 mL of 1:1 ethanol–water mixture at pH = 6.5 and 20 °C for 24 h until the red-violet betalain pigments were extracted. The solvent was evaporated at 40 °C, and betalain pigments were collected [13]. Different betalain pigments concentrations were prepared from stock solution (i.e. 300, 600, 900, and 1200 ppm) and stored until used.
Surface of aluminium
Rectangular sheets of aluminium with dimension of length = 30 cm, width = 10 cm, and height = 0.15 cm were abraded with emery paper up to 1200 grade and used to make smaller aluminium coupons. The purity of aluminium sheet = 99.7%. The Al-sheet was cut to smaller coupons with dimensions of length = 3 cm, width = 1 cm and height = 0.15 cm. The coupons were washed with deionized water and ethanol, respectively, and allowed to dry at ambient temperature. Later on, betalain pigments were adsorbed on the coupon's surface and the inhibition efficiency of the coating process was tested and evaluated using severe corrosive 1.0 M HCl solution. Aluminium coupons were soaked in different betalain concentrations of solutions (i.e. 300, 600, 900, and 1200 ppm) for one hour at different temperatures (i.e. 10, 20, and 30 °C) and different turbulent flows (i.e. 50, 100, 200, 300, 400, 500 rpm). After time completion, coupons were removed from solution and allowed to dry overnight and stored. Later on, the coupons were exposed to severe corrosive 1.0 M HCl solution for one hour, and the inhibition efficiency value (%IE) was determined as follows:
where \(w_{0}\) and \(w_{i}\) were the weight loss (in kg) of naked and coated aluminium coupons, respectively. The optimum conditions used for best inhibition efficiency values are available in Fig. 1.
Characterization techniques
Attenuated total reflectance Fourier transform infrared: Bruker alpha FTIR spectrometer armed with attenuated total reflectance unit was used to record the well-deconvoluted vibrational spectra in the 4000–400 cm−1 range. UV–Vis spectrophotometer: Shimadzu UV–Vis 2401 spectrophotometer was used to record the change of absorbance in 200–800 nm range. Heating control device was supplied with instrument to monitor absorbance change at elevated temperatures. Scanning electron microscope: Images of the aluminium surface were monitored at different magnifications using FEI Quanta model scanning electron microscope. Calibrated Radwag Wagi Elektroniczne (Poland) Semi-micron balance with a precision of 10 μg was adjusted and used to determine the weight loss measurements. All measurements were carried out in triplicate values, averaged, and used as ± SD values.
Results and discussion
Structural characterization of betalain pigments
Natural beetroot extracts of betalain pigments consist of betacyanins and betaxanthins components; betacyanins components are red–purple colour and consist of betanin and betanidin, whereas the betaxanthins components are yellow-orange and consist of indicaxanthin and betalamic acid, respectively (Fig. 1a). The chemical structure of betalain pigments was represented using attenuated total reflectance Fourier transform infrared spectrum as described in Fig. 2b; the stretching frequency of 3414 cm−1 showed the presence of secondary amine group (N–H). The O–H stretching frequency appeared as broad band at a maximum of 3354 cm−1, and the aromatic C–H stretching appeared at 3103 cm−1. The bands at 2977, 2929, and 2874 cm−1 indicated aliphatic C–H symmetry in stretching mode existing in glycosidic groups attached to betanin subgroups. The carbonyl group (C=O) of aldehyde in betalamic acid appeared at 1723 cm−1. The band at C=N bond existing in betanin, betanidin, and indicaxanthin pigments appeared at 1624 cm−1. The asymmetric and symmetric COO− frequencies existing in the four pigments appeared at 1610 and 1406 cm−1, respectively. The conjugated C=C band existing in the four pigments appeared at 1595 cm−1. Finally, the COC stretching of the glycosidic group of betanin appeared at 1044 cm−1, which manifested the backbone structure of the betalain pigments [14, 15].
Likewise, the different constituents of the betalain pigments were qualitatively determined using UV–Vis spectrophotometry as shown in Fig. 2c. Different absorption peaks in the range of 400–600 nm for different concentrations (300, 600, 900, or 1200) ppm were observed. The yellow-orange betaxanthins show one shoulder peak at 454 nm, which corresponds to betalamic acid pigment, and one sharp peak at 482 nm, which corresponds to indicaxanthin pigment. On the other hand, the red–purple betacyanins show two λmax peaks at 535 and 542 nm, which correspond to betanin and betanidin pigments, respectively. Such sharp peaks result from π − π* transitions of the four different betalain pigments, namely betanin, betanidin, betalamic acid and indicaxanthin coexisting with each other [16,17,18,19].
Optimum adsorption conditions of betalain pigments
The optimization of the different conditions meant to achieve optimum conditions for best inhibition of aluminium surface against severe corrosive HCl solution. For this purpose, different parameters such as betalain concentration, operating temperature, and turbulent flow were optimized for maximum inhibition efficiency values against severe corrosive HCl solution. Table 1 illustrates different green inhibitors for different metals with their maximum inhibition efficiency values.
The change of inhibition efficiency with betalain concentration is shown in Fig. 3a. The %IE value exponentially increased as betalain concentration increased. Clearly, the increased betalain concentration implied better coverage of aluminium surface, lower weight loss, better inhibition, and better resistance against corrosive HCl molecules. Ultimate inhibition efficiency was obtained at 1200 ppm with 98.4% value. Apparently, the intermolecular forces of betalain molecules with aluminium surface were much more favourable than between betalain molecules with themselves, which pave the way for layer-by-layer adsorption, better geometrical layer formation, and hence better resistance of the layer against corrosive HCl molecules. On the other hand, the effect of operating temperature had an adverse effect on the adsorption process (Fig. 3b), as temperature increased the %IE values deceased to 69.5% for the same inhibitor concentration. This can be explained due to large kinetic energy of aluminium surface that dismisses the betalain molecules away from aluminium surface leading to poorer coverage of the surface and hence poor inhibition against severe HCl molecules. Moreover, turbulent flow is an additional parameter that plays essential role in adsorption of the inhibitor molecules on the surface of aluminium. It measures the frequent structural settlement of the inhibitor on the surface of aluminium, and how successful the formed layer can protect the metal surface against corrosive HCl molecules. Obviously, the increase of turbulent flow led to increased inhibition efficiency (93.7%) up to 200 rpm, which then was sharply decreased to 46.7% at 500 rpm (Fig. 3c). In the range of 50–200 rpm, the inhibitor molecules gain enough kinetic energy to successfully settle on the metal surface, and the frequent speed of solution facilitated the adsorption of the inhibitor on the metal surface. However, in the range of 300–500 rpm a significant reduction in %IE due to high turbulent flow was obtained [27]. High turbulent flow implied high kinetic energy of inhibitor molecules during together with irregular flow of solution that allow desorption and encourage dislocation of inhibitor molecules away from metal surface and hence poor film coating and poor inhibition efficiency value.
Adsorption properties
Different adsorption isotherms were evaluated to fit to our data such as Langmuir, Temkin, and Freundlich, [28,29,30,31,32,33,34] respectively. Langmuir isotherm was described by the following equation;
where KL was the equilibrium constant of adsorption process and C was the betalain pigments concentration used (in ppm). The θ value is the fraction of surface covered by adsorbed molecules and can be determined as follows;
where \(w_{0}\) and \(w_{i}\) were the weight losses (in kg) of metal surface in the absence and in the presence of betalain pigments, respectively. The linearized form of Langmuir isotherm was as follows:
The linear plot of C/θ versus C can be used to determine equilibrium constant (KL) values from reciprocal of intercept value. On the other hand, Temkin adsorption isotherm equation was as follows:
where α-value was the molecular interaction parameter, KT was the binding constant of the adsorbate with the metal surface. The linear form of Temkin model is:
The linear plot of θ versus ln C can be used to determine α and KL from slope and intercept values, respectively. Freundlich adsorption isotherm equation was as follows:
where the 1/n value describes how easy the adsorption is, easy adsorption when 0 < 1/n < 1, moderate when 1/n = 1 or difficult adsorption when 1/n > 1. KF was the binding constant that describes how strong the betalain pigments bind to the aluminium surface. The 1.n and KF constant values were determined from slope and intercept of the linearized form of Freundlich as follows:
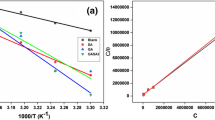
Delicate examination of the various adsorption isotherms of our data revealed linear behaviour as described in Fig. 4a–c. Different equilibrium constant values and adsorption parameters that describe the adsorption of betalain pigments on the surface of aluminium are summarized in Table 2.
Adsorption isotherms of betalain pigments on aluminium surface: a Langmuir isotherm at different temperatures, b Temkin isotherm at different temperatures, c Freundlich isotherm at different temperatures, d dynamic parameters; ln RC versus reciprocal temperature (1/T) at different concentrations, e thermodynamic parameters; ln KL versus reciprocal temperature (1/T)
Obviously, the equilibrium constant values (KL) decreased with increasing temperature, which indicates that desorption process dominates at elevated temperatures. Similarly, the Temkin and Freundlich binding constants (KT and KF) were in favour of poor adsorption of betalain pigments on the surface of aluminium at elevated temperature. The α-values were very close to zero value, which indicated good molecular interaction parameter of adsorbate with adsorbent [35]. Moreover, the values of 1/n were in the range of 0.1–0.3, which strongly indicates excellent and easy adsorption process of betalain pigments on the surface of aluminium [36]. Conclusively, the equilibrium constant values and the different adsorption parameters were in favour of successful adsorption and high affinity of betalain pigments to aluminium surface.
Dynamic parameters
The rate of corrosion (RC) is a very crucial parameter that can describe the success of inhibition process of aluminium surface against corrosive HCl. The rate of corrosion was determined from the following relation:
where W was the weight loss of an aluminium coupon, W/V value was the normalized weight loss (in kg/m3) per coupon exposed to corrosive 1.0 M HCl solution for one hour. The rate of corrosion was determined for each W/V value, and the apparent activation energy (Ea) for corrosion process was determined from the Arrhenius equation [37, 38]:
The apparent activation energy was determined from the slope of ln RC versus reciprocal temperature (1/T) plot, and R was the universal gas constant (8.314 J/mol.K). Figure 4d and Table 3 illustrate the dynamic parameters of the adsorption of betalain inhibitor on the aluminium surface. The rate of corrosion increased as temperature increased at different inhibitor concentrations. However, the apparent activation energy increased fourfold at 1200 ppm with respect to 300 ppm. This clearly indicated that layer-by-layer arrangement of the inhibitor on the surface of aluminium occurred, which allowed better coating and consistent inhibition layers, and hence higher resistance against corrosive HCl molecules.
Thermodynamic investigations
Thermodynamic parameters such as ΔG°, ΔH°, or ΔS° describe the type of sorption process (i.e. physisorption or chemisorption), energy enthalpy change, and entropy changes during the adsorption of betalain pigments on the surface of aluminium. Gibbs free energy (ΔG°) can be calculated from the relations [39, 40];
Where KL is the Langmuir equilibrium constant determined from Eq. 4. R is the universal gas constant (8.314 J/mol. K), and T is the operating temperature of adsorption (in K). As shown in Table 4, the Gibbs energy (ΔG°) values of the adsorption of betalain pigments on the aluminium surface demonstrated − 31 kJ/mol for the different temperatures. This clearly indicated that the adsorption of betalain pigments on the surface of aluminium was of physisorption nature [41].
Substitution of ΔG = ΔH − ΔS and rearrangement of equation 9 could be used to yield a linearized form of the equation, which was used to determine the ΔH° and ΔS° thermodynamic parameters as follows [42]:
The plot of ln KL versus 1/T can result with a straight line, for which the enthalpy change (ΔH°) and entropy change (ΔS°) can be determined from slope \(\left( { - \frac{{\Delta H^{^\circ } }}{R}} \right)\) and intercept \(\left( {\frac{{\Delta S^{^\circ } }}{R} - \ln 55.5} \right)\), respectively. The ΔH° and the ΔS° values were − 37.0 kJ/mol and − 20.5 J/mol.K, respectively, as described in Table 4 and Fig. 4e. Negative enthalpy change suggested exothermic nature of the metal dissolution process [43]. Furthermore, negative sign of entropy change suggested less entropy of betalain molecules adsorbed on the surface with respect to free betalain molecules in the solution [42]. Conclusively, the dynamic and thermodynamic parameters suggest successful adsorption of betalain molecules on the surface of aluminium based on electrostatic interaction of partially negative oxygen atoms of betalain with partially positive aluminium ions, leading to the formation of successful protective layers of betalain pigments.
Surface morphology
Morphologic changes of metal surfaces monitored by scanning electron microscope technique gained a great resonance and global attention due to microscale and nanoscale images that describe the corrosion punctures and scratches pre- and post-exposure to severe corrosive 1.0 M HCl solution. Furthermore, it can show how firm the coating material can resist acidic or saline environment [44,45,46]. The as-received aluminium coupon and film-coated aluminium surface pre- and post-exposure to 1.0 M HCl solution are explicitly shown in Fig. 5. Figure 5a demonstrates the great damage of the as-received aluminium surface (which cannot be seen by naked eye) due to bad humid storage, accidents through transportation, and atmospheric erosion. Around 2–10 μm microsized corrosion pitting, punctures, and cracks appeared on the surface. Such pitting could widen and become larger in time period due to atmospheric erosion. On the other hand, film coating of aluminium surface using 900 ppm betalain pigments can be seen in Fig. 4b, c, respectively. Obviously, the treatment with betalain pigment through film coating could yield a smooth surface of aluminium with no cracks, punctures, or corrosion pitting. Entire corrosion pitting and cracks that appeared in Fig. 4a have been well protected and cured by the betalain film coating. This gives a nice clue that betalain molecules were able to act as filler and fill the punctures and cracks appeared on aluminium surface, and to establish consistent and smooth film coating on the aluminium surface. However, the firmness and the durability of the film coating must be exposed to severe corrosive environment to examine and evaluate it. Therefore, the film-coated aluminium coupons were exposed to severe corrosive 1.0 M HCl solution for one hour (Fig. 4d, e). Apparently, the outcomes were stimulating, the uniformity and smoothness of the film coating did not alter, the surface was slightly scratched, and number of corrosion pitting and punctures was strongly minimized. These remarks recommend that betalain pigments coating was firm and durable during the exposure to severe and corrosive 1.0 M HCl solution and hence provides evidence on the technical feasibility for the use of betalain pigments as consistent and effective film coating of aluminium surface.
Conclusions
Betalain pigments extracted from green beetroots were used as sustainable eco-friendly film coating for aluminium surface. Chemical components of different functional groups forming betalain pigments were characterized using attenuated total reflectance Fourier transform infrared techniques, and the four pigments: betanin, betanidin betalamic acid, and indicaxanthin, were identified using UV–Vis spectrophotometry. Surface morphology of film coating of betalain pigments over aluminium surface revealed smooth, well-cured and filler-like coating, whereas after been exposed to corrosive 1.0 M HCl firm, durable and consistent film coatings were obtained. Various operating conditions examined to meet highest inhibition efficiency values revealed optimum conditions as follows: betalain concentration = 1200 ppm, operating temperature = 10 °C, turbulent flow = 200 rpm, and extreme inhibition efficiency = 98.4%. Several adsorption isotherms revealed equilibrium constant values and adsorption parameters in favour of the adsorption process. Dynamic investigations disclosed decay of rate of corrosion (RC) and increase of activation energy values (Ea) at high betalain concentration, Moreover, the thermodynamic parameters showed physisorption process, exothermic enthalpy, and low entropy properties. The extraordinary advantages of abundance, eco-friendly, low cost, sustainability, and technical feasibility of betalain pigments adapt it to be an uprising sustainable film coating for aluminium surfaces.
References
Chaubey N, Savita AQ, Chauhan DS, Quraishi MA (2021) Frontiers and advances in green and sustainable inhibitors for corrosion applications: a critical review. J Mol Liq 321:114385
Ahanotu CC, Onyeachu IB, Solomon MM, Chikwe IS, Chikwe OB, Eziukwu CA (2020) Pterocarpus santalinoides leaves extract as a sustainable and potent inhibitor for low carbon steel in a simulated pickling medium. Sustain Chem Pharm 15:100196
Arzt E, Quan H, McMeeking RM, Hensel R (2021) Functional surface microstructures inspired by nature – From adhesion and wetting principles to sustainable new devices. Prog Mater Sci 119:100778
Saeed MT, Saleem M, Niyazi AH et al (2020) Carrot (Daucus Carota L.) peels extract as an herbal corrosion inhibitor for mild steel in 1M HCl solution. Mod Appl Sci 14(2):97–112
Salleh SZ, Yusoff AH, Zakaria SK, Taib MAA, Seman AA, Masri MN, Mohamad M, Mamat S, Sobri SA, Ali A, Teo PT (2021) Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. J Cleaner Prod 304:127030
Addi BA, Addi AA, Shaban A, Addi EHA, Hamdani M (2021) Comparative electrochemical and spectroscopic analysis of the inhibition effect of molybdates and boiled red onion extract on the corrosion of tin in acidic solution. J Adhes Sci Technol 35(8):856–872. https://doi.org/10.1080/01694243.2020.1826827
Al-Akhras N, Mashaqbeh Y (2021) Potential use of eucalyptus leaves as green corrosion inhibitor of steel reinforcement. J Build Eng 35:101848
Ashassi-Sorkhabi H, Es’haghi M (2009) Corrosion inhibition of mild steel in hydrochloric acid by betalain as a green inhibitor. J Solid State Electrochem 13(8):1297–1301
Alejandra Guerrero-Rubio M, Hernández-García Samanta, García-Carmona Francisco, Gandía-Herrero Fernando (2021) Biosynthesis of a novel polymeric chitosan-betaxanthin and characterization of the first sugar-derived betalains and their effects in the in vivo model caenorhabditis elegans. Carbohydr Polym 252:117141
Das Trishitman PS, Negi NK Rastogi (2021) Concentration of beetroot juice colorant (betalains) by forward osmosis and its comparison with thermal processing. LWT 145:111522
Montiel-Sánchez M, García-Cayuela T, Gómez-Maqueo A, García HS, Pilar Cano M (2021) In vitro gastrointestinal stability, bioaccessibility and potential biological activities of betalains and phenolic compounds in cactus berry fruits (Myrtillocactus geometrizans). Food Chem 342:128087
Sharma R, Oberoi HS, Dhillon GS (2016) Fruit and vegetable processing waste: renewable feed stocks for enzyme production. In: Dhillon S, Kaur S (eds) Agro-industrial wastes as feedstock for enzyme production. Academic Press, pp 23–59
Ravichandran K, Saw NMMT, Mohdaly AAA, Gabr AMM, Kastell A, Riedel H, Cai Z, Knorr D, Smetanska I (2013) Impact of processing of red beet on betalain content and antioxidant activity. Food Res Int 50(2):670–675
Sutariya B, Saraf M (2017) Betanin, isolated from fruits of opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol 198(23):432–443
Li S, Bin Mu, Wang X, Kang Y, Wang A (2020) Fabrication of eco-friendly betanin hybrid materials based on Palygorskite and Halloysite. Materials 13:4649
Zhang D, Lanier SM, Downing JA, Avent JL, Lum J, McHale JL (2008) Betalain pigments for dye-sensitized solar cells. J Photochem Photobiol A 195:72–80
Quin C, Clark AE (2007) DFT characterization of the optical and redox properties of natural pigments relevant to dye-sensitized solar cells. Chem Phys Lett 438:26–30
Calogero G, Di Marco G, Cazzanti S, Caramori S, Argazzi R, Di Carlo A, Alberto Bignozzi C (2010) Efficient dye-sensitized solar cells using red turnip and purple wild Sicilian prickly pear fruits. Inter J Mol Sci 11:254–267
García-Salinas MJ, Ariza MJ (2019) Optimizing a simple natural dye production method for dye-sensitized solar cells: examples for betalain (bougainvillea and beetroot extracts) and anthocyanin dyes. Appl Sci 9:2515
Belakhdar A, Ferkous H, Djellali S, Sahraoui R, Lahbib H, Amor YB, Erto A, Balsamo M, Benguerba Y (2020) Computational and experimental studies on the efficiency of Rosmarinus officinalis polyphenols as green corrosion inhibitors for XC48 steel in acidic medium. Coll Surf A: Physicochem Eng Asp 606:125458
Zaher A, Chaouiki A, Salghi R, Boukhraz A, Bourkhiss B, Ouhssine M (2020) Inhibition of mild steel corrosion in 1M hydrochloric medium by the methanolic extract of Ammi Visnaga L. lam seeds. Int J Corros 2020:1–10
Farahati R, Morteza Mousavi-Khoshdel S, Ghaffarinejad A, Behzadi H (2020) Experimental and computational study of penicillamine drug and cysteine as water-soluble green corrosion inhibitors of mild steel. Progress Organ Coat 142:105567
Zhao Q, Guo J, Cui G, Han T, Wu Y (2020) Chitosan derivatives as green corrosion inhibitors for P110 steel in a carbon dioxide environment. Colloids Surf B Biointerfaces 194:111150. https://doi.org/10.1016/j.colsurfb.2020.111150
Chung I-M, Malathy R, Kim S-H, Kalaiselvi K, Prabakaran M, Gopiraman M (2020) Ecofriendly green inhibitor from Hemerocallis fulva against aluminum corrosion in sulphuric acid medium. J Adhes Sci Technol 34(14):1–24
Fares MM, Masadeh KH (2015) Glutamine-reinforced silica gel microassembly as protective coating for aluminium surface. Mater Chem Phys 162:124–130
Nathiya RS, Perumal S, Murugesan V, Raj V (2019) Evaluation of extracts of Borassus flabellifer dust as green inhibitors for aluminium corrosion in acidic media. Mater Sci Semicond Process 104:104674
Obat IB, Onyeachu OB, Umoren SA (2019) Alternative corrosion inhibitor formulation for carbon steel in CO2-saturated brine solution under high turbulent flow condition for use in oil and gas transportation pipelines. Corros Sci 159:108140
Laidler K, Meiser J, Sanctuary B (2000) Physical chemistry, 4th edn. Haughton Mifflin company, NY, pp 933–938
Rashidi NA, Yusupa S, Borhan A (2016) Isotherm and thermodynamic analysis of carbon dioxide on activated carbon. Procedia Eng 148:630–637
Ituen EB, Akaranta O, James OA (2015) 5-Hydroxytryptophan: a novel eco-friendly corrosion and oilfield microbial inhibitor. Soc Pet Eng. https://doi.org/10.2118/178370-MS
Fares MM, Maayta AK, Al-Mustafa JA (2013) Synergistic corrosion inhibition of aluminum by polyethylene glycol and ciprofloxacin in acidic media. J Adhes Sci Technol 27(23):2495–2506
Gerengi H, Schaefer K, Sahin HI (2012) Corrosion-inhibiting effect of Mimosa extract on brass-MM55 corrosion in 0.5 M H2SO4 acidic media. J Ind Eng Chem 18:2204–2210
Fares MM, Maayta AK, Al-Qudah MA (2013) Polysorbate20 adsorption layers below and above the critical micelle concentration over aluminum; cloud point and inhibitory role investigations at the solid/liquid interface. Surf Interface Anal 45:906–912
OZa BN, Sinha RS (1982) Thermometric study of corrosion behaviour of high strength Al-Mg alloy in phosphoric acid in presence of halides. Tanstact Saest 17(4):281–285
Umoren SA, Ogbobe O, Igwe IO, Ebenso EE (2008) Inhibition of mild steel corrosion in acidic medium using synthetic and naturally occurring polymers and synergistic halide additives. Corros Sci 50:1998–2006
Yonghong Wu (2017) The removal of methyl orange by periphytic biofilms: equilibrium and kinetic modeling. In: Periphyton. Functions and application in environmental remediation. Elsevier, pp 367–387. https://doi.org/10.1016/B978-0-12-801077-8.00016-8
Ogunleye OO, Arinkoola AO, Eletta OA, Agbede OO, Osho YA, Morakinyo AF, Hamed JO (2020) Green corrosion inhibition and adsorption characteristics of Luffa cylindrica leaf extract on mild steel in hydrochloric acid environment. Heliyon 6(1):e03205
Herrag L, Hammouti B, Elkadiri S, Aouniti A, Jama C, Vezin H, Bentiss F (2010) Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: experimental and theoretical investigations. Corros Sci 52:3042–3051
Aslam R, Mobin M, Aslam J, Lgaz H (2018) Sugar based N,N′-didodecyl-N,N′ digluconamideethylenediamine gemini surfactant as corrosion inhibitor for mild steel in 3.5% NaCl solution-effect of synergistic KI additive. Sci Rep 8:3690. https://doi.org/10.1038/s41598-018-21175-6
Fares MM, Maayta AK, Al-Qudah MM (2012) Pectin as promising green corrosion inhibitor of aluminum in hydrochloric acid solution. Corros Sci 60:112–117
Outirite M, Lagrenée M, Lebrini M, Traisnel M, Jama C, Vezin H, Bentiss F (2010) AC impedance, X-ray photoelectron spectroscopy and density functional theory studies of 3,5-bis(n-pyridyl)-1,2,4-oxadiazoles as efficient corrosion inhibitors for carbon steel surface in hydrochloric acid solution. Electrochim Acta 55:1670–1681
Fares MM, Maayta AK, Al-Mustafa JA (2012) Corrosion inhibition efficacy of iota-carrageenan natural polymer on aluminum in presence of zwitterion mediator in HCl media. Corros Sci 65:223–230
Dehri I, Ozcan M (2006) The effect of temperature on the corrosion of mild steel in acidic media in the presence of some sulphur-containing organic compounds. Mater Chem Phys 98:316–323
Wei W, Liu Z, Liang C, Han G-C, Han J, Zhang S (2020) Synthesis, characterization and corrosion inhibition behavior of 2-aminofluorene bis-Schiff bases in circulating cooling water. RSC Adv 10:17816
Ma Y, Han F, Li Z, Xia C (2016) corrosion behavior of metallic materials in acidic-functionalized ionic liquids. ACS Sustain Chem Eng 4(2):633–639
Chugh B, Singh AK, Thakur S, Pani B, Lgaz H, Chung I-M, Jha R, Ebenso EE (2020) comparative investigation of corrosion-mitigating behavior of thiadiazole-derived bis-schiff bases for mild steel in acid medium: experimental Theoretical, and Surface Study. ACS Omega 5(23):13503–13520
Acknowledgements
Authors wish to acknowledge Jordan University for Science & Technology, Deanship of Research, Project Number 20200117 for financial support and facilities.
Funding
This study was funded by Jordan University for Science & Technology, Deanship of Research (Grant Number = 20200117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author M.M. Fares has received research grant from Jordan University for Science & Technology. The authors declare that they have no conflict of interest.
Additional information
Handling Editor: M. Grant Norton.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fares, M.M., Bani-Domi, A. Sustainable betalain pigments as eco-friendly film coating over aluminium surface. J Mater Sci 56, 13556–13567 (2021). https://doi.org/10.1007/s10853-021-06179-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06179-4