Abstract
Spirulina, blue green algae is a rich source of proteins and vitamins with excellent antioxidant properties. Sunova spirulina powder an effective, green corrosion inhibitor was used to evaluate its inhibition efficiency towards mild steel in 1 M HCl medium. Weight loss studies of mild steel showed an inhibition efficiency of 96% for 600 ppm concentration of inhibitor solution and 12 h of immersion period at 303 K. The percentage of inhibition efficiency increased with a step up of 10 K raise in temperature from 303 to 333 K and thereafter decreased. The results obtained were further validated by inductively coupled plasma optical emission spectrometric (ICP-OES) measurements and electrochemical techniques that included Tafel polarisation, linear polarisation and AC impedance studies. Potentiodynamic polarisation study marked the inhibitor to be a mixed type inhibiting both cathodic and anodic reactions. The adsorption studies proved that the adsorption process was spontaneous and followed Langmuir adsorption isotherm. The thermodynamic activation and adsorption parameters calculated showed that the mechanism of inhibition involved a physisorption process initially and then it slightly shifted towards chemisorption process at higher temperature. The protective layer formed on the metal surface was studied using FTIR and SEM. The complex formation between the Fe2+ and the active constituents of the spirulina extract was verified using UV visible spectra and fluorescence spectra. The effect of inhibitor concentration and temperature on corrosion rate was tested statistically using two-way analysis of variance (ANOVA) technique.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Mild steel or low carbon steel due to its strength, malleability, weldability and optimum mechanical properties with potentially low cost is employed in diverse applications that extend from construction, automobile parts to pipes, sheets, wires or tubes. However, metals (iron/steel) have a natural tendency to revert back to the combined state by reacting with the surrounding atmosphere resulting in rust and scale formation on its surface. So a periodic chemical cleaning of any steel equipment is necessary for its efficient functioning. The chemical plants, boilers, oil and gas pipelines make use of acid solutions for cleaning, descaling, pickling and oil well acidizing processes [1, 2]. The cleaning processes may involve several litres of acid solutions and will be repeated every 5 to 8 years. Once the scale is removed and the bare metal is exposed, the metal rapidly undergoes a classic acid corrosion leading to further dissolution of the metal [2]. This can be prevented by using corrosion inhibitor blended acid solutions.
The corrosion inhibitors vary widely from inorganic complexes, rare earth elements, organic compounds containing elements such as N, O, P and S in their heterocyclic rings, to macrocyclic compounds like porphyrin, phthalocyanin, polymers and surfactants [3]. However, synthetic inorganic and organic corrosion inhibitors are found to cause environmental pollution and hence researchers are looking for green inhibitors [4]. A vast majority of plant extracts from different parts of the world are reported to possess excellent anticorrosive properties [5]. Green inhibitors are nontoxic, easily available, and economically viable and some of them are commercially used as alternatives for the chemical inhibitors [6]. The biofilm formed by the beneficial bacterial microorganism is also utilised as one of the novel corrosion control strategies [7]. Microalgae, an inhabitant of fresh water and marine system are diverse group of organisms with potential physiological and biochemical characteristics [8]. It is referred to as the green gold of the future as it comprises large variety of unexplored group of organisms whose utility is virtually untapped [9]. Spirulina platensis, a blue green algae is reported to be an excellent source of proteins [10], fatty acids [11], photosynthetic pigments [12], vitamins and phenolics [13] and showed high antioxidant properties [14]. It is commercially produced so as to be used as a popular food supplement. Spirulina is also projected as living cell factory for the biofuel production and is widely used in food, aquaculture and pharmaceutical industries [8]. Rajendran et al. have studied the corrosion inhibition efficiency of spirulina for mild steel and mild steel coated zinc in artificial saliva [15]. Sribharathy and Susai Rajendran have reported the corrosion inhibition efficiency of an aqueous extract of spirulina and sodium metavanadate solution [16]. Kamal and Sethuraman have studied the corrosion inhibition efficiency of Spirulina platensis powder extracted from absolute ethanol for mild steel at different temperatures in both 1 M hydrochloric acid and 1 M sulphuric acid [17]. This investigation has brought to light another important application of this microalga. The alcoholic extract of the Spirulina platensis with 500 ppm concentration showed inhibition efficiency of 76% for mild steel by impedance studies. Xhanari et al. have reviewed the effectiveness of beta carotene in mitigation of corrosion of aluminium alloy [18]. Badawy et al. have explained the corrosion inhibition behaviour of beta carotene obtained from algal secretion. The inhibitor solution with 50 ppm concentration when tested for Cu–Al–Ni alloys in sulphide polluted chloride solutions showed 95% inhibition efficiency [19].
The present study was carried out using the acid extract of commercially available Sunova Spirulina (SS) capsules to explore its enhanced corrosion inhibition efficiency. The composition of SS capsule as claimed by the company in 100 g of the powder is 60 g of protein, 5 µg of vitamin B12, < 200 mg of beta carotene and slightly higher than 30 mg of iron [20]. The corrosion study was carried out using weight loss, electrochemical and spectrophotometric methods. The surface morphology was studied using SEM. The inhibitor film formed on the metal surface was characterised using IR, UV–Vis spectrophotometer and spectrofluorophotometer.
2 Experimental
2.1 Preparation of the Inhibitor Solution
1 g of the Sunova Spirulina (SS) powder was refluxed with 100 mL of 1 M hydrochloric acid at 353 K for 3 h and was kept overnight. The clear, supernatant liquid was then filtered and the filtrate was made up to 500 mL in a standard flask using 1 M hydrochloric acid. This was used as the stock solution for the corrosion inhibition efficiency study for mild steel.
2.2 Preparation of the Mild Steel Specimen
The mild steel coupons used for the present study had the following percentage composition: Fe-99.62, Mn-0.184, W-0.042, Al-0.032, Cu-0.021, C-0.017, P-0.013, Ni-0.010, Cr-0.010, Mo-0.008, S-0.008, V-0.008, Si-0.007, Nb-0.006, Pb-0.005, N-0.005, Ti-0.004. The specimen was cut into 5 × 1 × 0.2 cm dimension, mechanically polished and the surface abraded using fine grade emery sheets. The polished metal was degreased using acetone, dried and placed in a desiccator.
2.3 Weight Loss Study
The weight loss studies were carried out as per ASTM G31 procedure. Accurately weighed metal coupons were immersed in 100 mL beakers containing 1 M hydrochloric acid with and without the inhibitor for a stipulated period. The immersion time was varied from 3, 6, 12 and 24 h. The experiment was repeated by varying the temperature from 303 to 343 K for an immersion period of 30 min. After each study, the coupon was again weighed accurately to find the weight loss. The experiments were performed in naturally aerated conditions and no stirring was employed. The following Eqs. (1, 2, 3) were used to calculate the inhibition efficiency, surface coverage and corrosion rate of the inhibitor.
where W0 and Wi represent the weight loss in grams for blank and inhibitor solution, respectively. ρ is the density of mild steel in g/cm3, A is the area of the coupon in cm2 and t is the time of immersion in hours.
2.4 Electrochemical Studies
CH Electrochemical workstation CHI608E model was used for the electrochemical studies namely AC impedance, LPR measurements and Tafel polarisation. The instrument makes use of three electrode cell assembly constituting platinum counter electrode, saturated calomel reference electrode and mild steel working electrode with a geometric area of 1 cm2. The measurements were carried out after a steady state open circuit potential was established. Impedance studies were performed by applying a frequency range of 100 kHz to 1 Hz with AC signal amplitude perturbation of 0.005 V. Polarisation curves were obtained by changing the potential from − 0.2 V cathodically to + 0.2 V anodically with respect to open circuit potential and at a scan rate of 10 mV/sec.
2.5 Spectrophotometric Studies
2.5.1 ICP Optical Emission Spectra
The instrument ICP-OES spectrometer, Perkin Elmer Optima 7000 model was employed to estimate the iron content present in the blank and the inhibitor solutions obtained after the 12 h of immersion study. The inhibition efficiency obtained by weight loss studies can be further validated by this method [21]. The sample solutions enter the capillary tube of the instrument through suction. After a waiting period of 15 s the sample solutions were analysed using certified reference material for iron. The iron content in the diluted sample solutions were measured at a wavelength of 238.204 nm. From the iron content the inhibition efficiency (IE) was calculated from the Eq. (4)
where C0 (mg/ litre) and C (mg/ litre) are the concentrations of the iron present in the blank and the inhibitor solutions, respectively.
2.5.2 FTIR Spectra
Fourier transform infrared spectrophotometer Shimadzu IR affinity1S model was used to characterise the Sunova Spirulina powder, its acid extract and the inhibitor coated metal. The protective layer coated on the mild steel specimen after 6 h of immersion in the solution containing 600 ppm of the inhibitor was used for the analysis. The IR spectra were recorded from 4000 to 500 cm−1 with a resolution of 4 cm−1.
2.5.3 UV–Vis and Fluorescence Spectra
UV spectrophotometer Shimadzu UV-1800 model and spectrofluorophotometer Shimadzu RF- 5301P model were used to record the UV–Vis absorbance and fluorescence spectra, respectively. The solutions obtained after the immersion of mild steel in 1 M HCl for 6 h with and without the inhibitor (600 ppm) were used for the studies. UV–Vis spectra were recorded from 200 to 800 nm and for fluorescence spectra the emission intensities were recorded from 300 to 800 nm.
2.6 Surface Analysis Studies
Field emission scanning electron microscope Carl Zeiss Sigma V model was employed for the surface analysis study of the polished metal, inhibited and uninhibited metal. Sputtering was done using gold for 120 s at 10 mA.
3 Results and Discussion
3.1 Effect of Inhibitor Concentrations and Immersion Periods
The inhibition efficiency of corrosion inhibitors are extensively evaluated by the weight loss technique [18]. The hetero atoms present in the active constituents of the inhibitor act as adsorption centres leading to the formation of a protective film on the metal surface. This film acts as a barrier between the metal and the aggressive medium thus inhibiting the corrosion [22]. The extent of protection increases with the inhibitor concentration as revealed by the decrease in the weight loss of the mild steel specimen [23]. Table 1 shows the performance of the acid extract of the Sunova spirulina of varying concentrations from 100 to 600 ppm for different immersion periods at 303 K. It was found that the inhibition efficiency reached a maximum of 96.32% for 600 ppm and 12 h of immersion time. The increased adsorption of the active molecules on the metal surface results in an enhanced surface coverage of the metal [24, 25]. The inhibitor efficiency was found to increase with the increase in immersion period up to 12 h and thereafter it decreases slightly. The decrease may be attributed to the collapse of the protective film due to its thickness exposing the metal to the acid solution. Figure 1 shows the variation of the corrosion rate with concentration of the inhibitor at different immersion periods. The corrosion rate increases with increase in the immersion period both in the absence and presence of the corrosion inhibitor. However, for a particular immersion period the inhibitor concentration shows a significant role in reducing the corrosion rate.
3.2 Effect of Temperature
The nature of adsorption of the inhibitor molecules on the surface of the metal can be studied by monitoring the inhibition efficiency of the inhibitor at different temperatures. Table 2 shows an increase in the trend of IE % of SS extract of same concentration with a step up of 10 K raise in temperature from 303 to 333 K and half an hour of immersion period. The inhibition efficiency was found to reach a maximum of 91.30% at 333 K for 600 ppm concentration. This trend reveals that a stable protective layer was formed on the metal surface due to the chemical interaction between the active constituents of the inhibitor and the metal surface. This reveals the nature of adsorption process involved in the corrosion inhibition was chemisorption [26]. However, at 343 K there was a decrease in the inhibition efficiency % which may be due to the breaking of the adsorbed layer leading to smaller segments, exposing the surface to the aggressive medium [27]. Figure 2 reveals an increase in the corrosion rate with increase in temperature both in the absence and presence of the inhibitor. This is due to the increase in the thermal agitation of the acid medium that abviously will enhance the metal dissolution [28]. However, it can be noted that the corrosion rate decreases with the increase in SS concentration for a particular temperature due to the formation of stable protective layer on the metal surface [29].
3.2.1 Thermodynamic Activation Parameters
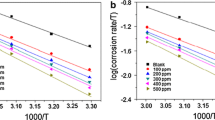
Temperature plays an important role in influencing the rate of electrochemical corrosion. The relationship between the temperature and the corrosion rate is given by the Arrhenius Eq. (5).
where CR is the corrosion rate, Ea, the apparent activation energy, A, the Arrhenius pre-exponential factor, R, the molar gas constant and T, the absolute temperature. The transition state Eq. (6) is used to calculate enthalpy of activation (ΔH*) and entropy of activation (ΔS*) for the corrosion reaction.
where N is the Avagadro number and ‘h’ is the Planck’s constant. The thermodynamic activation parameters Ea, A, ΔH* and ΔS* listed in Table 3 were obtained from the linear plots of log CR versus 1/T and log (CR/T) versus 1/T. The Ea value for mild steel in 1 M HCl was found to be 66.33 kJ/mole which agrees well with the literature values 50–100 kJ/mole [30]. The lower values of Ea for the inhibitor solutions than that of the blank supports the formation of chemisorbed layer of the inhibitor on the surface of the mild steel [31]. According to Eq. (5) the mild steel corrosion can be influenced by Arrhenius pre-exponential factor A and apparent activation energy Ea. Greater variation of A with the inhibitor concentration compared to Ea indicates that A may be the dominant factor in influencing the corrosion rate [32]. The lower values of ΔH* than that of Ea reveals that a gaseous reaction namely the hydrogen evolution reaction was involved in the corrosion process, correlated to reduction in total reaction volume [33]. The difference between Ea and ΔH* values is 2.68 kJ /mole that signifies unimolecular reaction in the corrosion process [34, 35]. The positive sign of the enthalpy of activation reveals that the metal dissolution process was endothermic in presence of the inhibitor. The sign of the ΔS* values changed when moving from blank to the inhibitor solution. This is attributed to the non-spontaneity of the metal dissolution process in presence of the inhibitor. The negative sign of ΔS* reflects orderly adsorption of the inhibitor molecules on the metal surface which was initially moving freely in the bulk of the solution [32].
3.2.2 Adsorption Isotherm
The effectiveness of the inhibitor in corrosion mitigation of mild steel in 1 M HCl solution was investigated by the use of adsorption isotherms as it correlates the surface coverage with the concentration of the inhibitor. The adsorption isotherms namely Langmuir, Temkin, Freundlich, El-Awady and Florry-Huggins isotherms were attempted. Langmuir isotherm showed a best fit as was determined by the regression values R2. The modified Langmuir isotherm equation is
where is inhibitor concentration, is adsorption equilibrium constant, and θ is the degree of surface coverage of the inhibitor. Figure 3 shows the modified Langmuir plot of C versus C/θ which is nearly linear and the slope was found to vary from 1.03 to 1.10 between the temperature 303 K and 343 K. This suggests the monolayer adsorption of the main constituents of SS on the mild steel surface and there is no interaction between them [36]. These observations point out that the inhibitor which was physisorbed at lower temperatures gets firmly held on the surface of the metal by chemisorption with the raise in temperature [32] The intercept of the modified Langmuir plot is equal to 1/ads and is related to the standard Gibbs free energy by the Eq. (8).
where R is molar gas constant, 55.5 is molar concentration of water molecules at electrode and electrolyte interface during the adsorption process and T is absolute temperature. Table 4 shows the values of Kads and ΔGoads. High values of Kads with increase in temperature indicates the formation of strong and stable adsorbed layer and consequently higher inhibition efficiency [36, 37]. The value of ΔGoads up to − 20 kJ mol−1 indicates physical adsorption while a value more than − 40 kJ mol−1 corresponds to chemisorption [38].. The calculated values of ΔGoads for different temperatures vary from − 15.28 to − 23.93 kJ mol−1. This shows that the adsorption process involves physisorption which slightly shifted towards chemisorption at higher temperatures. The negative value of ΔGoads supports the spontaneity of the adsorption process. Based on Van’t Hoff Eq. (9), ΔHoads was calculated and found to be − 50.02 kJ/mole.
The negative value of ΔHoads signifies physisorption or chemisorption process [39]. According to the literature the enthalpy value for a physisorption process is less than 41.86 kJ/mole while for chemisorption it approaches 100 kJ/mole [40]. This indicates that the electrostatic interaction between the protonated inhibitor molecules and Cl− ions could have allowed the physisorption of the inhibitor on the surface. This may be followed by the transfer of electrons from the active constituents of the inhibitor molecules to the empty ‘d’ orbitals of iron [32].
3.2.3 Statistical Analysis
The null hypotheses stating there is no significant difference in the corrosion rate due to different inhibitor concentration levels and due to different temperatures were tested using two-way analysis of variance (ANOVA) [41]. The results are given in Table 5.
Since the F-calculated values are greater than the F table values, i.e. 6.85 > 2.36 and 4.29 > 2.71, the null hypotheses are rejected at 5% level. The study infers that the corrosion rate is significantly different for different temperatures as well as different inhibitor concentration levels. This means that the inhibitor concentration and temperature have significant effect on corrosion rate.
3.3 Electrochemical Studies
3.3.1 Tafel Polarisation
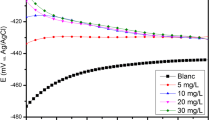
Figure 4 shows the polarisation curves obtained for mild steel in 1 M HCl solution in the presence and absence of the inhibitor. Both the cathodic and anodic curves shift towards the lower current densities on addition of the inhibitor pointing that the anodic metal dissolution and the cathodic hydrogen evolution partial reactions are efficiently inhibited by the inhibitor [42]. Table 6 provides the values of corrosion potential (Ecorr), corrosion current densities (Icorr), cathodic tafel slope (βc) and anodic tafel slope (βa). According to the report if the difference in the Ecorr values between the uninhibited and inhibited solution is greater than ± 85 mV then the inhibition can be labelled as anodic or cathodic type [43]. In the present study, the maximum displacement in Ecorr values is 24.5 mV which indicates a mixed type inhibition. The inhibition efficiency calculated using Icorr values was found to increase with the inhibitor concentration and reached a maximum of 91.27% at 600 ppm concentration of the inhibitor. This may be attributed to both anodic and cathodic inhibition performance of the inhibitor via adsorption on the metal surface blocking the active sites [44]. Tafel slopes (βa) and (βc) were found to change with inhibitor concentration indicating mixed type of corrosion inhibition [45].
3.3.2 Linear Polarisation Study
Linear polarisation measurements help to monitor the corrosion rate and the performance of the inhibitor. Table 6 shows that the linear polarisation resistance (LPR) values were higher for the inhibitor solutions than that of the blank solution. The LPR(inhibitor) values were found to increase with the increase in inhibitor concentration [46]. Using Eq. (10) the inhibition efficiency (IE %) was calculated.
The results were in agreement with the values obtained from weight loss studies.
3.3.3 Electrochemical Impedance Study
Impedance measurements give the characteristics and the kinetics of the in situ electrochemical reactions occurring at the metal/acid interface in the presence and in the absence of the corrosion inhibitor. Figure 5 shows a: Nyquist plot, b and c: Phase angle and Bode plots drawn using the results obtained from the electrochemical impedance measurements. A single capacitive loop in the Nyquist and only one time constant/ single relaxation process in phase angle plot reveals that the formation of the electrical double layer at the metal solution interface and corrosion of mild steel are controlled by the single charge transfer process [47]. The corrosion mechanism remains unchanged in the absence and presence of the inhibitor as revealed by the similar capacitive loop appearance. However, the diameter of the capacitive loop is found to increase with the increase in the inhibitor concentration. This can be correlated due to the increased efficacy of the inhibitor to be adsorbed on the metal solution interface forming a protective film and mitigate corrosion [48]. Further evidence comes from the Bodes plot which shows an increase in the absolute impedance value at lower frequencies with increase in the inhibitor concentration [49]. The inhomogeneity of the metal surface that arises from surface roughness, impurities, adsorption of the inhibitor molecules, formation of porous layers, distribution of the activity centres is reflected in the depressed semicircle of the capacitive loop in Nyquist plot [50]. The non-ideal capacitance behaviour is further supported by the phase angle plot where the increase in the phase angle with the inhibitor concentration was lower than − 90° [51]. This led to a necessity of replacing double layer capacitance in the Randles circuit by a constant phase element (CPE) for the best fit of the depressed semicircle. The various impedance parameters obtained by best fitting the experimental data in the equivalent circuit (Fig. 6) are given in Table 7. The best fit is revealed by the Chi-squared value [52]. The impedance of the CPE is given by the following expression [53]
where the Y0 refers to the magnitude of CPE, j is an imaginary number which is equal to square root of -1, ω is the angular frequency and n is the phase shift that corresponds to the inhomogeneity of the surface. The Rp values displayed in Table 7 denotes the polarisation resistance which constitutes charge transfer resistance, inhibitor film resistance, diffused layer resistance and resistance of all accumulated species [54]. The interfacial double layer capacitance (Cdl) values was calculated using the following relationship [42]
The Rp values is found to increase while the Cdl values decrease with increase in concentration as shown in Table 7. This can be corroborated to the retardation of the charge transfer process due to the adsorption of the inhibitor at the metal solution interface [55].
3.3.4 ICP- OES Measurements
Table 8 shows the iron content of the blank and the inhibited solutions obtained after the weight loss studies using inductively coupled plasma (ICP) optical emission spectrometry (OES). The decrease in the amount of iron with the increase in the concentration of the inhibitor supports the effectiveness of the inhibitor in preventing the dissolution of iron into the solution. This may be due to the adsorption of the active constituents of the inhibitor onto the metal surface [56,57,58]. The inhibitor efficiency calculated from the iron content using the Eq. (4) was in agreement with the results obtained by other methods.
3.4 UV Visible, FTIR and Spectrofluorometric Spectral studies
Figure 7a shows the UV–Vis spectra of the blank solution and the inhibitor solution obtained after 6 h of immersion of the mild steel specimen. This technique is suitable for the identification of formation of the complex ion in solution. The absorption maxima was found to be shifted towards longer wavelength in the presence of the inhibitor. This indicates the complex formation between Fe2+ and the active constituent of the inhibitor solution [59, 60].
Figure 7b shows the FTIR spectra of the spirulina powder, inhibitor extract and the inhibitor coated metal. This technique is employed to ascertain that the corrosion inhibition is due to the interaction between the metal and the inhibitor molecules [61]. The S–O stretching occurring at 1543 cm−1 has a high intensity in the powder which reduces in intensity in the acid extract probably due to protonation and this stretching shifts to a lower wave number 1516 cm−1 in the inhibitor coated metal. This can be attributed to the binding of the R–S–O unit to the metal. This is further corroborated when we consider the S–O stretching at 1409 cm−1 which being low in intensity in the powder and the acid extract, increases in intensity in the inhibitor coated metal. The P–O stretching occurs at 1020 cm−1 in the powder and this shift to 1046 cm−1 in the acid extract and then to 1064 cm−1 in the inhibitor coated metal. This would mean that the P–O group gets protonated in the acid medium as well as in the inhibitor coated metal surface suggesting electrostatic attraction (physisorption) of the inhibitor molecules on the metal surface [62]. The IR absorption at 1640 cm−1 in the powder shifts to 1658 cm−1 in the acid extract and then to 1622 cm−1 in the inhibitor coated metal surface matches well with the scissor bending mode of –NH2 of amide group implying the involvement of the latter in the inhibition activity [62].
Figure 7c shows the fluorescence spectra of the acid extract of the inhibitor and the inhibitor solution obtained after 6 h of immersion of the mild steel specimen. The absorption peak between 400 and 500 nm corresponds to beta carotene and that at 700 nm corresponds to chlorophyll [63]. The decrease in the intensity of absorbance peak of beta carotene obtained for the inhibitor solution containing Fe2+ ions shows the interaction between beta carotene and the metal ion.
3.5 Surface Morphology
Figure 8 shows the SEM images of (a) pure metal (b) metal in 1 M HCl and (c) metal immersed in the 600 ppm concentration of acid extract of the Sunova spirulina for 6 h at 298 K. On comparison, it clearly showed the corrosive attack of the acid medium on the metal surface and the corrosion rate visibly reduced on the surface of the metal in presence of the inhibitor. This is due to the uniform deposition of the inhibitor on the surface of the metal.
3.6 Mechanism
The experimental results clearly reveal the enhanced corrosion inhibition efficiency of the acid extract of the Sunova spirulina powder as compared to that of its alcoholic extract study by others.[17]. This is perhaps due to the adsorption of the active constituents of the spirulina extract onto the metal surface. The active constituents in the extract are the monosaccharides, amino acids and beta carotene. These constituents contain the hetero atoms in their functional groups such as –OH, − NH2, R–S–O, and P–O which get protonated in acid medium. These protonated groups are then electrostatically attracted towards Cl− adsorbed metal surface. The π electrons of beta carotene must be attracted towards the partially empty ‘d’ orbitals of Fe2+ resulting physisorption. This is followed by the complex formation as indicated by the spectrophotometric techniques. These interactions help in retaining the inhibitor on the metal surface even at higher temperatures.
4 Conclusion
The acid extract of Sunova spirulina powder was observed to be an effective green corrosion inhibitor for mild steel in 1 M HCl than the ones investigated with alcoholic extract of the spirulina powder by others. The inhibition efficiency increases with the increase in the concentration as more number of active molecules of the inhibitor get adsorbed on the metal surface and also may be due to the formation of Fe2+ beta carotene complex on the metal surface. The increase in IE % with raise in temperature upto 333 K suggests chemical interaction between the inhibitor molecules and the metal surface. This is further supported by the Ea and enthalpy values. Adsorption studies show that it obeyed modified Langmuir isotherm and support the monolayer adsorption. The negative value of ΔGoads indicates the spontaneity of adsorption. The higher value of Kads with increase in temperature infers increased adsorption rather than desorption. Potentiodynamic polarisation studies reveal that the inhibition was mixed type inhibiting both the anodic metal dissolution and cathodic hydrogen evolution. The impedance measurements indicated that there was an increase both in the surface coverage of the inhibitor and the thickness of the electrical double layer reflecting the efficiency of the inhibitor. The UV–Vis, FTIR spectral studies and surface analytical study using SEM agree to the formation of the protective layer on the metal surface.
References
Li LF, Caenen P, Celis JP (2008) Effect of hydrochloric acid on pickling of hot-rolled 304 stainless steel in iron chloride-based electrolytes. Corros Sci 50:804–810. https://doi.org/10.1016/j.corsci.2007.09.006
Ken Dibble GS, Wansbrough, H (2003) Chemical cleaning of metals. VIII-Metals-H-Cleaning-2, NZ Inst. Chem., pp 1–9
Raja PB, Ismail M, Ghoreishiamiri S et al (2016) Reviews on corrosion inhibitors: a short view. Chem Eng Commun 203:1145–1156. https://doi.org/10.1080/00986445.2016.1172485
Krishnaveni K, Ravichandran J (2014) Effect of aqueous extract of leaves of Morinda tinctoria on corrosion inhibition of aluminium surface in HCl medium. Oral Oncol 50:2704–2712. https://doi.org/10.1016/S1003-6326(14)63401-4
Al-Otaibi MS, Al-Mayouf AM, Khan M et al (2014) Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab J Chem 7:340–346. https://doi.org/10.1016/j.arabjc.2012.01.015
Yuli Y, Emriadi, Novesar J, Gunawarman (2015) Asian Journal of Chemistry. Asian J Chem 27:875–881
Zuo R (2007) Biofilms: Strategies for metal corrosion inhibition employing microorganisms. Appl Microbiol Biotechnol 76:1245–1253. https://doi.org/10.1007/s00253-007-1130-6
Priyadarshani I, Rath B (2012) Commercial and industrial applications of micro algae—a review. J Algal Biomass Util 3:89–100
Wolkers H, Barbosa MJ, Kleinegris DMM, Bosma R, Wijffels RH (2011) Microalgae: the green gold of the future?: large-scale sustainable cultivation of microalgae for the production of bulk commodities. UR-Food & Biobased Research, Wageningen, p 32
Colla LM, Oliveira Reinehr C, Reichert C, Costa JAV (2007) Production of biomass and nutraceutical compounds by Spirulina platensis under different temperature and nitrogen regimes. Bioresour Technol 98:1489–1493. https://doi.org/10.1016/j.biortech.2005.09.030
Sajilata MG, Singhal RS, Kamat MY (2008) Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulina platensis. Food Chem 109:580–586. https://doi.org/10.1016/j.foodchem.2008.01.005
Madhyastha HK, Vatsala TM (2007) Pigment production in Spirulina fussiformis in different photophysical conditions. Biomol Eng 24:301–305. https://doi.org/10.1016/j.bioeng.2007.04.001
Machu L, Misurcova L, Ambrozova JV et al (2015) Phenolic content and antioxidant capacity in algal food products. Molecules 20:1118–1133. https://doi.org/10.3390/molecules20011118
Piñero Estrada J (2001) Antioxidant activity of different fractions of Spirulina platensis protean extract. Farm 56:497–500. https://doi.org/10.1016/S0014-827X(01)01084-9
Rajendran S, Paulraj JR (2017) Corrosion behaviour of metals in artificial saliva in presence of spirulina powder. African J Dent 5:063–070
Sribharathy V, Rajendran S (2012) Corrosion inhibition by green inhibitor : sodium metavanadate –spirulina system correspondence. Chem Sci Rev Lett 1:25–29
Kamal C, Sethuraman MG (2012) Spirulina platensis—a novel green inhibitor for acid corrosion of mild steel. Arab J Chem 5:155–161. https://doi.org/10.1016/j.arabjc.2010.08.006
Xhanari K, Finšgar M, Knez Hrnčič M et al (2017) Green corrosion inhibitors for aluminium and its alloys: a review. RSC Adv 7:27299–27330. https://doi.org/10.1039/C7RA03944A
Badawy WA, El-rabiei MM (2014) The use of beta-carotene as environmentally safe inhibitor for Cu- Al-Ni alloyscorrosion in sulf ide polluted chloride solutions. Chem Mater Res 6:107–115
Sanat Products Ltd. https://www.sanat.co.in/health-care-products/1/sunova-spirulina-capsules
McCafferty E (2005) Validation of corrosion rates measured by the Tafel extrapolation method. Corros Sci 47:3202–3215. https://doi.org/10.1016/j.corsci.2005.05.046
Palumbo G, Berent K, Proniewicz E, Banaś J (2019) Guar gum as an eco-friendly corrosion inhibitor for pure aluminium in 1-M HCl solution. Materials (Basel) 12:2620. https://doi.org/10.3390/ma12162620
Raghavendra N (2019) Use of expired naftifine drug as corrosion inhibitor for copper in hydrochloric acid. J Adv Electrochem 5:177–179
Rugmini Ammal P, Prajila M, Joseph A (2018) Effective inhibition of mild steel corrosion in hydrochloric acid using EBIMOT, a 1, 3, 4-oxadiazole derivative bearing a 2-ethylbenzimidazole moiety: electro analytical, computational and kinetic studies. Egypt J Pet 27:823–833. https://doi.org/10.1016/j.ejpe.2017.12.004
Athar M, Ali H, Quraishi MA, Quraishi MA (2016) Corrosion inhibition of carbon steel in hydrochloric acid by organic compounds containing heteroatoms. Br Corros J 0599:1–5. https://doi.org/10.1179/000705902225002376
Huong DQ, Duong T, Nam PC (2019) Eff ect of the Structure and Temperature on Corrosion Inhibition of Thiourea Derivatives in. 1 0 M HCl Solution. ACS Omega. https://doi.org/10.1021/acsomega.9b01599
Menaka R, Subhashini S (2016) Chitosan schiff base as eco-friendly inhibitor for mild steel corrosion in 1 M HCl. J Adhes Sci Technol 30:1622–1640. https://doi.org/10.1080/01694243.2016.1156382
Alhaffar MT, Umoren SA, Obot IB, Ali SA (2018) Isoxazolidine derivatives as corrosion inhibitors for low carbon steel in HCl solution: experimental, theoretical and effect of KI studies. RSC Adv 8:1764–1777. https://doi.org/10.1039/c7ra11549k
Sanni O, Popoola API, Fayomi OSI (2019) Temperature effect, activation energies and adsorption studies of waste material as stainless steel corrosion inhibitor in sulphuric acid 0.5 M. J Bio Tribo Corros 5:1–8. https://doi.org/10.1007/s40735-019-0280-2
Riggs OL Jr, Hurd RM (1967) Temperature coefficient of corrosion inhibition. Corros. NACE 23:252–258
Menaka R, Subhashini S (2017) Chitosan schiff base as effective corrosion inhibitor for mild steel in acid medium. Polym Int. https://doi.org/10.1002/pi.5245
Tang L, Mu G, Liu G (2003) The effect of neutral red on the corrosion inhibition of cold rolled steel in 1.0 M hydrochloric acid. Corros Sci 45:2251–2262. https://doi.org/10.1016/S0010-938X(03)00046-5
Noor AE (2007) Temperature effects on the corrosion inhibition of mild steel in acidic solutions by aqueous extract of fenugreek leaves. Int J Electrochem Sci 2:996–1017
Attar T, Larabi L, Harek Y (2014) Inhibition effect of potassium iodide on the corrosion of carbon steel (XC 38) in acidic medium. Int J Adv Chem 2:139–142. https://doi.org/10.14419/ijac.v2i2.3272
Ben Aoun S (2017) On the corrosion inhibition of carbon steel in 1 M HCl with a pyridinium-ionic liquid: chemical, thermodynamic, kinetic and electrochemical studies. RSC Adv 7:36688–36696. https://doi.org/10.1039/c7ra04084a
Chakravarthy MP, Mohana KN (2014) Adsorption and corrosion inhibition characteristics of some nicotinamide derivatives on mild steel in hydrochloric acid solution. ISRN Corros 2014:1–13. https://doi.org/10.1155/2014/687276
Lagren M (2002) Study of the mechanism and inhibiting 4H–1, 2, 4-triazole on mild steel corrosion in acidic media. Corros Sci 44:573–588. https://doi.org/10.1016/S0010-938X(01)00075-0
Umoren SA, Eduok UM, Solomon MM, Udoh AP (2016) Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab J Chem 9:S209–S224. https://doi.org/10.1016/j.arabjc.2011.03.008
Benabdellah M, Tounsi A, Khaled KF, Hammouti B (2011) Thermodynamic, chemical and electrochemical investigations of 2-mercapto benzimidazole as corrosion inhibitor for mild steel in hydrochloric acid solutions. Arab J Chem 4:17–24. https://doi.org/10.1016/j.arabjc.2010.06.010
Sanja Martinez IS (2002) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in the low carbon steel/mimosa tannin/sulfuric acid system. Appl Surf Sci 199:83–89
Yaro AS, Khadom AA, Wael RK (2013) Apricot juice as green corrosion inhibitor of mild steel in phosphoric acid. Alexandria Eng J 52:129–135. https://doi.org/10.1016/j.aej.2012.11.001
Arukalam IO, Madu IO, Ijomah NT et al (2014) Acid corrosion inhibition and adsorption behaviour of ethyl hydroxyethyl cellulose on mild steel corrosion. J Mater 2014:1–11. https://doi.org/10.1155/2014/101709
Preethi Kumari P, Shetty P, Rao SA (2017) Electrochemical measurements for the corrosion inhibition of mild steel in 1 M hydrochloric acid by using an aromatic hydrazide derivative. Arab J Chem 10:653–663. https://doi.org/10.1016/j.arabjc.2014.09.005
Li WH, He Q, Zhang ST et al (2008) Some new triazole derivatives as inhibitors for mild steel corrosion in acidic medium. J Appl Electrochem 38:289–295. https://doi.org/10.1007/s10800-007-9437-7
Al-Amiery AA, Kadhum AAH, Kadihum A et al (2014) Inhibition of mild steel corrosion in sulfuric acid solution by new schiff base. Materials (Basel) 7:787–804. https://doi.org/10.3390/ma7020787
Karthikaiselvi R, Subhashini S (2014) Study of adsorption properties and inhibition of mild steel corrosion in hydrochloric acid media by water soluble composite poly (vinyl alcohol-o-methoxy aniline). J Assoc Arab Univ Basic Appl Sci 16:74–82. https://doi.org/10.1016/j.jaubas.2013.06.002
El Aoufir Y, Sebhaoui J, Chaouiki A et al (2018) Two novel benzodiazepines as corrosion inhibitors for carbon steel in hydrochloric acid: experimental and computational studies. J Bio Tribo Corros. https://doi.org/10.1007/s40735-018-0169-5
Singh A, Caihong Y, Yaocheng Y et al (2019) Analyses of new electrochemical techniques to study the behavior of some corrosion mitigating polymers on n80 tubing steel. ACS Omega 4:3420–3431. https://doi.org/10.1021/acsomega.8b02983
Zehra S, Mobin M, Aslam J, Parveen M (2018) Assessment of glycine derivative N-benzylidine-2((2-oxo-2-(10H-phenothiazine-10yl)ethyl)amino) acetohydrazide as inhibitor for mild steel corrosion in 1 M HCl solution: electrochemical and theoretical approach. J Adhes Sci Technol 32:317–342. https://doi.org/10.1080/01694243.2017.1354669
Popova A, Raicheva S, Sokolova E, Christov M (1996) Frequency dispersion of the interfacial impedance at mild steel corrosion in acid media in the presence of benzimidazole derivatives. Langmuir 12:2083–2089
Acid H, Lgaz H, Masroor S et al (2020) Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid. Metals (Basel) 10:1–14
Parveen M, Mobin M, Zehra S, Aslam R (2018) L-proline mixed with sodium benzoate as sustainable inhibitor for mild steel corrosion in 1M HCl: an experimental and theoretical approach. Sci Rep 8:1–18. https://doi.org/10.1038/s41598-018-24143-2
Xu X, Singh A, Sun Z et al (2017) Theoretical, thermodynamic and electrochemical analysis of biotin drug as an impending corrosion inhibitor for mild steel in 15% hydrochloric acid. R Soc Open Sci. https://doi.org/10.1098/rsos.170933
Singh P, Ebenso EE, Olasunkanmi LO et al (2016) Electrochemical, theoretical, and surface morphological studies of corrosion inhibition effect of green naphthyridine derivatives on mild steel in hydrochloric acid. J Phys Chem C 120:3408–3419. https://doi.org/10.1021/acs.jpcc.5b11901
Belarbi Z, Dominguez Olivo JM, Farelas F et al (2019) Decanethiol as a corrosion inhibitor for carbon steels exposed to aqueous CO2. Corrosion 75:1246–1254. https://doi.org/10.5006/3233
Alaneme KK, Olusegun SJ, Adelowo OT (2016) Corrosion inhibition and adsorption mechanism studies of Hunteria umbellata seed husk extracts on mild steel immersed in acidic solutions. Alexandria Eng J 55:673–681. https://doi.org/10.1016/j.aej.2015.10.009
Shah AM, Rahim AA, Hamid SA, Yahya S (2013) PGreen inhibitors for copper corrosion by Mangrove tannin. Int J Electrochem Sci 8:2140–2153
Prabakaran M, Kim SH, Sasireka A et al (2017) β-Sitosterol isolated from rice hulls as an efficient corrosion inhibitor for mild steel in acidic environments. New J Chem 41:3900–3907. https://doi.org/10.1039/c6nj03760g
Abdel-Gaber AM, Abd-El-Nabey BA, Sidahmed IM et al (2006) Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros Sci 48:2765–2779. https://doi.org/10.1016/j.corsci.2005.09.017
Aloysius A, Ramanathan R, Christy A et al (2018) Experimental and theoretical studies on the corrosion inhibition of vitamins—thiamine hydrochloride or biotin in corrosion of mild steel in aqueous chloride environment. Egypt J Pet 27:371–381. https://doi.org/10.1016/j.ejpe.2017.06.003
Gadow HS, Motawea MM (2017) Investigation of the corrosion inhibition of carbon steel in hydrochloric acid solution by using ginger roots extract. RSC Adv 7:24576–24588. https://doi.org/10.1039/c6ra28636d
Dotto GL, Vieira MLG, Esquerdo VM, Pinto LAA (2013) Equilibrium and thermodynamics of azo dyes biosorption onto Spirulina platensis. Braz J Chem Eng 30:13–21. https://doi.org/10.1590/S0104-66322013000100003
Kleinegris DMM, van Es MA, Janssen M et al (2010) Carotenoid fluorescence in Dunaliella salina. J Appl Phycol 22:645–649. https://doi.org/10.1007/s10811-010-9505-y
Acknowledgements
One of the authors S.J. Hepziba wishes to thank Centre for Research, CHRIST (Deemed to be University) for providing the financial support and Ms. Jilna Jomy I MSc chemistry for her involvement in carrying out a part of the research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jessima, S.J.H.M., Subhashini, S. & Arulraj, J. Sunova spirulina Powder as an Effective Environmentally Friendly Corrosion Inhibitor for Mild Steel in Acid Medium. J Bio Tribo Corros 6, 71 (2020). https://doi.org/10.1007/s40735-020-00370-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00370-x