Abstract
In this paper, Bi3+- and Eu3+-co-doped ScVO4 had been investigated as an alternative red-emitting phosphor under near-ultraviolet excitation. Structural and spectroscopic characterizations were carried out. The results show that the samples can efficiently absorb near-ultraviolet light and produce very bright red emission. In particular, the optimized sample shows much stronger emission intensity than the commercial phosphor Y2O2S: 5% Eu3+ in the excitation range of 365–395 nm, and can reach a strength 5.4 times of the latter under 385 nm excitation. It also has better CIE chromaticity coordinates. These suggest ScVO4: Bi3+, Eu3+ as a promising red-emitting phosphor for white light-emitting diodes based on near-ultraviolet light-emitting diode chips.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid-state white light-emitting diodes (LEDs) developments have been blooming since the landmark achievement of bright-blue LEDs came out in the mid-1990s. A typical white LED, covering the broadband yellow-emitting phosphor YAG: Ce3+ on a blue LED chip, has been widely applied in many fields, owing to its superior properties such as high efficiency, energy-saving, friendly to the environment and long lifetime. Unfortunately, the major drawback of lacking red light component limits such white-lighting fixture to have a poor color rendering index (CRI, usually <75) and high correlated color temperature (CCT, 4000–8000 K) [1,2,3,4,5,6,7]. To generate a warm white light (CRI >80; CCT <4000 K), there is much room to improve. Many strategies have been proposed and that coating red–green–blue (RGB) tricolor phosphors on near-ultraviolet (NUV) LED chips (365–395 nm) is considered as one of the optimal [8].
In contrast to blue and green components, red-emitting phosphors still demand great attentions to enhance their performance [9]. Divalent Eu ion (Eu2+)-activated nitrides can give off very bright red light, which, however, is always quite far into deep red region where human eye is insensitive. Moreover, they could absorb visible light covering as far as the red spectral scope thus may suffer from color change and lower luminous efficiency (efficiency of light conversion subject to the human eye sensitivity) [6, 10]. Trivalent Eu ion (Eu3+) is considered as an ideal source of red light with excitation lines at NUV region. Traditional high-efficient red-emitting phosphors activated by Eu3+ ions such as Y2O2S: Eu3+ and YVO4: Eu3+, however, show a dramatic decrease in excitation efficiency in the NUV region and a tiny, narrow absorption around 395 nm, not suitable for NUV-activated white light applications. Therefore, host materials that can efficiently absorb NUV light and then transfer to red-emitting center is imperative.
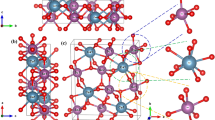
For orthovanadates, the polarization of the oxygen ions resulting from the introduction of foreign ions in the host lattice influences the position of charge-transfer bands. By replacing Y3+ with trivalent cation with a smaller radius, such as Sc3+ and Lu3+, the absorption band of LnVO4 (Ln = Lu or Sc) doped with Eu3+ had a large red shift, which would be more significant upon introduction of Bi3+ ion [11, 12]. A sketch (Fig. 1) presents the energy absorption and transfer mechanism in LnVO4: Bi3+, Eu3+. Besides the famous charge-transfer (CT) transition between O2− and V5+ (O–V), the metal–metal CT transition between Bi3+ and V5+ is also taken into account in view of the 6s level of Bi3+ that lies above the highest filled 2p level of O2− and the electron in the 6s level is apt to transfer to the empty 3d level of V5+. The absorption band is accordingly extended to violet range upon introduction of Bi3+. Moreover, the high energy levels of Eu3+ are embedded into the conduction band (V-3d states), so the energy transfer to Eu3+ could be highly efficient.
In this work, through a conventional high-temperature solid-state reaction, we synthesize samples of ScVO4: Bi3+, Eu3+ and optimize the doping proportion to achieve the best luminous efficiency. Under NUV light excitation, the obtained products show an excellent performance which will be discussed as follows.
Experimental
The samples of ScVO4: Bi3+, Eu3+ were synthesized through a conventional high-temperature solid-state reaction in air. The started materials were Sc2O3 (99.99%), Bi2O3 (AR), V2O5 (99.99%), Eu2O3 (99.99%). First, the raw materials were mixed according to a stoichiometric ratio and ground in an agate mortar. Then, the mixture was transferred into a ceramic crucible. The presintering step was performed at 750 °C for 4 h in a muffle furnace. After that, the samples were ground again and sintered at 1000 °C for 5 h. Finally, the obtained samples were ground to be powders for the subsequent measurements.
The XRD data were recorded via X-ray diffractometer (MAC Science Co. Ltd MXP18AHF), using nickel-filtered Cu Kα radiation (λ = 1.5418 Å) in the range of 2θ = 10–70º. The excitation and emission spectra were recorded with a Hitachi 850 fluorescence spectrophotometer using a 150-W xenon lamp as the excitation source. The decay curves were measured with a Tektronix TDS2024 digital storage oscilloscope under the excitation of 355 nm pulse laser yielded in a Q-switched flash-pumped Nd: YAG laser with the pulse duration of 10 ns.
Results and discussion
XRD characterization
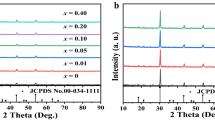
The XRD patterns of the samples are shown in Fig. 2, along with the standard data of ScVO4 (PDF-6-260). All the strong diffraction peaks agree well with the standard data, which belong to the zircon-type structure of space group I41/amd. Bi3+ and Eu3+ ions are supposed to occupy the Sc3+ sites, and because of larger ionic radii of Bi3+ and Eu3+ than Sc3+, the peaks shift slightly to small angle with increasing the doping proportion of Bi3+ or Eu3+. It is noteworthy that two tiny impurity peaks at 29° and 25° emerge when the doping increases to a relatively heavy level, which are, respectively, ascribed to the main diffraction peak of BiVO4 and EuVO4. Such impurity will shrink the expected concentration of the concerned ion in the final product, suggesting heavy doping in this host is unsuitable.
Luminescent properties
Figure 3 shows the luminescent properties of ScVO4 doped with Eu3+ and Bi3+ samples, composed of excitation spectra (a) monitored the emission at 616 nm (Eu3+, 5D0 → 7F2) and emission spectra under 395 nm excitation for samples with different doping concentrations of Bi3+ (b) and Eu3+ (c). In the excitation spectra, besides the weak narrow peaks from 450 to 550 nm corresponding to the Eu3+ 4f–4f transitions, there are intense broadbands covering from 200 to 430 nm which are attributed to the overlap of O–Eu, O–V and Bi–V CT transitions, as proposed earlier [11]. It is of significance that upon introduction of Bi3+ ions in the host a striking shoulder bursts from 350 to 430 nm attaching to the dominant O–V CT transition centered at 326 nm. This shoulder has been identified as the Bi–V metal–metal CT transition, ascribed to electron transfer from Bi-6s state to V-3d state [11]. It should be noted that the charge-transfer transition is allowed by the spin and parity selection rules, which is responsible for the efficient absorption of NUV light, and here overwhelmingly outshines the parity-forbidden intra-4f transition of Eu3+. It should also be worthy of mention that for many Eu3+-activated red-emitting materials, the near-ultraviolet absorption around 395 nm is usually attributed to the transition of 7F0 → 5L6, which is always weak unless the doping is heavy. Moreover, shielded by the filled 5s 2 and 5p 6 shell, the intra-4f transition is generally narrow and sharp, leading the excitation efficiency to be vulnerable to the tiny emission wavelength shift of the NUV LED chip at various temperatures. Fortunately, owing to the sensitization of Bi3+, the current material ScVO4: Bi3+, Eu3+ has shown a broad excitation band covering NUV, indicating it a promising candidate for excellent NUV-excited red-emitting phosphor.
On the other hand, the presence of energy transfer from CT band to Eu3+ ions can be verified by the observation of the charge-transfer band in the excitation spectra of Eu3+ ions. When the doping concentration of Bi3+ increases, the O–V CT band decreases monotonously, while the Bi–V CT band rises to the maximum until the doping concentration of Bi3+ reaches 2%. As elucidated in Fig. 1, two relaxation paths are considered for the excitation energy in this material: One is quenching in Bi3+ ions, and the other is being emitted by Eu3+ ions. The decline of O–V CT band in the Eu3+ excitation spectra is ascribed to the increase in the energy transfer from O–V CT band to Bi3+, and part of the excitation energy would subsequently be quenched in Bi3+ ions. The excitation energy transferred to Eu3+ ions is thus reduced, resulting in a decline of O–V CT band in the excitation spectra.
The rising of Bi–V band is owing to the increase in the absorption of Bi–V CT band and the energy transfer possibility to Eu3+ ions, resulting in a stronger Eu3+ emission, i.e., a higher Bi–V CT band. However, when the doping concentration of Bi3+ exceeded 2%, energy transfer from Bi–V CT band to Eu3+ ions would be reduced due to the fast increase in nonradiative relaxation in Bi3+ ions, leading to the suppression of Bi–V CT band in the excitation spectra. So, it is conclusive that the excitation energy prefers to quench in Bi3+ ions rather than transfer to Eu3+ in high doping concentration of Bi3+. In other words, the doping of Bi3+ should not be high in this host material for good luminescence. A similar phenomenon was also reported in Ref. 13 for Bi3+ singly doped ScVO4 samples. Only in the case of very low Bi3+ doping concentration (usually lower than 2 or 1%), exciting into O–V CT band could give off the Bi3+ broadband 3P1 → 1S0 emission centered at 635 nm. This emission drops quickly with increasing the doping concentration of Bi3+ because the nonradiative relaxation would dominate [13].
Under 395 nm light excitation, the samples doped with Eu3+ emit very bright red light, corresponding to 5D0 → 7F2 transition of Eu3+. As seen in Fig. 3b, the Eu3+ electric dipole transition 5D0 → 7F2 is much stronger than magnetic dipole transition 5D0 → 7F1. It is because the Sc3+ site in ScVO4 has very large deviation from centrosymmetry. Such site surrounding is desired for Eu3+-activated red-emitting phosphor to gain a suitable color rendering index. The samples doped with 2 and 4% Bi3+ yield apparently the stronger red emission, submerged by a very week broadband. In view of the V–O CT band emission has a quenching temperature far below room temperature and always locates around 465 nm, the week bands mentioned above are accordingly considered to originate from Bi3+ 3P1 → 1S0 transition centered at 635 nm [13], and here is too weak to significantly alter the energy distribution of emission. So, it can be concluded that the excitation energy could be efficiently transferred to Eu3+ when the doping concentration of Bi3+ is below 2%. For those with higher doping concentration, the quenching in Bi3+ ions grows rapidly to dominate the majority of the relaxation, resulting in much weaker Eu3+ emission. This is in accordance with the situation in excitation spectra.
The dependence of integrated emission intensity on the doping concentrations of Eu3+ or Bi3+ is plotted in Fig. 4a, b, respectively. The ideal doping level of Eu3+ is 4%. As shown in Fig. 4c, the decay lifetimes of 5D0 → 7F2 emission of Eu3+ at 616 nm for 2% Bi3+-doped ScVO4 samples are almost invariant for different Eu3+ concentrations. The above results indicate that there is no back energy transferring from Eu3+ to Bi3+. Therefore, the Eu3+ luminescence quenching in high doping concentration may be due to the formation of slight EuVO4, as shown in the XRD patterns.
A comparison on luminescence performance between the optimized ScVO4: Bi3+, Eu3+ and the commercial red-emitting phosphor Y2O2S: 5% Eu3+ is shown in Fig. 5. Distinct characteristics can be seen in both excitation and emission spectra. In the scope from 360 to 410 nm in the excitation spectra, obviously the optimized sample ScVO4: 2% Bi3+, 4% Eu3+ with the contribution of Bi–V CT band shows more efficient excitation than Y2O2S: 5% Eu3+. Accordingly, with NUV light excitation, ScVO4: 2% Bi3+, 4% Eu3+ shows more bright red emission than Y2O2S: 5%Eu3+ does. Concretely speaking, the former yields red emissions 4.6, 5.4, 3.1 and 1.7 times of the latter, respectively, under 395, 385, 375 and 365 nm excitations. Moreover, the main emission peak of ScVO4: 2%Bi3+, 4%Eu3+ is located at 616 nm, which is an ideal peak position to provide good color rendering [7], whereas Y2O2S: 5% Eu3+ gives off a relatively deep red emission mainly at 627 nm. Besides, the CIE chromaticity coordinates for ScVO4: 2% Bi3+, 4% Eu3+ are calculated to be (0.65, 0.35), closer to the National Television System Committee standard value (0.67, 0.33) for red-emitting phosphor, compared with those of Y2O2S: 5% Eu3+ (0.64, 0.36). These results propose ScVO4: 2% Bi3+, 4% Eu3+ to be a very competitive candidate for red component in tricolor-based white LEDs.
Conclusion
In summary, a series of ScVO4: Bi3+, Eu3+ samples were successfully synthesized, and their luminescent properties were investigated in detail to achieve the best luminous efficiency. The experimental results show that the samples exhibit very efficient excitation in NUV range and can be excited even at 430 nm. All samples show the characteristic red emission of Eu3+ at 616 nm corresponding to 5D0 → 7F2 transition. And the optimized one produces an emission intensity 5.4 times of the commercial phosphor Y2O2S: 5%Eu3+ under 385 nm excitation. Moreover, it also has a better CIE chromaticity coordinates than the latter, closer to the National Television System Committee standard value for red-emitting phosphor. These results suggest that ScVO4: Bi3+, Eu3+ is promising for NUV-excited white LEDs in illumination applications.
References
Pust P, Schmidt PJ, Schnick W (2015) A revolution in lighting. Nat Mater 14:454–458
George NC, Denault KA, Seshadri R (2013) Phosphors for solid-state white lighting. Annu Rev Mater Res 43:481–501
Sheu JK, Chen FB, Wang YC, Chang CC, Huang SH, Liu CN, Lee ML (2015) Warm-white light-emitting diode with high color rendering index fabricated by combining trichromatic InGaN emitter with single red phosphor. Opt Express 23:A232–A239
Ye S, Xiao F, Pan YX, Ma YY, Zhang QY (2010) Phosphors in phosphor-converted white light-emitting diodes recent advances in materials, techniques and properties. Mat Sci Eng R 71:1–34
Chen L, Lin CC, Yeh CW, Liu RS (2010) Light converting inorganic phosphors for white light-emitting diodes. Materials 3:2172–2195
Pust P, Weiler V, Hecht C, Tucks A, Wochnik AS, Henss AK, Wiechert D, Scheu C, Schmidt PJ, Schnick W (2014) Narrow-band red-emitting Sr[LiAl3N4]: Eu2+ as a next-generation LED-phosphor material. Nat Mater 13:891–896
Coltrin ME, Armstrong AM, Brener I, Chow WW, Crawford MH, Fischer AJ, Kelley DF, Koleske DD, Lauhon LJ, Martin JE, Nyman M, Schubert EF, Shea-Rohwer LE, Subramania G, Tsao JY, Wang GT, Wierer JJ, Wright JB (2014) Energy frontier research center for solid-state lighting science: exploring new materials architectures and light emission phenomena. J Phys Chem C 118:13330–13345
Deng KM, Gong T, Chen YH, Duan CK, Yin M (2011) Efficient red-emitting phosphor for near-ultraviolet-based solid-state lighting. Opt Lett 36:4470–4472
Zhu HM, Lin CC, Luo WQ, Shu ST, Liu ZG, Liu YS, Kong JT, Ma E, Cao YG, Liu RS, Chen XY (2014) Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat Commun 4:4312-1–4312-10
Pust P, Wochnik AS, Baumann E, Schmidt PJ, Wiechert D, Scheu C, Schnick W (2014) Ca[LiAl3N4]:Eu2+-a narrow-band red-emitting nitridolithoaluminate. Chem Mater 26:3544–3549
Wei XT, Wen J, Li S, Huang S, Cheng J, Chen YH, Duan CK, Yin M (2014) Red-shift of vanadate band-gap by cation substitution for application in phosphor-converted white light-emitting diodes. Appl Phys Lett 104:181904-1–181904-4
Blasse G, Bril A (1969) Luminescence of phosphors based on host lattices ABO4 (A is Sc, In; B is P, V, Nb). J Chem Phys 50:2974–2980
Kang FW, Yang XB, Peng MY, Wondraczek L, Ma ZJ, Zhang QY, Qiu JR (2014) Red photoluminescence from Bi3+ and the influence of the oxygen-vacancy perturbation in ScVO4: a combined experimental and theoretical study. J Phys Chem C 118:7515–7522
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2016YF0701001), the National Natural Science Foundation of China (Grants Nos. 11574298, 61635012, 11274299, 11374291) and the University Science Research Project of Anhui Province (Grant No. KJ2017A791).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chi, F., Qin, Y., Hu, F. et al. Efficient red-emitting phosphor ScVO4 doped with Bi3+ and Eu3+ for near-ultraviolet-activated solid-state lighting. J Mater Sci 52, 11592–11597 (2017). https://doi.org/10.1007/s10853-017-1329-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1329-6