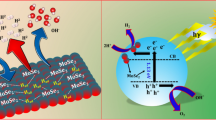
Abstract
A facile two-step hydrothermal approach is adopted to synthesize MoSe2/N-doped RGO (NG) composites with the N/C atomic percentage changing from 1.13 to 5.16 at%. In the composites, nanoclusters of MoSe2 nanosheets are dispersed on plicated NG nanosheets. The electrochemical measurement suggests that the MoSe2/NG composites exhibit enhanced electro-catalytic HER activity as compared to MoSe2 and MoSe2/RGO. Moreover, as the N/C ratio of NG is increased, the activity of MoSe2/NG increases firstly and then decreases. At low N/C ratio, the impact of interfacial energy barrier between MoSe2 and NG is negligible and the electron transfer is substantial, so the activity of the MoSe2/NG composites increases with carrier concentration in NG. However, at high N/C ratio, the energy barrier blocks the electron transfer from NG to MoSe2 remarkably. Consequently, the MoSe2/NG composites with an intermediate N/C ratio have the highest activity. Owing to the synergistic effect of NG and MoSe2, the Tafel slope of the composites is reduced from 114.69 to 78.45 mV dec−1 by 32% as compared to pure MoSe2. The results provide us valuable information for efficient design of transition metal dichalcogenide catalysts for electro-catalytic hydrogen evolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With growing population and expanding industrialization in the world, the demands for energy have been promptly increased in recent years [1]. To date, gigantic consumption of fossil fuel seriously threatens the environmental security. So it is quite urgent to develop eco-friendly energy sources replacing fossil fuels. Hydrogen energy is believed to be one of promising clean and renewable energies [2,3,4], Electro-catalytic hydrogen evolution reaction (HER) is an effective method to produce hydrogen with high efficiency [5]. Platinum (Pt) has been proved to be state-of-the-art catalyst for electro-catalytic HER, but the large-scale application is limited by its extremely high cost [6,7,8]. It is very necessary to explore cheap and efficient electro-catalysts.
MoSe2 is a typical transition metal dichalcogenides (TMDs) with lamellar structure in which each Se-Mo-Se sandwiched layer is held together by van der Waals interaction [9]. Few-layer counterparts can be fabricated by hydrothermal method [10], mechanical exfoliation [11] and chemical vapor deposition (CVD) [12, 13]. Recently, it was found that MoSe2 might be a promising electro-catalyst for HER, and the unsaturated Se edges in MoSe2 are electro-catalytically active [14, 15]. According to the DFT calculations, the hydrogen binding energy at S/Se edges of MoS2 and MoSe2 is −34.6 and −13.1 meV/f.u., respectively. So the binding of hydrogen atoms on Se edge is weaker than that on S edge, and thus a higher exchange current for hydrogen evolution was predicted for MoSe2 [17]. Moreover, the Fermi energy (E F) of MoSe2 is more close to the normal hydrogen electrode with respect to MoS2. As a result, 2H–MoSe2 is more active than 2H–MoS2 for HER, which was proved by the HER experiments [16, 17]. However, the electrical conductivity of both MoSe2 and MoS2 is not good, which restricts their activity to some degree [18, 19]. As well known, graphene sheets possess good electrical conductivity and very large specific surface area. If MoSe2 or MoS2 are dispersed on graphene sheets, their conductivity should be significantly improved. So graphene sheets are regarded as the ideal support [19,20,21,22,23]. Since N-doping can effectively regulate the electronic states and chemical features, N-doped RGO (NG) as one of the derivatives of graphene usually exhibits better performance than graphene [24, 25] and has attracted intensive attention. For instance, pyrrolic nitrogen atoms in graphene lattice were proved to be effective to activate and reduce the oxygen molecules in oxygen reduction reaction (ORR), that is, N-doping might regulate the electronic states and chemical properties of graphene [26]. In addition, NG was also regarded to be promising in HER, because nitrogen atoms in NG have strong interaction with H+ for HER [25]. For example, CdS/NG hybrid structure showed enhanced catalytic activity for H2 evolution. The doped N facilitates the reaction kinetics of HER, and further substantially improves the activity [27]. Similar effects have been reported in other systems, such as, Fe2P/NG nanocomposites and MoS2/NG hybrid [28, 29]. But the optimal N component and the mechanism is not well known.
In this paper, a facile two-step hydrothermal approach is adopted to synthesize MoSe2/NG composites with N/C ratio changing from 1.13 to 5.16 at%. It is expected that NG in the composites act as a channel for electron transfer, and the electronic states and conductivity are regulated by changing the atomic ratio of N/C. X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), Raman spectra, field emission scanning electron microscopy (FESEM) and high-resolution transmission electron microscopy (HRTEM) are used to characterize the structures of MoSe2/NG composites. The dependence of electro-catalytic activity on the carrier concentration in NG is studied, and the influence on the HER performance is discussed in details.
Experimental
Synthesis of samples
Chemical reagents
All chemical reagents, including graphite powders, sodium nitrate (NaNO3), sulfuric acid (H2SO4, 98%), potassium permanganate (KMnO4), hydrogen peroxide (H2O2, 30%), sodium molybdate dihydrate (Na2MoO4·2H2O), hydrazine hydrate (N2H4·H2O, 85%), selenium powder (Se), ammonia solution (NH3·H2O, 30%), are analytical grade and are used without further purification.
Synthesis of NG
Firstly, the GO sheets were fabricated from graphite powders by the modified Hummer’s method [30]. Then 15 mg GO was dispersed into 30 mL distilled water with magnetic stirring for 5 min and with sonication for 1 h, and 100 μL 30% ammonia solution as the nitrogen source was added into the above solution. Subsequently, different amount of N2H4·H2O was introduced to adjust the atomic ratio of N/C. Finally, the homogeneous solution was transferred into a 50-mL Teflon-lined autoclave and kept at 160 °C for 6 h. The precipitates were collected, washed by distilled water three times and by ethanol two times, and then dried at 80 °C in a vacuum oven overnight to prepare four NG samples, NG1, NG2, NG3, and NG4. The atomic ratio of N/C was measured by XPS spectra.
Synthesis of MoSe2/NG
Five milligrams of as-synthesized NG was dispersed into 10 mL distilled water with magnetic stirring for 5 min and with sonication for 1 h, then 0.484 g Na2MoO4·2H2O was added into the solution with stirring for another 5 min to form uniform solution. 0.316 g Se powder was dissolved into 5 ml hydrazine hydrate and maintained in another 10-mL flask for 24 h. Finally, the mixed solution was transferred into 50-mL Teflon-lined autoclave and kept at 220 °C for 12 h. The precipitates were collected, washed by distilled water three times and by ethanol two times, and dried at 80 °C in a vacuum oven overnight. MoSe2/RGO composites and pure MoSe2 were also prepared for comparison. To this end, RGO rather than NG was adopted in the above preparation route and pure MoSe2 in absence of NG.
Microstructure characterization
XRD (SHIMADZU XRD-7000S diffractometer) was adopted to analyze the phase structure of the as-prepared samples. FESEM (FEI Quanta 600S) and HRTEM (JEOL JEM 2100F) were adopted to characterize the morphology and microstructure of the samples. N2 adsorption–desorption isotherms were measured on Micromeritics ASAP2020. The specific surface areas were determined by the Brunauer–Emmett–Teller (BET) method. Raman spectra were examined by a Horiba HR800 spectrometer with a 633-nm laser as the excitation light source. XPS measurements were performed on Thermo Scientific K-Alpha XPS spectrometer, and the binding energies were corrected by referencing the peak of C 1s at 284.80 eV. PL spectra were detected with a PTI QM40 spectrometer using a 532-nm line from a Xenon lamp.
Electrochemical evaluation
One milligram of catalysts (MoSe2/NG, MoSe2/RGO, and MoSe2) was suspended in solution containing 400 μL ethanol and 10 μL of 5% nafion solution by sonication for 40 min. Then the uniform inks were drop-casted on carbon fiber paper (CFP) with the electrode area of 1 cm2 and dried in air for 12 h. The electrochemical experiments were done in an Autolab PGSTAT 128 N station via a standard three-electrode configuration. The CFP electrode was adopted as the working electrode, and a graphite rod and Ag/AgCl were used as counter and reference electrodes, respectively. Pt/C with the same amount of MoSe2/NG loaded on the CFP was also used as working electrode for comparison. The Ag/AgCl reference electrode was calibrated to be −0.226 V (vs. RHE) by E(RHE) = E(Ag/AgCl) + 0.197 + 0.059 pH. The electro-catalysis was measured using linear sweep voltammetry (LSV) from 0.2 to −0.5 V (vs. RHE) with a scanning rate of 5 mV/s in 0.5 M H2SO4. The electrochemical impedance spectroscopy (EIS) was measured in the frequency range from 100 kHz to 1 Hz centered at −0.2 V (vs. RHE) with an amplitude of 10 mV. The Ohmic loss in all the data (except for EIS) was corrected. The Nyquist plots were employed to fit the Rs of MoSe2, MoSe2/RGO, MoSe2/NG1, MoSe2/NG2, MoSe2/NG3, MoSe2/NG4, and the values were 1.30, 1.57, 1.49, 1.67, 1.64, and 1.53 Ω, respectively. Cyclic voltammetry (CV) was used to measure the electrochemical double-layer capacitance at non-Faradaic potential and to estimate the effective electrode surface area. The scan rates are 10, 20, 50, 100, and 200 mV s−1. The stability of electro-catalysts was examined by continuous cycling between 0 V and −0.4 V (vs. RHE) at a scanning rate of 100 mV s−1 for 1000 cycles. Before measurement, the electrolyte was deaerated with high-purity N2 for 30 min.
Results and discussion
Figure 1a shows the XPS spectra of the NG samples. The peaks at 284.8 and 400.3 eV can be assigned to the binding energies of C 1s and N 1s. Figure 1b–e displays the high-resolution XPS (HR-XPS) spectra of N 1s. Evidently, nitrogen dopants have been successfully introduced into the samples; moreover, the N 1s peak is enhanced gradually from samples NG1 to NG4, indicating the increased N component. The atomic percentage of N/C in NG1, NG2, NG3, and NG4 is 1.13, 1.94, 3.0, and 5.16 at%, respectively, and the results are listed in Table 1. The HR-XPS peak of N 1s can be resolved into three peaks at 398.4, 400.1, and 401.3 eV corresponding to pyridinic-N, pyrrolic-N, and graphitic-N, respectively, [31] as listed in Table 1. More than 50% N exists in the formation of pyrrolic-N, about 20% in graphitic-N, and 10% in pyridinic-N. The pyridinic-N in NG sheets might result in new electronic states at Fermi level and improve the electric conductivity [32].
Figure 2 shows the XRD patterns of the as-synthesized MoSe2, MoSe2/RGO, and MoSe2/NG composites. The diffraction peaks of MoSe2 can be perfectly indexed to the (002), (100), (103), (105), and (110) planes of hexagonal 2H-MoSe2 (JCPDS 29-0914) [33]. However, no diffraction peak of RGO or NG is observed in MoSe2/RGO and MoSe2/NG composites, and the peaks of composites are similar to those in pure MoSe2. It indicates that the RGO or NG is not well stacked during the hydrothermal process [34], and RGO and NG affect the growth of MoSe2 nanosheets little. The broad diffraction peaks suggest nanosize feature of MoSe2 sheets [10]. The full width at half maximum (FWHM) of diffraction peaks of MoSe2/RGO and MoSe2/NG composites is listed in Table S1[Electronic Supplementary Information], and it is slightly larger than that of pure MoSe2 owing to the lowered crystallinity in the composites since RGO and NG sheets promote the nucleation and thus reduce the size of MoSe2 nanosheets.
Figure 3 shows the Raman spectra of the samples. The Raman peaks at 237 and 281 cm−1 are from the out-of-plane A 1g and in-plane \( E_{2g}^{1} \) vibration modes in hexagonal MoSe2 [10] In all the samples, the intensity of \( E_{2g}^{1} \) mode is lower than that of A 1g mode, characteristics of edge-rich feature in MoSe2 nanosheets [35]. The peaks at 1334 and 1584 cm−1 corresponding to the D- and G-bands of graphene can be identified from the spectra of MoSe2/RGO and MoSe2/NG samples [36, 37]. So the MoSe2/RGO and MoSe2/NG composites are successfully fabricated via a two-step hydrothermal method. The intensity ratio of I D/I G was usually adopted to evaluate the structural disorder and defects in graphene [38,39,40]. The I D/I G ratio of MoSe2/RGO, MoSe2/NG1, MoSe2/NG2, MoSe2/NG3, and MoSe2/NG4 is determined as 1.03, 1.14, 1.19, 1.21, and 1.28, respectively. Obviously, the I D/I G ratio increases with the N/C ratio gradually, that is, N-doping leads to structural disorder in NG, which is consistent with the reported results [41].
Figures 4a–f displays the SEM images of pure MoSe2, MoSe2/RGO, MoSe2/NG1, MoSe2/NG2, MoSe2/NG3 and MoSe2/NG4, respectively. As shown in Fig. 4a, pure MoSe2 exists in nanoclusters which are composed of nanosheets. The lamellar RGO and NG with plicated feature are clearly observed in MoSe2/RGO and MoSe2/NG composites; moreover, MoSe2 nanoclusters are tightly intertwined with RGO and NG sheets. It is worth noting that the plicated feature becomes more remarkable in MoSe2/NG composites, particularly, in the composites with the higher N/C ratio. N2 adsorption–desorption isotherm curves are measured to evaluate the specific surface area of the as-prepared samples according to the BET method, and the results are displayed in Figure S1. The specific surface area of MoSe2, MoSe2/RGO, MoSe2/NG1, MoSe2/NG2, MoSe2/NG3, and MoSe2/NG4 is 22.4, 29.5, 34.2, 29.3, 32.2, and 31.8 m2 g−1, respectively. Obviously, the composites have much larger specific surface area as compared to the pure MoSe2. Taking the MoSe2/NG3 composite as an example, Fig. 5a–c show the TEM and HRTEM images, and SAED patterns. It can be seen from Fig. 5a that the diameter of MoSe2 nanoclusters is 120~150 nm. Ultra-thin NG3 sheets are distinguished from the TEM images. Lamellar structures with an interplanar spacing of 0.66 nm corresponding to (002) planes of MoSe2 can be identified from the HRTEM image (Fig. 5b). The SAED patterns in Fig. 5c suggest that MoSe2 in MoSe2/NG3 sample is polycrystalline, and the diffraction rings correspond to (100), (103), and (110) planes of hexagonal 2H–MoSe2, as shown in XRD patterns.
The electro-catalytic HER performance of MoSe2, MoSe2/RGO, and MoSe2/NG is evaluated by using LSV. Commercial Pt/C catalyst on CFP is also examined for comparison. Ohmic resistance (iR) affects the intrinsic performance, Fig. 6a shows the LSV data after correction. Among all the samples, pure MoSe2 samples exhibit the smallest current density, but the current density is substantially enhanced in MoSe2/RGO and MoSe2/NG composites. The overpotential required at a current density of 10 mA cm−2 (η 10) is usually regarded as a benchmark to evaluate the activity of HER catalysts. The η 10 of MoSe2/RGO, MoSe2/NG1, MoSe2/NG3, and MoSe2/NG4 is lower than that of pure MoSe2 (−0.261 V vs. RHE). In particular, MoSe2/NG2 has the lowest η 10 of −0.229 V (vs. RHE). To further check the activity of the samples, the overpotential required at a current density of 50 mA cm−2 (η 50) is measured, and the results are listed in Table 1. As compared to pure MoSe2 (−0.417 V vs. RHE), the η 50 of MoSe2/RGO composites (−0.345 V vs. RHE) is lowered by 17%. That is, the synergistic effect of RGO and MoSe2 improves the activity greatly. The η 50 value of the MoSe2/NG composites is further reduced. So N-doping into graphene could enhance the activity further. Notably, MoSe2/NG2 exhibits the best activity owing to the smallest η 50. Tafel slope is an important parameter describing the HER performance [42]. The smaller Tafel slope corresponds to the lower overpotential required for an increased current density [43]. The Tafel plots are derived from the polarization curves, and the linear portion is fitted as following:
in which η is the overpotential, j is the current density, and b is the Tafel slope [15]. As shown in Fig. 6b and Table 1, the Tafel slope of MoSe2/RGO is calculated as 90.87 mV dec−1, which is smaller than that of pure MoSe2 (114.69 mV dec−1) by 20%. The Tafel slope is further reduced to 82.36, 78.45, 84.06, and 81.57 mV dec−1 for MoSe2/NG1, MoSe2/NG2, MoSe2/NG3, and MoSe2/NG4 composites, respectively. Among them, MoSe2/NG2 possesses the smallest Tafel slope, indicating the best HER activity. The results are in agreement with the above LSV analysis. Importantly, the Tafel slope can be used to distinguish the pathways of the reaction in the HER process in acidic electrolyte [44, 45]. In the primary discharge step, Volmer reaction occurs: \( {\text{H}}_{3} {\text{O}}^{ + } + {\text{e}}^{ - } \to {\text{H}}_{\text{ads}} + {\text{H}}_{2} {\text{O}} \), which is followed by either an electrochemical desorption step (Heyrovsky reaction): \( {\text{H}}_{\text{ads}} + {\text{H}}_{3} {\text{O}}^{ + } + {\text{e}}^{ - } \to {\text{H}}_{2} + {\text{H}}_{2} {\text{O}} \), or a recombination step (Tafel reaction): \( {\text{H}}_{\text{ads}} + {\text{H}}_{\text{ads}} \to {\text{H}}_{2} \). At the slope of about 120 mV dec−1, Volmer reaction is indeed the rate-limiting process for HER, but Heyrovsky or Tafel reaction is the rate-limiting procedure at a Tafel slope of 40 and 30 mV dec−1. According to the fitted data, Volmer reaction seems to be the rate-limiting process in pure MoSe2 samples, but Volmer–Heyrovsky or Volmer–Tafel mechanism for MoSe2/RGO and MoSe2/NG composites. Obviously, the synergistic effect of RGO/NG and MoSe2 changes the rate-limiting mechanism. For comparison, the HER performances of various MoS2 and MoSe2 related materials have been added in Table S2 (Electronic Supplementary Information). As compared to the reported results, the small overpotential and Tafel slope indicate good electro-catalytic activity for HER of the as-prepared MoSe2/NG composites in this work.
The double-layer capacity, C dl, is used to determine the electrochemically active surface area (ECSA) of catalyst. Figure 6c shows the C dl calculated from the CV curves (Figure S2). The C dl value of MoSe2/RGO and MoSe2/NG composites are substantially increased as compared to pure MoSe2, which can be ascribed to the dispersed MoSe2 nanosheets and more active sites exposed, owing to the large specific surface area. So, they exhibit enhanced HER activity. Nearly the same C dl in MoSe2/RGO and MoSe2/NG composites indicates no dependence of C dl on the N/C ratio. Electrochemical impedance spectroscopy (EIS) is measured to study the reactions at the electrode/solution interface and the electron transfer kinetics in the HER process [42]. The Nyquist plots are displayed in Fig. 6d, and a facile Randle equivalent circuit is plotted in the inset of Fig. 6d to model the impedance data [46]. The values of R s and charge transfer resistance (R ct) are listed in Table 1. R s is all about 1.5 Ω, and the low value indicates an intimate contact between catalysts and CFP substrate. The R ct derived from the low frequency zone is related to the electro-catalytic kinetics at the interface between electro-catalysts and electrolyte, and a lower R ct corresponds to a faster electron transfer [47]. As listed in Table 1, the MoSe2/RGO and MoSe2/NG composites have smaller R ct than pure MoSe2, among them, that of the MoSe2/NG2 composite is the smallest one (6.82 Ω). So the electron transfer at electrode/solution interface in MoSe2/NG2 is fastest, resulting in the best performance.
In fact, RGO and NG sheets have been adopted as the supports to improve the electro-catalytic HER activity. The good electrical conductivity of NG sheets makes it more effective than RGO. Figure 7a presents the Mott–Schottky curves. Accordingly, the carrier concentration in NG is fitted, and the results are listed in Table S3. The carrier concentration gradually increases with N/C ratio owing to more pyridinic-N-doped in NG, [32] and the activity of MoSe2/NG should increase correspondingly. But the experimental results deviate from this trend slightly. As displayed in Fig. 7b, the slope of the Mott–Schottky curve of pure MoSe2 is negative, characteristic of p-type feature. It changes from p-type to n-type upon N-doping, which is confirmed by the positive slope of Mott–Schottky curve of NG (Fig. 7a). A p–n junction might be formed when p-MoSe2 is stacked on n-NG. So a narrow depletion layer emerges at the interface between MoSe2 and NG (Fig. 7c), resulting in an energy barrier, which will block the electron transfer from NG to MoSe2 during electro-catalytic HER process. PL spectra are measured to examine the change of energy barrier at interface. If the energy barrier is higher, the photo-induced electron and holes will be more effectively separated from each other and the intensity of PL peak will be lowered. As shown in Fig. 7d, the intensity of PL peak of MoSe2/NG decreases with increasing N/C ratio gradually. It can be inferred that the energy barrier between MoSe2 and NG increases with N/C ratio, namely, the electron transfer at interface is not efficient at high N/C ratio. So both the electrical conductivity of NG and the energy barrier at interface affect the HER activity. At low N/C ratio (1.13 and 1.94 at%), the activity of MoSe2/NG increases with carrier concentration in NG because of negligible energy barrier and enhanced electron transfer. However, at high N/C ratio (3.0 and 5.16 at%), the energy barrier at interface will block the electron transfer from NG to MoSe2 substantially so that the activity of MoSe2/NG3 and MoSe2/NG4 with high carrier concentration, on the contrary, is lowered as compared to MoSe2/NG2. To assess the stability of MoSe2/NG2 composites in the HER process, [48] continuous HER test for 1000 cycles are conducted. The polarization curves before and after 1000 cycles are shown in Fig. 8. The HER activity decreases only a little after 1000 cycles, so the stability of MoSe2/NG2 in HER is good.
Conclusion
In summary, MoSe2/NG composites are synthesized through a facile two-step hydrothermal approach. XPS, XRD, FESEM, TEM, and Raman spectra are adopted to characterize the structures and morphologies. In the composites, nanoclusters of MoSe2 nanosheets are dispersed on plicated NG nanosheets. As the N/C ratio is increased, the plicated feature of NG becomes more remarkable, and the carrier concentration in NG increases gradually. The electrochemical measurement shows the synergistic effect of N-doping in RGO and MoSe2 can greatly improve the activity for HER. The activity of MoSe2/NG depends not only on the carrier concentration in NG but also on the interfacical energy barrier between MoSe2 and NG. At low N/C ratio, the activity of MoSe2/NG increases with carrier concentration in NG because of the negligible energy barrier and remarkable electron transfer. However, at high N/C ratio, the energy barrier becomes high enough to block the electron transfer substantially. Consequently, the highest activity can be obtained in MoSe2/NG composites with an intermediate N/C ratio. It suggests us that an appropriate N-doping is indispensible to improve the HER activity of TMDs/RGO hybrid structures.
References
Zhuang M, Ou X, Dou Y et al (2016) Polymer-embedded fabrication of Co2P nanoparticles encapsulated in N, P-doped graphene for hydrogen generation. Nano Lett 16:4691–4698. doi:10.1021/acs.nanolett.6b02203
Xie J, Zhang H, Li S et al (2013) Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv Mater 25:5807–5813. doi:10.1002/adma.201302685
An L, Huang L, Zhou P, Yin J, Liu H, Xi P (2015) A self-standing high-performance hydrogen evolution electrode with nanostructured NiCo2O4/CuS heterostructures. Adv Funct Mater 25:6814–6822. doi:10.1002/adfm.201503784
Zhou W, Lu J, Zhou K et al (2016) CoSe2 nanoparticles embedded defective carbon nanotubes derived from MOFs as efficient electrocatalyst for hydrogen evolution reaction. Nano Energy 28:143–150. doi:10.1016/j.nanoen.2016.08.040
Zhong X, Sun Y, Chen X, Zhuang G, Li X, Wang J-G (2016) Mo doping induced more active sites in urchin-like W18O49 nanostructure with remarkably enhanced performance for hydrogen evolution reaction. Adv Funct Mater 26:5778–5786. doi:10.1002/adfm.201601732
Dai X, Li Z, Du K et al (2015) Facile synthesis of in–situ nitrogenated graphene decorated by few-layer MoS2 for hydrogen evolution reaction. Electrochim Acta 171:72–80. doi:10.1016/j.electacta.2015.05.017
Kong D, Wang H, Lu Z, Cui Y (2014) CoSe2 nanoparticles grown on carbon fiber paper: an efficient and stable electrocatalyst for hydrogen evolution reaction. JACS 136:4897–4900. doi:10.1021/ja501497n
Zhang Y, Zuo L, Zhang L et al (2016) Cotton wool derived carbon fiber aerogel supported few-layered MoSe2 nanosheets as efficient electrocatalysts for hydrogen evolution. ACS Appl Mater Interfaces 8:7077–7085. doi:10.1021/acsami.5b12772
Zhang Y, Chang TR, Zhou B et al (2014) Direct observation of the transition from indirect to direct bandgap in atomically thin epitaxial MoSe2. Nat Nanotechnol 9:111–115. doi:10.1038/nnano.2013.277
Tang H, Dou KP, Kaun CC, Kuang Q, Yang SH (2014) MoSe2 nanosheets and their graphene hybrids: synthesis, characterization and hydrogen evolution reaction studies. J Mater Chem A 2:360–364. doi:10.1039/C3ta13584e
Larentis S, Fallahazad B, Tutuc E (2012) Field-effect transistors and intrinsic mobility in ultra-thin MoSe2 layers. Appl Phys Lett 101:223104. doi:10.1063/1.4768218
Xia J, Huang X, Liu L-Z et al (2014) CVD synthesis of large-area, highly crystalline MoSe2 atomic layers on diverse substrates and application to photodetectors. Nanoscale 6:8949–8955. doi:10.1039/c4nr02311k
Shim GW, Yoo K, Seo S-B et al (2014) Large-area single-layer MoSe2 and its van der Waals heterostructures. ACS Nano 8:6655–6662. doi:10.1021/nn405685j
Lei Z, Xu S, Wu P (2016) Ultra-thin and porous MoSe2 nanosheets: facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction. PCCP 18:70–74
Xu C, Peng S, Tan C et al (2014) Ultrathin S-doped MoSe2 nanosheets for efficient hydrogen evolution. J Mater Chem A 2:5597–5601. doi:10.1039/c4ta00458b
Gong Q, Cheng L, Liu C et al (2015) Ultrathin MoS2(1–x )Se2x alloy nanoflakes for electrocatalytic hydrogen evolution reaction. ACS Catal 5:2213–2219. doi:10.1021/cs501970w
Gupta U, Naidu BS, Maitra U et al (2014) Characterization of few-layer 1T-MoSe2 and its superior performance in the visible-light induced hydrogen evolution reaction. APL Mater 2:092802. doi:10.1063/1.4892976
Yuan HY, Li JY, Yuan C, He Z (2014) Facile synthesis of MoS2@CNT as an effective catalyst for hydrogen production in microbial electrolysis cells. Chemelectrochem 1:1828–1833. doi:10.1002/celc.201402150
Liao L, Zhu J, Bian X et al (2013) MoS2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv Funct Mater 23:5326–5333. doi:10.1002/adfm.201300318
Gopalakrishnan K, Pramoda K, Maitra U, Mahima U, Shah MA, Rao CNR (2015) Performance of MoS2-reduced graphene oxide nanocomposites in supercapacitors and in oxygen reduction reaction. Nanomater Energy 4:9–17. doi:10.1680/nme.14.00024
Ma C-B, Qi X, Chen B et al (2014) MoS2 nanoflower-decorated reduced graphene oxide paper for high-performance hydrogen evolution reaction. Nanoscale 6:5624–5629
Zhang Z, Lu B, Hao J, Yang W, Tang J (2014) FeP nanoparticles grown on graphene sheets as highly active non-precious-metal electrocatalysts for hydrogen evolution reaction. Chem Commun 50:11554–11557
Pramoda K, Gupta U, Ahmad I, Kumar R, Rao CNR (2016) Assemblies of covalently cross-linked nanosheets of MoS2 and of MoS2–RGO: synthesis and novel properties. J Mater Chem A 4:8989–8994. doi:10.1039/c6ta00645k
Zhong J, Deng J-J, Mao B-H et al (2012) Probing solid state N-doping in graphene by X-ray absorption near-edge structure spectroscopy. Carbon 50:335–338. doi:10.1016/j.carbon.2011.08.046
Pan Y, Yang N, Chen Y et al (2015) Nickel phosphide nanoparticles-nitrogen-doped graphene hybrid as an efficient catalyst for enhanced hydrogen evolution activity. J Power Sources 297:45–52. doi:10.1016/j.jpowsour.2015.07.077
Gopalakrishnan K, Rao CNR (2015) Remarkable performance of heavily nitrogenated graphene in the oxygen reduction reaction of fuel cells in alkaline medium. Mater Res Exp 2:095503. doi:10.1088/2053-1591/2/9/095503
Jia L, Wang D-H, Huang Y-X, Xu A-W, Yu H-Q (2011) Highly durable N-Doped graphene/CdS nanocomposites with enhanced photocatalytic hydrogen evolution from water under visible light irradiation. J Phys Chem C 115:11466–11473. doi:10.1021/jp2023617
Huang Z, Lv C, Chen Z, Chen Z, Tian F, Zhang C (2015) One-pot synthesis of diiron phosphide/nitrogen-doped graphene nanocomposite for effective hydrogen generation. Nano Energy 12:666–674
Zhao K, Gu W, Zhao L, Zhang C, Peng W, Xian Y (2015) MoS2/Nitrogen-doped graphene as efficient electrocatalyst for oxygen reduction reaction. Electrochim Acta 169:142–149. doi:10.1016/j.electacta.2015.04.044
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. JACS 80:1339. doi:10.1021/ja01539a017
Du C, Huang H, Feng X, Wu S, Song W (2015) Confining MoS2 nanodots in 3D porous nitrogen-doped graphene with amendable ORR performance. J Mater Chem A 3:7616–7622. doi:10.1039/c5ta00648a
Ma J, Habrioux A, Luo Y et al (2015) Electronic interaction between platinum nanoparticles and nitrogen-doped reduced graphene oxide: effect on the oxygen reduction reaction. J Mater Chem A 3:11891–11904. doi:10.1039/c5ta01285f
Mao S, Wen ZH, Ci SQ, Guo XR, Ostrikov K, Chen JH (2015) Perpendicularly oriented MoSe2/graphene nanosheets as advanced electrocatalysts for hydrogen evolution. Small 11:414–419. doi:10.1002/smll.201401598
Chang K, Mei Z, Wang T, Kang Q, Ouyang S, Ye J (2014) MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 8:7078–7087. doi:10.1021/nn5019945
Kong DS, Wang HT, Cha JJ et al (2013) Synthesis of MoS2 and MoSe2 films with vertically aligned layers. Nano Lett 13:1341–1347. doi:10.1021/Nl400258t
Zhang L, Sun L, Liu S, Huang YH, Xu KW, Ma F (2016) Effective charge separation and enhanced photocatalytic activity by the heterointerface in MoS2/reduced graphene oxide composites. RSC Adv 6:60318–60326. doi:10.1039/c6ra10923c
Pham TT, Santos CN, Joucken F, Hackens B, Raskin J-P, Sporken R (2016) The role of SiC as a diffusion barrier in the formation of graphene on Si(111). Diam Relat Mater 66:141–148. doi:10.1016/j.diamond.2016.04.011
He L, Jing L, Luan Y, Wang L, Fu H (2014) Enhanced visible activities of α-Fe2O3 by coupling N-doped graphene and mechanism insight. ACS Catal 4:990–998. doi:10.1021/cs401122e
Iamprasertkun P, Krittayavathananon A, Sawangphruk M (2016) N-doped reduced graphene oxide aerogel coated on carboxyl-modified carbon fiber paper for high-performance ionic-liquid supercapacitors. Carbon 102:455–461. doi:10.1016/j.carbon.2015.12.092
Wang C, Kang J, Sun H, Ang HM, Tade MO, Wang S (2016) One-pot synthesis of N-doped graphene for metal-free advanced oxidation processes. Carbon 102:279–287. doi:10.1016/j.carbon.2016.02.048
Asedegbega-Nieto E, Perez-Cadenas M, Morales MV et al (2014) High nitrogen doped graphenes and their applicability as basic catalysts. Diam Relat Mater 44:26–32. doi:10.1016/j.diamond.2014.01.019
Huang J, Chen F, Zhang Q et al (2015) 3D silver nanoparticles decorated zinc oxide/silicon heterostructured nanomace arrays as high-performance surface-enhanced raman scattering substrates. ACS Appl Mater Interfaces 7:5725–5735. doi:10.1021/am507857x
Wang K, Xi D, Zhou C et al (2015) CoSe2 necklace-like nanowires supported by carbon fiber paper: a 3D integrated electrode for the hydrogen evolution reaction. J Mater Chem A 3:9415–9420. doi:10.1039/c5ta01143d
Conway BE, Tilak BV (2002) Interfacial processes involving electrocatalytic evolution and oxidation of H2, and the role of chemisorbed H. Electrochim Acta 47:3571–3594. doi:10.1016/S0013-4686(02)00329-8
Li Y, Wang H, Xie L, Liang Y, Hong G, Dai H (2011) MoS2 nanoparticles grown on graphene: an advanced catalyst for the hydrogen evolution reaction. JACS 133:7296–7299. doi:10.1021/ja201269b
Chen X, Liu G, Zheng W et al (2016) Vertical 2D MoO2/MoSe2 core-shell nanosheet arrays as high-performance electrocatalysts for hydrogen evolution reaction. Adv Funct Mater 26:8537–8544. doi:10.1002/adfm.201603674
Wang K, Ye Z, Liu C et al (2016) Morphology-controllable synthesis of cobalt telluride branched nanostructures on carbon fiber paper as electrocatalysts for hydrogen evolution reaction. ACS Appl Mater Interfaces 8:2910–2916. doi:10.1021/acsami.5b10835
Zhou H, Yu F, Huang Y et al (2016) Efficient hydrogen evolution by ternary molybdenum sulfoselenide particles on self-standing porous nickel diselenide foam. Nat Commun 7:12765. doi:10.1038/ncomms12765
Acknowledgements
This work was jointly supported by National Natural Science Foundation of China (Grant Nos. 51471130, 51771144, 51501012, 51601142), Natural Science Foundation of Shaanxi Province (No.2017JZ015),the fund of the State Key Laboratory of Solidification Processing in NWPU (SKLSP201708), and Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, L., Sun, L., Huang, Y. et al. Hydrothermal synthesis of N-doped RGO/MoSe2 composites and enhanced electro-catalytic hydrogen evolution. J Mater Sci 52, 13561–13571 (2017). https://doi.org/10.1007/s10853-017-1417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1417-7