Abstract
Polymeric nanocomposites containing Fe3O4 nanoparticles were prepared through a chemical route under different precursor-to-solvent ratios and were submitted to structural and morphologic characterization. The embedded nanoparticles, containing pure magnetite and characterized by considerable polydispersity, are rather homogeneously dispersed in the matrix. The magnetic properties of two representative samples were analyzed in detail between T = 5 K and room temperature. Magnetic effects clearly associated with the Verwey monoclinic to cubic transition with transition temperatures distributed in the interval 95–120 K were put in evidence. On heating through this region, the coercive field and the maximum susceptibility of hysteresis loops display marked downward/upward steps, respectively, while the high-field magnetization is not affected at all; a comparable upward step is measured in the FC/ZFC curves. Reporting the maximum susceptibility as a function of the reciprocal of the coercive field in the interval from T = 95 to T = 120 K, and using the predictions for single-domain nanoparticles with randomly distributed axes of uniaxial and cubic anisotropy (the former/latter case being applicable below/above the Verwey transition, respectively), the evolution of the transformed cubic-anisotropy fraction upon heating has been studied, and the distribution of Verwey transition temperatures related to the sample polydispersity has been accurately determined. The low-temperature value of the uniaxial anisotropy constant is obtained from coercive field measurements and found to be comparable to, albeit slightly higher than the corresponding quantity measured in bulk crystalline magnetite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Verwey transition, a well-known [1], still debated [1, 2] effect occurring in bulk magnetite near 125 K is revealed by a dramatic change in the electronic transport properties and by a corresponding change in magnetic properties such as magnetic anisotropy [1] and magnetostriction [3].
The recent years have witnessed a remarkable surge of research on sub-micrometric magnetite; in particular, Fe3O4 nanoparticles and related nanocomposites/nanocomplexes have attracted considerable attention owing to the combination of efficient preparation techniques [4,5,6,7,8], low toxicity [9] and ever-increasing potential of applications in strategic fields such as sensors, Information and Communication Technologies (ICTs), environment and biomedicine [8, 10,11,12,13,14,15,16].
As for all magnetic nanosystems, the magnetic properties of Fe3O4 nanoparticles are strongly affected by size effects; the measured differences from the bulk material are often ascribed to surface effects: for instance, increasing the surface-to-volume ratio typically results in a reduction in the room-temperature saturation magnetization [17, 18] and an enhancement of magnetic anisotropy [19, 20] with respect to their bulk values.
In sharp contrast, the Verwey transition in magnetite particles appears as being rather elusive an effect. In fact, a number of sometimes contradictory results have been reported in the recent literature, including strong reduction [21,22,23,24] or even complete disappearance [25, 26] of the Verwey transition temperature TV in small particles. A comprehensive study of the effect of nanoparticle size on TV has been performed on nanocrystals grown around 5-nm seeds through seed-mediated nanocrystal growth [27]. In that case, the Verwey transition was found to be size-independent down to 20 nm with a progressive reduction in the transition temperature from 20 to 8 nm before suddenly disappearing below 6 nm.
However, size is not the only parameter influencing the value of TV. The observation of the Verwey transition is related also to details of the nanoparticle preparation process, such as the presence of a magnetic field [26]. The particle shape plays an important role on the Verwey transition in ultrasmall nanoparticles [28]. As recently pointed out [29], the quality of magnetite (both structural and chemical) plays a most important role in determining the existence of the Verwey transition. In fact, a significant reduction in TV has been measured in macroscopic magnetite single crystals as a function of increasing off-stoichiometry status [29]. The observation of TV in nanometer-sized magnetite has been recently proven to be connected to the high quality of the sample [27, 30].
In this paper, a study based on structural, morphologic and magnetic data is conducted on a set of nanocomposites containing different amounts of polydisperse magnetite nanoparticles with sizes in the 5–70 nm range. The magnetic behavior of representative nanocomposite samples above and below the Verwey transition is explained making use of morphologic data; the changes in magnetic properties occurring at the Verwey transition are described in some detail. The ensuing analysis indicates that in this class of composites, a continuous distribution of TV values exists, suggesting that the embedded nanoparticles undergo the Verwey transition at different temperatures.
Experimental
Materials and methods
All chemicals were high-purity grade and were used as received without any further purification. Bisphenol A diglycidyl ether (DGEBA, Dow D.E.R.™ 332 with an epoxide equivalent weight of 172–176), diethylenetriamine (DETA) and acetone (AcO) were purchased from Sigma-Aldrich (Milan, Italy). Acetylacetone (AcAcH), iron(III)-chloride hexahydrate (FeCl3·6H2O) and sodium acetate (NaOAc) were purchased from Carlo Erba (Milan, Italy).
Magnetite synthesis
The magnetite precursor, iron(III)-acetylacetonate (Fe(AcAc)3), was synthetized starting from FeCl3·6H2O and NaOAc, as described in Messori et al. [31]. According to non-hydrolytic sol–gel (NHSG) route, designed by Niederberger and Pinna [32] and already successfully used in the magnetic nanoparticles synthesis with alcohol solvents [18, 33, 34], in a 100 mL Schlenk tube 1.00 g (2.83 mmol) (Fe(AcAc)3) was dissolved in different amounts of DETA, in order to evaluate the effect of the precursor-to-solvent ratio on the powder properties. The precursor-to-solvent ratio was varied in the molar range 0.1–0.9. Composition details are reported in Table 1.
The reaction was left stirring at room temperature for 15 min and then heated to 200 °C in an oil bath for 48 h.
After reaction, a stable suspension was obtained. To better characterize the inorganic phase, the obtained powders were re-dispersed in AcO with an ultrasonic bath and centrifuged at 4000 rpm for 60 min; the powders were washed, centrifuged until the obtainment of a colorless liquid phase and finally dried under reduced pressure.
Powder characterization
X-ray powder diffraction (XRPD) data for qualitative and quantitative phase analysis were collected using a Bragg–Brentano θ–2θ diffractometer (X’Pert PRO PANalytical, Netherlands) equipped with a gas proportional detector.
Nanoparticle size and morphology were examined by transmission electron microscopy (TEM) investigation, performed on a Tecnai 12 Gspirit electron microscope (FEI Company, USA), using an accelerating voltage of 120 kV and LaB6 as electron source.
Experimental histograms of nanoparticle size distribution were obtained by TEM image analysis. The grain size was approximated by the equivalent circular diameter (or Heywood diameter, E.C.D), representing the diameter of the circle having the area equivalent to the nanoparticle area [35]. The resulted histograms were fitted by the log-normal distribution function, using a nonlinear curve-fitting software tool (OriginLab Microcal Northampton, USA).
Nanocomposite preparation
Nanocomposites, based on epoxy matrix, were prepared by in situ polymerization employing the reactive suspension method [20, 36, 37].
Epoxy resins containing Fe3O4 nanoparticles were prepared by the step polymerization of the bisphenol A diglycidyl ether (DGEBA) epoxy monomer with DETA in the presence of Fe3O4 synthesized via NHSG process. The formulations were prepared by adding the amine suspension of magnetite nanoparticles (prepared as previously described) in the range between 1 and 8 phr (parts of Fe3O4 per hundred parts of resins) of actual Fe3O4 content with respect to the epoxy resin.
Composition details and sample codes of the obtained nanocomposites are summarized in Table 2.
A typical formulation was prepared by mixing magnetite suspension and epoxy monomer by using a magnetic stirrer (15 min mixing time) and an ultrasonic bath (15 min mixing time).
The formulations were casted into silicone molds having cavities with dimension 8 × 1 × 0.3 cm3. All the formulations were cured at 100–120 °C for 3 h and post-cured at 130–150 °C for 30 min.
Nanocomposite characterization
TEM analysis was performed on nanocomposite samples in order to evaluate the distribution and dispersion of magnetic nanoparticles into the polymer matrix and correlate the microstructural features with the magnetic functional properties of the nanocomposite. Nanocomposite samples for microscopic analysis were sectioned into ultrathin slices (100–150 nm) at − 30 °C using a cryoultramicrotome Leica UC6 (Leica Microsystems, Austria). Sample sections were collected on dimethylsulfoxide–water solution and then deposited on 200 mesh copper grids.
Infra-red spectroscopy (IR) characterization was performed to evaluate the epoxy groups conversion; an Avatar 330 FT-IR Thermo Nicolet spectrometer was used, equipped with a diamond crystal and operating in the ATR mode from 4000 to 500 cm−1 (64 scans and resolution of 1 cm−1).
Magnetic measurements
Magnetic hysteresis loops and FC/ZFC curves were measured on two nanocomposite samples using a LOT-Quantum Design SQUID magnetometer operating in the temperature range 2–300 K and in the field range 0–7 × 104 Oe. FC/ZFC curves were taken using an applied field Happ = 30 Oe and an average heating rate of 6 K/min. The behavior of hysteresis loops in the temperature region around TV was studied using a narrow temperature step. Samples were positioned on a quartz sample holder whose diamagnetic signal was orders of magnitude lower than the magnetic signal from the nanomaterials.
Results
Structure and morphology of nanoparticles/nanocomposites
XRD patterns of Mag-DETA_x powders are reported in Fig. 1. Independent of the precursor-to-solvent ratio, all synthesized powders are composed by magnetite (JCPDS file 01-075-0449) as crystalline phase. No significant contribution from other iron oxide phases was detected by Raman spectroscopy [36]. In particular, the Raman spectrum of nanoparticles, already shown in Ref. [36], exhibits the characteristic bands of magnetite at 668 cm−1 assigned to the A1g vibrational mode. The absence of iron oxides different from magnetite is also supported by the high saturation magnetization values (see Table 3).
TEM images reveal that magnetite particles are nanometer-sized crystals with irregular and anisotropic shapes, such as tetragonal and hexagonal structures (Fig. 2). It can be noted that in addition to nanoparticles with sizes in the 10 nm range, larger nanostructures, between 35 and 70 nm in size, are also present. These larger particles seem to decrease in number and size as the precursor–solvent ratio increases.
DGEBA monomers can polymerize to form a cross-linked structure via step-growth polymerization by reacting with amines, phenols, mercaptans, isocyanates or acids. Amines are the most commonly used curing agents/hardeners for epoxides, and the case of step-growth polymerizations is mainly represented by epoxy–amine reactions, that can be considered the key-reaction in the “reactive suspension method” for the system Fe3O4–DETA–DGEBA.
In particular, DETA has five reactive sites and the polymerization reaction between DETA and DGEBA can lead to a highly cross-linked 3D network as shown in Scheme 1.
The development of the three-dimensional network resulting from polymerization reaction during the nanocomposite preparation was followed by IR spectroscopy. The degree of epoxy groups conversion (determined by FT-IR analysis, according to the decrement of the peak centered at 914 cm−1 corresponding to the asymmetric stretching of oxirane ring) is relatively high and almost equal (more than 90%) for all the composite materials, as shown by IR spectra (Fig. 3). This suggests that the ring-opening reaction was not affected by the presence of both the nanoparticles and by-products of the reaction between Fe(AcAc)3 and DETA.
TEM micrographs of epoxy–magnetite nanocomposites show the presence of a dispersed phase attributable to the magnetite nanoparticles (Fig. 4). Even if some particle agglomeration phenomenon is apparent, a sufficiently homogeneous distribution and dispersion of the filler was reached for the majority of the samples, avoiding the use of any coupling agent and/or time-consuming, complex dispersion treatments for samples preparation.
TEM image analysis has been performed to get information about the size distribution of nanoparticles embedded in the polymeric matrix. Experimental histograms and fitting curves are shown in Fig. 5. The mean E.C.D. for all samples is reported in Table 3. Histograms for Mag-DETA_1 and Mag-DETA_2 samples are compatible with a bimodal distribution, while samples Mag-DETA_4 and Mag-DETA_8 are characterized by a substantially monomodal distribution with an asymmetry toward larger sizes.
Magnetic properties
The room-temperature saturation magnetization of all nanopowders (obtained by fitting the high-field region of the M(H) curves to the standard approach to saturation curve for a ferri/ferromagnetic material [38]) are given in Table 3. The measured values are in the range 55–61 emu/g, in good agreement with typical results for magnetite nanoparticles of similar size [20, 39]. The reduction with respect to the bulk value has been related to the presence of a magnetically “dead” layer at the nanoparticle surface [17, 18].
The magnetic properties of two representative nanocomposite samples (Mag-DETA_2, Mag-DETA_8) have been investigated. The choice of samples was suggested by the nanoparticle distributions as reported in Fig. 5 and Table 3: sample Mag-DETA_2 displays a bimodal size distribution and a greater mean size of the small particles, whereas nanoparticles embedded in sample Mag-DETA_8 have a basically monomodal distribution and a lower mean size. The room-temperature saturation magnetization of these nanocomposites is given in Table 4.
The FC/ZFC curves of nanocomposites Comp-DETA_n (n = 2.8), normalized to the room-temperature value, are shown in Fig. 6.
The absence of a definite peak in the ZFC curve, the occurrence of a rather flat FC curve and a merging temperature coincident with room temperature are typical hallmarks of nanoparticles blocked over the whole measured temperature range, either because of their size [40] or because of interactions [17, 37, 41, 42]; however, both FC and ZFC curves exhibit a marked upward step on heating just below T = 120 K, clearly related to the Verwey transition. TV can be estimated from the inflexion point of the ZFC curves; the resulting values are reported in Table 3. (The same results are obtained from the FC curves.) It should be noted that TV = 123 K in high-quality, bulk magnetite crystals [30, 43]. Considering the experimental relationship between the observation of TV in nanometer-sized magnetite and the high quality of the sample [30], it can be concluded that a fraction of rather high-quality magnetite crystals is present in our systems, in agreement with TEM images (Fig. 4).
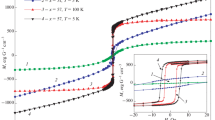
Magnetic hysteresis loops of the two samples, measured above and below TV (at T = 75 K and 150 K, respectively), are reported in Fig. 7 (top panels). The most distinctive features observed on heating from below to above TV are a strong reduction in the coercive field Hc associated with a corresponding increase in the slope of loops (dM/dH). Full hysteresis loops (from Hmin = −70 kOe to Hmax = +70 kOe) at room temperature are shown in Fig. 7 (bottom panel). When the reduced magnetization M/Ms is plotted, the loops are almost superimposed. In both samples, a clearly non-saturating behavior of the magnetization is observed up to field values as large as 70 kOe. This behavior indicates that a fraction of particles is in the superparamagnetic state at room temperature. In fact, using the typical value of the cubic anisotropy of magnetite around room temperature (K1 ≅ 1–3 × 105 erg/cm3 [44]) and taking into account the particle size distributions of Fig. 5, the blocking temperature of the smaller particles in the distribution is estimated to be well below room temperature.
The hysteresis loop parameters have been investigated with special attention to the critical region around \( \left\langle {T_{\text{V}} } \right\rangle \), and are summarized in Fig. 8. The high-field magnetization MHF (Happ = 70 kOe) is shown in panel (a): No particular change in MHF is observed around \( \left\langle {T_{\text{V}} } \right\rangle \). The different magnetic signals from the two samples are related to the different contents of magnetite in the polymeric matrix. (The magnetic volume fraction × in these samples is estimated from the data of Table 2 and is reported in Table 4.)
On the contrary, the coercive field Hc exhibits a sharp decrease on heating from 95 K to about 120 K (see Fig. 8b). Such a behavior is clearly related to the Verwey transition, although it is definitely less sharp and less strong than in bulk magnetite single crystals [45]; the effect we measure around \( \left\langle {T_{\text{V}} } \right\rangle \) is more apparent in sample Comp-DETA_2 than in sample Comp-DETA_8. Outside this region, the measured Hc(T) curve exhibits features typical of the coercive field of an assembly of polydisperse magnetic nanoparticles which undergo blocking over an extended range of temperatures, typically resulting in smooth, continuous decrease in Hc with increasing measurement temperature [46]. It should be noted that a nonzero coercive field is measured even at room temperature in both samples, in agreement with the presence of large particles which are still blocked there; however, Hc is definitely lower in sample Comp-DETA_8, which contains a higher fraction of smaller particles which are in the superparamagnetic state at 300 K (see Fig. 7).
A quite complementary behavior is observed on the maximum magnetic susceptibility of the loop χMax = (dM/dH)Max (Fig. 8c). In sample Comp-DETA_2, this quantity is almost constant both above and below the Verwey-temperature region, while it almost doubles on heating from 95 to 120 K. A similar, though slightly less sharp effect is observed in sample Comp-DETA_8, where χMax exhibits some change even outside this region.
Valuable information about the distribution of Verwey temperatures in our samples can be obtained by plotting χMax as a function of the reciprocal of the coercive field Hc in a temperature region around TV, as shown in Fig. 9. The limits of the investigated temperature interval were taken at T = 95 K and T = 120 K, i.e., just before and after the jumps of Hc and χMax (Fig. 8b, c).
In fact, a functional relationship between maximum susceptibility and coercive field of the type:
is expected in systems where the magnetization process occurs by coherent rotation of the magnetization vector away from the easy axis by effect of an applied field [38]. The same condition applies to single-domain, blocked nanoparticles also (Stoner–Wohlfarth model). In Eq. (1), Ms is the intrinsic magnetization of nanoparticles (see Table 2) and α is a numeric proportionality constant which depends on the value and symmetry of the crystal anisotropy [38].
The magnetite nanoparticles embedded in these nanocomposites can be considered as being predominantly in the single-domain state, the crossover between single and multiple-domain configurations being estimated by different theoretical calculations to occur around 60–80 nm in magnetite particles [47,48,49]. Comparison with the histograms reported in Fig. 5 reveals that basically all particles are single domain in sample Comp-DETA_8, whereas a very small fraction of multiple-domain nanoparticles could be present in sample Comp-DETA_2. In the following, the contribution from multiple-domain particles, if any, will be neglected.
In the region of interest, the single-domain nanoparticles contained in the two samples can be either in the magnetically blocked or in the superparamagnetic state. The shape of the FC/ZFC curves (Fig. 6) clearly indicates that in both samples, the average blocking temperature is above room temperature; however, a fraction of superparamagnetic particles exists at room temperature, as indicated by the non-saturating behavior of the high-field magnetization (Fig. 7); in addition, the presence of a superparamagnetic fraction at even lower temperatures, and specifically around TV, cannot be excluded because of the considerable polydispersity of these systems. In principle, therefore, one should separately consider both types of nanoparticles (i.e., blocked and superparamagnetic) when the region around TV is studied.
As known, above the Verwey transition the magnetic anisotropy has cubic symmetry, with easy axes corresponding to the \( \left\langle {111 } \right\rangle \) directions of the cubic cell (the anisotropy constant K1 being negative at high temperature), while below TV the magnetic anisotropy becomes uniaxial with easy axis corresponding to the c-axis of the monoclinic cell [50, 51]. These changes in symmetry have basically no influence on the small fraction of particles which possibly are in the superparamagnetic state around TV: The temperature behavior of the magnetic anisotropy constants below and above TV [44, 51] clearly indicates that a particle already in the superparamagnetic state below TV remains in this state above TV.
As a consequence, we assume that the contribution of superparamagnetic particles to the measured magnetic properties remains basically the same over the narrow interval under consideration (T = 95 K to T = 120 K), playing no substantial role in the observed jumps of both χMax and Hc, which must be entirely ascribed to effects taking place in the magnetically blocked particles only.
In a system of blocked, single-domain nanoparticles with uniaxial anisotropy Ku and randomly distributed easy directions, the predictions of the Stoner–Wohlfarth model [38] apply:
Therefore, in the uniaxial case the α coefficient of Eq. (1) is easily calculated to be \( \alpha_{\text{u}} = 0.55 \). On the contrary, in a system of blocked single-domain nanoparticles with negative cubic anisotropy (K1 < 0) and randomly distributed easy directions, the following predictions [52] apply:
resulting in a much higher α coefficient (\( \alpha_{\text{c}} = 2.96 \)). The difference between αu and αc is deemed responsible for the difference observed in both samples between the values taken at T = 95 K and the ones taken at T = 120 K (Fig. 9). In fact, in this narrow temperature interval, the saturation magnetization can be considered as a constant (see Fig. 8a).
In the interval 95–120 K, the value of χMax steadily increases with increasing H −1c , following an almost linear law, as clearly observed in Fig. 9. Such an effect can be explained by supposing that the Verwey transition does not occur at the same temperature in all magnetite particles of the studied nanocomposites. If one considers that the fraction of nanoparticles exhibiting cubic anisotropy (fc) is equal to zero at T = 95 K and equal to one at T = 120 K, the monotonic increase in χMax with H −1c is easily associated with the growth of the transformed cubic-anisotropy fraction fc from 0 to 1. The growth curve fc(T) is obtained assuming a linear relationship between the values of χMax at each temperature in the interval 95–120 K and the value of fc at the same temperature, leading to the expression:
This growth curve turns out to be S-shaped and is shown in Fig. 10 (top panel) for both samples. The full lines are Richards curves (generalized logistic curves) which appear to accurately fit the experimental data.
Top panel: transformed cubic-anisotropy fraction fc as a function of measurement temperature for both nanocomposite samples (symbols: data obtained from Eq. (4); lines: fitting Richards growth curves). Bottom panel: normalized Verwey-temperature distribution function p(TV) for both samples
In sample Comp-DETA_8, characterized by a monomodal particle distribution and a lower mean particle size, a larger transformed fraction is observed at all temperatures below 115 K.
The derivative with respect to temperature of each fitting curve of Fig. 10 (top panel) gives the elementary transformed fraction in the temperature interval between T and T + dT; this corresponds to the fraction of particles which undergo the Verwey transition in the same temperature interval, i.e., whose Verwey transition temperature is between T and T + dT. As a consequence, the derivative of each fitting curve with respect to temperature is proportional to the actual distribution of the Verwey transition temperatures p(TV) present in either sample \( \left( {p\left( {T_{\text{V}} } \right) \propto \left. {\frac{{{\text{d}}f_{\text{c}} }}{{{\text{d}}T}}} \right|_{{T = T_{\text{V}} }} } \right) \). The resulting normalized p(TV) curves are shown in Fig. 10 (bottom panel). It should be noted that the fitting function proposed to represent fc(T) is not fully accurate in Comp-DETA_8, resulting in a rather unphysical tail of the p(TV) curve above T = 120 K (dashed line) which should therefore be discarded. The mode of the distribution is at Tm = 113.2 K and Tm = 112.2 K, and the mean value is \( \left\langle {T_{\text{V}} } \right\rangle \) = 110.5 K and \( \left\langle {T_{\text{V}} } \right\rangle \) = 109.4 K in samples Comp-DETA_2 and Comp-DETA_8, respectively. The agreement between the values estimated from the inflection points of ZFC curves and the ones obtained by the present analysis (particularly with the modal temperatures Tm) is striking. It should be explicitly reminded that ZFC curves represent the magnetic response of a demagnetized sample submitted to a very small field, whereas χMax and Hc are quantities measured on a major hysteresis loop. The agreement between the TV values estimated analyzing very different magnetic states of a sample strengthens the proposed procedure of analysis.
Finally, an estimate of the uniaxial anisotropy constant Ku of the studied nanoparticles at low temperature (T = 5 K) is obtained from the value of the coercive field (Fig. 8b). Inserting in Eq. (2) Hc (T = 5 K) ≅ 878/862 Oe, Ms ≅ 321/392 emu/cm3 (taken from the values measured on nanopowders (Table 2) extrapolated to T = 5 K), one gets Ku ≅ 2.94 × 105/3.51 × 105 erg/cm3 in samples Comp-DETA_2/8, respectively. Both values are slightly higher than the low-temperature Ku of bulk monoclinic magnetite (Ku = 2.1 × 105 emu/cm3) [44]; this is a general behavior, usually related to surface effects [20]; in the present case, the difference between the two estimates is compatible with the lower mean nanoparticle size in sample Comp-DETA_8 with respect to sample Comp-DETA_2.
Conclusion
Significant changes in magnetic properties connected to the Verwey transition have been observed in two nanocomposite polymeric materials containing polydisperse magnetite particles. Such particles were synthesized starting from the same precursors through the same chemical route, but are characterized by different size distributions and mean sizes. In both investigated materials, the transition takes place over a temperature interval of about 15 K and the average Verwey transition temperature is slightly below the value attributed to high-quality, bulk magnetite. A detailed study of the region around \( \left\langle {T_{\text{V}} } \right\rangle \) indicates that the changes in the maximum magnetic susceptibility and of the coercive field are ascribed to blocked nanoparticles only, whereas small particles which are expected to be in the superparamagnetic state do not play any important role. In blocked particles, the transition from uniaxial to cubic magnetic anisotropy takes place gradually over the temperature interval from T = 95 to T = 120 K. This behavior indicates a continuous distribution of Verwey temperatures described by the function p(TV) which has been directly obtained from the magnetic analysis. The existence of a distribution of Verwey transition temperatures is almost certainly related to the polydispersity of the investigated nanoparticle systems; however, it is not possible to directly link the p(TV) function to the actual distribution of nanoparticle sizes in the absence of more precise information about the role of particle size on the value of the Verwey transition temperature and of the physical mechanisms (related to, e.g., stoichiometry, particle shape, surface, defectiveness) which influence the transition in nanometer-sized magnetite.
References
Walz F (2002) The Verwey transition—a topical review. J Phys Condens Matter 14:R285–R340. https://doi.org/10.1088/0953-8984/14/12/203
García J, Subías G (2004) The Verwey transition—a new perspective. J Phys Condens Matter 16:R145. https://doi.org/10.1088/0953-8984/16/7/R01
Tsuya N, Arai KI, Ohmori K (1977) Effect of magnetoelastic coupling on the anisotropy of magnetite below the transition temperature. Phys B + C 86–88:959–960. https://doi.org/10.1016/0378-4363(77)90759-8
Tartaj P, Morales P, Veintemillas-verdaguer S, Gonz T (2003) The preparation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys 36:R182–R197
Sun S, Zeng H, Robinson DB et al (2004) Monodisperse MFe2O4 (M = Fe Co, Mn) nanoparticles. J Am Chem Soc 126:273–279. https://doi.org/10.1021/JA0380852
Pinna N, Grancharov S, Beato P et al (2005) Magnetite nanocrystals: nonaqueous synthesis, characterization, and solubility. Chem Mater 17:3044–3049. https://doi.org/10.1021/CM050060+
Chaichi M, Sharif F, Mazinani S (2017) Preparation and evaluation of magnetic field-induced orientation on magnetic nanoparticles on PVA nanocomposite films. J Mater Sci 53:5051–5062. https://doi.org/10.1007/s10853-017-1923-7
Li J, Chen Y, Wu Q et al (2018) Synthesis of sea-urchin-like Fe3O4/SnO2 heterostructures and its application for environmental remediation by removal of p-chlorophenol. J Mater Sci. https://doi.org/10.1007/s10853-018-2899-7
Jalil WBF, Pentón-Madrigal A, Mello A et al (2017) Low toxicity superparamagnetic magnetite nanoparticles: one-pot facile green synthesis for biological applications. Mater Sci Eng C 78:457–466. https://doi.org/10.1016/j.msec.2017.04.066
Sen T, Shimpi NG, Mishra S, Sharma R (2014) Polyaniline/γ-Fe2O3 nanocomposite for room temperature LPG sensing. Sens Actuators B Chem 190:120–126. https://doi.org/10.1016/j.snb.2013.07.091
Loh KS, Lee YH, Musa A et al (2008) Use of Fe3O4 nanoparticles for enhancement of biosensor response to the herbicide 2,4-dichlorophenoxyacetic acid. Sensors 8:5775–5791. https://doi.org/10.3390/s8095775
Mohammed L, Gomaa HG, Ragab D, Zhu J (2017) Magnetic nanoparticles for environmental and biomedical applications: a review. Particuology 30:1–14. https://doi.org/10.1016/j.partic.2016.06.001
Ghazanfari MR, Kashefi M, Shams SF, Jaafari MR (2016) Perspective of Fe3O4 nanoparticles role in biomedical applications. Biochem Res Int 2016:1–32. https://doi.org/10.1155/2016/7840161
Sarkar S, Guibal E, Quignard F, SenGupta AK (2012) Polymer-supported metals and metal oxide nanoparticles: synthesis, characterization, and applications. J Nanoparticle Res 14:715. https://doi.org/10.1007/s11051-011-0715-2
Pang J, Li Z, Li S et al (2018) Folate-conjugated zein/Fe3O4 nanocomplexes for the enhancement of cellular uptake and cytotoxicity of gefitinib. J Mater Sci 53:14907–14921. https://doi.org/10.1007/s10853-018-2684-7
Kim KJ, Park JW (2017) Stability and reusability of amine-functionalized magnetic-cored dendrimer for heavy metal adsorption. J Mater Sci 52:843–857. https://doi.org/10.1007/s10853-016-0380-z
Caruntu D, Caruntu G, O’Connor CJ (2007) Magnetic properties of variable-sized Fe3O4 nanoparticles synthesized from non-aqueous homogeneous solutions of polyols. J Phys D Appl Phys 40:5801–5809. https://doi.org/10.1088/0022-3727/40/19/001
Sciancalepore C, Gualtieri AF, Scardi P et al (2018) Structural characterization and functional correlation of Fe3O4 nanocrystals obtained using 2-ethyl-1,3-hexanediol as innovative reactive solvent in non-hydrolytic sol-gel synthesis. Mater Chem Phys 207:337–349. https://doi.org/10.1016/j.matchemphys.2017.12.089
Knobel M, Nunes WC, Socolovsky LM et al (2008) Superparamagnetism and other magnetic features in granular materials: a review on ideal and real systems. J Nanosci Nanotechnol 8:2836–2857. https://doi.org/10.1166/jnn.2008.15348
Sciancalepore C, Bondioli F, Messori M et al (2015) Epoxy nanocomposites functionalized with in situ generated magnetite nanocrystals: microstructure, magnetic properties, interaction among magnetic particles. Polymer (UK) 59:278–289. https://doi.org/10.1016/j.polymer.2014.12.047
López Maldonado KL, De La Presa P, Flores Tavizón E et al (2013) Magnetic susceptibility studies of the spin-glass and Verwey transitions in magnetite nanoparticles. J Appl Phys 113:2013–2016. https://doi.org/10.1063/1.4797628
Markovich G, Fried T, Poddar P et al (2002) Observation of the Verwey transition in Fe3O4 nanocrystals. MRS Proc 746(Q4):1. https://doi.org/10.1557/PROC-746-Q4.1
Goya GF, Berquó TS, Fonseca FC, Morales MP (2003) Static and dynamic magnetic properties of spherical magnetite nanoparticles. J Appl Phys 94:3520–3528. https://doi.org/10.1063/1.1599959
Hevroni A, Bapna M, Piotrowski S et al (2016) Tracking the Verwey transition in single magnetite nanocrystals by variable-temperature scanning tunneling microscopy. J Phys Chem Lett 7:1661–1666. https://doi.org/10.1021/acs.jpclett.6b00644
Arelaro AD, Brandl AL, Lima E et al (2005) Interparticle interactions and surface contribution to the effective anisotropy in biocompatible iron oxide nanoparticles used for contrast agents. J Appl Phys 97:2003–2006. https://doi.org/10.1063/1.1853931
Wang J, Chen Q, Li X et al (2004) Disappearing of the Verwey transition in magnetite nanoparticles synthesized under a magnetic field: implications for the origin of charge ordering. Chem Phys Lett 390:55–58. https://doi.org/10.1016/j.cplett.2004.04.005
Lee J, Kwon SG, Park J-G, Hyeon T (2015) Size dependence of metal-insulator transition in stoichiometric Fe3O4 nanocrystals. Nano Lett 15:4337–4342. https://doi.org/10.1021/acs.nanolett.5b00331
Mitra A, Mohapatra J, Meena SS et al (2014) Verwey transition in ultrasmall-sized octahedral Fe3O4 nanoparticles. J Phys Chem C 118:19356–19362. https://doi.org/10.1021/jp501652e
Shepherd JP, Koenitzer JW, Aragón R et al (1991) Heat capacity and entropy of nonstoichiometric magnetite Fe3(1−delta)O4: the thermodynamic nature of the Verwey transition. Phys Rev B 43:8461. https://doi.org/10.1103/PhysRevB.43.8461
Schmitz-Antoniak C, Schmitz D, Warland A et al (2016) Reversed ageing of Fe3O4 nanoparticles by hydrogen plasma. Sci Rep 6:2–7. https://doi.org/10.1038/srep20897
Sangermano M, Allia P, Tiberto P et al (2013) Photo-cured epoxy networks functionalized with Fe3O4 generated by non-hydrolytic sol-gel process. Macromol Chem Phys 214:508–516. https://doi.org/10.1002/macp.201200494
Niederberger M, Pinna N (2009) Metal Oxide nanoparticles in organic solvents synthesis, formation, assembly and application. Springer, Berlin
Sciancalepore C, Rosa R, Barrera G et al (2014) Microwave-assisted nonaqueous sol-gel synthesis of highly crystalline magnetite nanocrystals. Mater Chem Phys 148:117–124
Sciancalepore C, Bondioli F, Manfredini T, Gualtieri A (2015) Quantitative phase analysis and microstructure characterization of magnetite nanocrystals obtained by microwave assisted non-hydrolytic sol–gel synthesis. Mater Charact 100:88–97. https://doi.org/10.1016/j.matchar.2014.12.013
Xu R, Di Guida OA (2003) Comparison of sizing small particles using different technologies. Powder Technol 132:145–153. https://doi.org/10.1016/S0032-5910(03)00048-2
Sciancalepore C, Bondioli F, Messori M (2017) Non-hydrolytic sol–gel synthesis and reactive suspension method: an innovative approach to obtain magnetite–epoxy nanocomposite materials. J Sol-Gel Sci Technol 81:69–83. https://doi.org/10.1007/s10971-016-4095-z
Barrera G, Sciancalepore C, Messori M et al (2017) Magnetite-epoxy nanocomposites obtained by the reactive suspension method: Microstructural, thermo-mechanical and magnetic properties. Eur Polym J 94:354–365. https://doi.org/10.1016/j.eurpolymj.2017.07.022
Chikazumi S (1997) Physics of ferromagnetism. Oxford University Press, Oxford
Lin C-R, Chiang R-K, Wang J-S, Sung T-W (2006) Magnetic properties of monodisperse iron oxide nanoparticles. J Appl Phys 99:08N710. https://doi.org/10.1063/1.2172891
Allia P, Barrera G, Tiberto P (2018) Linearized rate-equation approach for double-well systems: cooling- and temperature-dependent low-field magnetization of magnetic nanoparticles. Phys Rev B 98:134423. https://doi.org/10.1103/PhysRevB.98.134423
Uva M, Mencuccini L, Atrei A et al (2015) On the mechanism of drug release from polysaccharide hydrogels cross-linked with magnetite nanoparticles by applying alternating magnetic fields: the case of DOXO delivery. Gels 1:24–43. https://doi.org/10.3390/gels1010024
Ningthoujam RS, Vatsa RK, Prajapat CL et al (2010) Interaction between amorphous ferromagnetic Co–Fe–B particles in conducting silver matrix prepared by chemical reduction route. J Alloys Compd 492:40–43. https://doi.org/10.1016/j.jallcom.2009.11.119
Craco L, Laad MS, Müller-Hartmann E (2006) Verwey transition in Fe3O4 investigated using LDA + DMFT. Phys Rev B 74:064425. https://doi.org/10.1103/PhysRevB.74.064425
Abe K, Miyamoto Y, Chikazumi S (1976) Magnetocrystalline anisotropy of low temperature phase of magnetite. J Phys Soc Jpn 41:1894–1902
Özdemir Ö (2000) Coercive force of single crystals of magnetite at low temperatures. Geophys J Int 141:351–356. https://doi.org/10.1046/j.1365-246X.2000.00081.x
Mercante LA, Melo WWM, Granada M et al (2012) Magnetic properties of nanoscale crystalline maghemite obtained by a new synthetic route. J Magn Magn Mater 324:3029–3033. https://doi.org/10.1016/j.jmmm.2012.04.049
Moskowitz BM, Banerjee SK (1979) Grain size limits for pseudosingle domain behavior in magnetite: implications for paleomagnetism. IEEE Trans Magn 15:1241–1246. https://doi.org/10.1109/TMAG.1979.1060319
Argyle KS, Dunlop DJ (1984) Theoretical domain structure in multidomain magnetite particles. Geophys Res Lett 11:185–188
Kirschvink JL, Lowenstam HA (1979) Mineralization and magnetization of chiton teeth: paleomagnetic, sedimentologic, and biologic implications of organic magnetite. Earth Planet Sci Lett 44:193–204. https://doi.org/10.1016/0012-821X(79)90168-7
Kakol Z, Honig JM (1989) Influence of deviations from ideal stoichiometry on the anisotropy parameters of magnetite Fe3(1-)O4. Phys Rev B 40:9090–9097. https://doi.org/10.1103/PhysRevB.40.9090
Reznıcek R, Chlan V, Stepankova H et al (1983) Magnetocrystalline anisotropy of magnetite. J Magn Magn Mater 31–34:813–814. https://doi.org/10.1016/0304-8853(83)90698-4
Usov NA, Peschany SE (1997) Theoretical hysteresis loops for single-domain particles with cubic anisotropy. J Magn Magn Mater 174:247–260. https://doi.org/10.1016/S0304-8853(97)00180-7
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrera, G., Tiberto, P., Sciancalepore, C. et al. Verwey transition temperature distribution in magnetic nanocomposites containing polydisperse magnetite nanoparticles. J Mater Sci 54, 8346–8360 (2019). https://doi.org/10.1007/s10853-019-03510-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03510-y