Abstract
Acetone detection at the parts-per-billion (ppb) level is achieved in this work using selective and optimized Fe doping of the three-dimensional (3D) flower-like SnO2 hierarchical microstructures. These structures were successfully synthesized via a one-step hydrothermal route. Detailed information about the crystal structure, surface morphology and composition of the Fe-doped SnO2 microstructures was investigated using X-ray diffraction (XRD), energy-dispersive spectroscopy (EDS), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS) techniques. Gas sensing experiments were conducted on the as-prepared Fe-doped SnO2 sensors. The measured results show that the incorporation of Fe into the SnO2 structure can greatly enhance the gas sensing properties of SnO2 sensors under the optimum working temperature (200 °C). Specifically, the 1.0 mol% Fe-doped SnO2 microstructures exhibit the highest response, fast response/recovery time, lowest detection limit and good selectivity and long-term stability. The results demonstrate that the developed Fe-doped SnO2 gas sensor has great potential for ppb-level acetone detection in many practical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acetone is a highly flammable gas which has been widely used in industries and scientific laboratories. Research indicates that people exposure to the high acetone concentration (more than 173 ppm) may cause low blood pressure, bronchial irritation, difficulty breathing and abdominal pain [1, 2]. Therefore, it is very important to detect the concentration of acetone at the workplace to ensure safety and health. Apart from that, being an important breath biomarker, the exhaled acetone level has been recognized to possess tight and quantitative connection with the human diabetes. Generally, gaseous acetone concentration in breath varies between 300 and 900 ppb in healthy people, while the concentration in diabetes patients is reported to exceed 1.8 ppm [3]. Thus, in order to achieve accurate disease diagnosis using exhaled breath sensors, the detection limit should be at ppb level, especially in highly humid atmospheres. Up to now, although considerable efforts have been devoted to acetone detection using analysis equipment such as gas chromatography/mass spectrometry (GC/MS) [4] and optical spectroscopy [5], these techniques still suffer from disadvantages such as bulky size, high cost and time-consuming measurement. These drawbacks limit wider applications of the existing acetone detection techniques [6]. Therefore, it is highly desirable to develop high-performance miniature sensors for the rapid and selective detection of acetone in practical applications.
Recently, considerable efforts have been made to develop gas sensors using various oxide semiconductors including SnO2 [7, 8], WO3 [9, 10], In2O3 [11], ZnO [12], NiO [13], MnO2 [14], V2O5 [15]. In particular, SnO2 (an n-type semiconductor with a wide band gap (3.6 eV)) is one of the most studied due to its better performance and high chemical stability [16]. However, most sensors based on bulky SnO2 still suffer from numerous problems associated with relatively poor sensitivity, long response/recovery time and low reliability [17, 18]. They still can’t meet these growing demands for fast-responding, accurate and reliable sensors with ppb-level detection. Therefore, great efforts have been dedicated to further improve the acetone sensing capabilities, such as improvement of the specific surface area, formation the heterojunction structure and doping with extrinsic dopants [19,20,21]. Noteworthy, doping with extrinsic dopants has been demonstrated as a facile and effective way to improve the sensitivity of SnO2-based sensors. It was recently reported that Zn-doped SnO2 nanostructures exhibited tremendous sensing performance toward glycol where the sensor’s response to 100 ppm glycol was about two times higher than that of undoped SnO2 sensor [22]. It was also demonstrated that NiO-doped SnO2 polyhedra has a significantly enhanced response of 6.7–30 ppm ethanol at 280 °C with the response and recovery times were shorter than 0.6 and 10 s, which are much better than that of pure SnO2 [23]. In addition, there are many other reports about doping nanostructured SnO2 resulted in the significant improvement of the sensor’s characteristics [24, 25]. All of those reports confirm the feasibility of successful doping of SnO2 by nanostructured dopants to achieve the desired improvements. Despite the tremendous research on the influence of doping on sensing performances, only few of the recent reports touched on the optimization of Fe content which might be critical for SnO2 gas sensing performance.
Herein, both pure SnO2 and different contents of Fe-doped 3D flower-like hierarchical SnO2 microstructures were successfully synthesized by the hydrothermal process. Their crystal structure, surface morphology and composition were subsequently characterized. Meanwhile, the acetone sensing experiments were conducted on the pure and Fe-doped SnO2 sensing materials. The measured results clearly show that the incorporation of Fe into the structure of SnO2 can greatly improve the gas sensing performances of SnO2-based acetone sensors. In particular, the 1.0 mol% Fe-doped SnO2 microstructures exhibited the highest response, best selectivity and excellent long-term stability, which indicate that the Fe-doped 3D SnO2 microstructures can be utilized as a promising sensing material for detection of ppb-level acetone gas.
Experimental
Materials and reagents
Tin chloride dihydrate (SnCl2·2H2O ≥ 99.9%) and ferric chloride hexahydrate (FeCl3·6H2O ≥ 99.0%) were purchased from Sigma–Aldrich (Shanghai, China). Sodium citrate dihydrate (C6H5Na3O7·2H2O ≥ 99.0%) was obtained from Aladdin Chemistry Co., Ltd. (Shanghai, China). All chemical reagents in this experiment were of analytical grade and used without further purification.
Synthesis of flower-like hierarchical SnO2 microstructures
Pure and Fe-doped SnO2 microstructures were synthesized by hydrothermal method. Typically, 4 mmol SnCl2 2H2O, 10 mmol C6H5Na3O7 2H2O and a definite amount of FeCl3 6H2O (Fe/Sn = 0.5 mol%, 1.0 mol%, 3.0 mol%) were dissolved into 40 mL of an ethanol–water (2:3, v/v) solution magnetic string for 10 min to form a homogeneous solution. Subsequently, the resultant mixture solution was transferred into a 100-mL Teflon-lined stainless steel autoclave and heated at 210 °C for 12 h. After it was cooled down to room temperature, the obtained precipitates were washed thoroughly by adding deionized water and ethanol, followed by centrifugation at 6000 rpm for 5 min. Finally, the products were annealed at 600 °C for 2 h with a ramping rate of 3 °C min−1. Furthermore, the pure SnO2 microstructures were also synthesized using the same method without addition of FeCl3 6H2O in the solution.
Characterization
The obtained SnO2 microstructures were examined in terms of their morphological, crystalline structural, compositional properties. The crystal structure and the phase of the as-synthesized products were measured by X-ray diffractometer (XRD, DRIGC-Y 2000A) with Cu-Kα1 radiation (λ = 1.5406 Å) in the range of 20°–80°. The surface morphologies and microstructures of the obtained samples were investigated by field-emission scanning electron microscopy (FESEM, JEM-7100F, operated at 20 kV) and a high-resolution transmission electron microscope (HRTEM, JEM-2010, 200 kV with electron diffraction). The chemical composition elements of the product were analyzed by the energy-dispersive spectroscopy (EDS, QUANTAX200). The surface composition and the chemical state of samples were studied by X-ray photoelectron spectroscope (XPS, ESCALAB 250Xi) with Al Ka X-ray radiation at 15 kV. All XPS spectra were accurately calibrated using the C 1 s peak of graphitic carbon at 284.6 eV as a reference. The current–voltage (I-V) characteristics of prepared sensors were tested using the semiconductor device analyzer (Agilent Technologies B1500A).
Fabrication and measurement of gas sensors
The structure of the gas sensor belongs to the side-heated type. The fabrication process of acetone sensor is as follows: The calcined product was firstly mixed with suitable amount of ethyl cellulose and terpineol (weight ratio 2:1:8) and hand-ground in an agate mortar to form a small amount of slurry. Secondly, the paste was coated on the surface of cylindrical Al2O3 ceramic tube installed with a pair of Au electrodes and Pt wires. After that, the sensing elements were dried at the room temperature and annealed at 600 °C for 2 h. Then, a Ni–Cr heating wire was inserted into the tube to control the operating temperature. Finally, the sensing element was welded onto the pedestal formation the gas sensor. The gas sensing properties of the as-prepared pure and Fe-doped SnO2 sensors were tested using CGS-1TS intelligent analysis system (Elite, Beijing, China). The relative humidity in the measurement atmosphere was maintained at about 35 ± 2%. The sensor response (S) is defined as the ratio (Ra/Rg) of the sensor resistance in air (Ra) to that in target gases (Rg). The time taken for the sensor to achieve 90% of the total resistance change was defined as the response time in the case of adsorption or the recovery time in the case of desorption [26].
Results and discussion
Structural and morphological characteristics
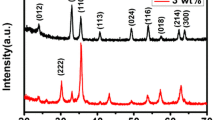
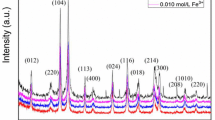
Figure 1a shows the XRD patterns of all samples after annealing at 600 °C. The measured results exhibit that all of the detected diffraction peaks are well matched with the tetragonal SnO2 structure (JCPDS card no. 41-1445). In addition, no obvious XRD peaks of Fe element are observed in the Fe-doped SnO2 microstructures, which may be due to the low concentration of Fe in the samples. Figure 1b shows the magnified region of the (110) peaks for the Fe-doped SnO2 microstructures. All the measured diffraction angles shift to larger value with the increasing Fe concentration. Table S1 (Supporting information) illustrates the details of the peak position, which indicates that the Sn ions were partially substituted by Fe ions in the samples. Similar results have been previously demonstrated [21, 27, 28]. In the air, Fe3O4 can be converted into Fe2O3 via heat treatment at 450 °C or higher, which indicates that Fe2+ can be converted into Fe3+ at temperatures above 450 °C [29]. All samples prepared were subsequently calcined at 600 °C for 2 h in air prior to gas sensing tests, to ensure that the doping into SnO2 lattice was Fe3+ ions. In addition, the element composition for 3.0 mol% Fe-doped SnO2 was examined by EDS analysis (Fig. 1c). As a result, only Fe, Sn and O elements existed in the sample, which suggests a high level of purity of the sintered microstructures. Meanwhile, the chemical compositions of Fe-doped SnO2 samples were qualitative analyzed as shown in Fig. S2 (Supporting information), and the atomic percentage of Fe element in 0.5, 1.0, 3.0 mol% Fe-doped SnO2 samples is 0.438, 0.812 and 2.842 at.%, respectively.
The surface morphologies and microstructures of pure SnO2 and Fe-doped SnO2 were observed by SEM as shown in Fig. 2. The results show that the flower-like pure SnO2 microstructures appear to be assembled together by numerous SnO2 nanosheets with the average size of approximately 2–3 μm (Fig. 2a). After the introduction of the Fe dopant, it seems that there was no apparent influence of the dopant on both the morphology and dimensions of the developed SnO2 microstructures, as shown in Fig. 2b–d. Furthermore, the SEM micrograph and EDS elemental mapping were performed on the 3.0 mol% Fe-doped SnO2 sample and the results are illustrated in Fig. 2e–h. The corresponding elemental mapping images of tin, oxygen and iron reveal an identical spatial distribution, which suggests that the Fe element is immobilized at the surface of the SnO2 microstructures.
The morphology and microstructure of the synthesized SnO2 samples were further characterized by TEM and high-resolution TEM (HRTEM). Figure 3a depicts the representative TEM images of 3.0 mol% Fe-doped SnO2. The HRTEM image of the Fe-doped SnO2 microstructure is shown in Fig. 3b, which indicates that the inter-planar spacing of the lattice fringes is 0.238 and 0.264 nm, respectively, corresponding to the (200) and (101) crystal planes of tetragonal SnO2 structure. As shown in Fig. 3c, the lattice fringe spacing of the small nanoparticle on the SnO2 nanosheet was observed to be 0.208 nm corresponding to the (202) plane of α-Fe2O3, while the lattice fringe spacing in nanosheet was 0.335 nm corresponding to the (110) plane of SnO2. A higher-magnification HRTEM image of the same sample (Fig. 3d) exhibits sets of lattice fringes with an inter-planar distance of 0.367 nm, which is attributed to the (110) plane of α-Fe2O3. These results reveal that the Fe2O3 nanoparticles were successfully incorporated into the surface of SnO2, which is consistent with the previous XRD and EDS analysis.
The chemical composition and chemical valence of the 3.0 mol% Fe-doped SnO2 sample were measured by XPS as shown in Fig. 4. In the full range of the XPS spectrum (Fig. 4a), the binding energies correspond to different energy levels of O, Sn and C, which are clearly observed. However, no obvious impurities other than carbon could be found in the spectrum. From Fig. 4b, we can find that the peak positions of Sn 3p3/2 at 716.25 eV located at around 716.25 eV and 464.1 eV, respectively, which is attributed to Sn4+ in the SnO2 lattice. Noteworthy, two very weak peaks at the lower binding energy of 711.3 and 724.6 eV were corresponded to the characteristic doublet of Fe 2p3/2 and Fe 2p1/2 spectrum of Fe2O3, implying that the oxide state of iron is trivalent [30]. Moreover, the binding energy of 56.2 eV could be ascribed to Fe 3p, as depicted in Fig. 4c. With respect to O 1 s (Fig. 4d), its core-level spectrum was resolved into three peaks at 530.3, 531 and 532 eV, respectively. The peak at the lower binding energy of 530.3 eV corresponded to the SnO2 lattice oxygen. The medium binding energy of 531 eV is assigned to the O2− ions in the oxygen deficient regions. For the high binding energy (532 eV), it could be attributed to the presence of oxygen ions chemisorbed on the SnO2 surface. As discussed above, the XPS results further confirmed that the Fe dopants were incorporated into SnO2 products.
Gas sensing properties
It is well known that the working temperature plays a critical role in the response of a semiconductor gas sensor [31]. That’s why initially both pure SnO2 and Fe-doped SnO2-based sensors were investigated for the detection of 100 ppm acetone at different operating temperatures ranging from 125 to 275 °C, in order to optimize the working temperature where the highest sensing capabilities can be obtained. Figure 5 shows that the responses of all sensors increased as the temperature increased to 200 °C, and then decreased dramatically. This phenomenon can be ascribed to both thermodynamics and kinetics of the acetone adsorption and desorption on the surfaces of the sensors [32, 33]. When the semiconductor-based sensor works at the lower temperatures, the thermal energy of the gas molecules is too low to react with the surface-adsorbed oxygen species [34, 35]. However, as the temperature increases, the thermal energy obtained is high enough to overcome the potential barrier of the surface reaction. Moreover, the electron concentration increases due to the conversion of adsorbed oxygen species, which facilitates the chemical reaction and leads to a stronger response. On the contrary, under the higher temperatures, the adsorption ability of gas molecules decreased causing the low utilization rate, which in turn leads to the decrease in the sensor’s response. Clearly, 200 °C is the optimum working temperature for the as-fabricated samples, and unless we stated otherwise, all following measurements were carried out at 200 °C. In addition, it was discovered that the 1.0 mol% Fe-doped SnO2 sensor exhibits the highest response to 100 ppm acetone under the optimum working temperature of 200 °C, which is almost three times higher than the response of the pure SnO2-based sensor. Meanwhile, the response of the sensor based on SnO2 microstructures to 100 ppm ethanol was also measured under the different operating temperatures. The results have also show that the 1.0 mol% Fe-doped SnO2 sensor has excellent sensing properties for ethanol (Fig. S3 in Supporting information).
To further investigate the sensing properties of as-prepared gas sensors, dynamic resistance transient experiments were conducted on both pure SnO2 and Fe-doped SnO2-based sensors at the optimum working temperature. Figure 6a–d displays the dynamic transient curves for all sensors for different acetone concentrations (1, 5, 10, 50, 100, 200, 400 and 1000 ppm) at 200 °C. It can be seen that the resistance of the gas sensors decreases sharply when the target gas (acetone) was injected into the chamber. It reverts to the initial value after exposure to fresh air. Noteworthy, the measured resistances of the sensors also decreased significantly with the increase in acetone concentration. The initial resistance of as-prepared sensors increases with the increase in Fe content in SnO2, which indicates that the introduction of Fe element has indeed a significant influence on sensor’s characteristics. Meanwhile, there was no apparent “baseline drift” observed for all sensors, which confirmed the great repeatability of their performance. In addition, all as-fabricated gas sensors were further tested investigate to the different ethanol concentrations and the results are presented in Fig. S4 (Supporting information). All of the above results have clearly demonstrated that the sensor based on Fe-doped SnO2 3D microstructure exhibits superior sensing capabilities compared with the sensor based on pure SnO2.
To find out the lowest acetone detection level for the Fe-doped SnO2 3D microstructure, further tests were carried out at the optimum working temperature of 200 °C and the main results are shown in Fig. 7. It is evident that the sensors based on Fe-doped SnO2 3D microstructures exhibit better response/recovery to ppb level of acetone compared with sensors based on pure SnO2. Specifically, 1.0 mol% Fe-doped SnO2 sensor showed superior acetone sensing performance than the others, and the measured response reached to 1.55 even at 100 ppb acetone at 200 °C. The lowest acetone detection limit (LOD) was estimated with Ra/Rg > 1.2 being used as the criterion for gas detection [36, 37]. When the acetone concentration is 100 ppb, the corresponding sensitivity (Ra/Rg) = 1.55 > 1.2. Therefore, it is appeared to indicate the detection limit of the sensor based on 1.0 mol% Fe-doped SnO2 could be as low as 100 ppb. Consequently, this doped nanomaterial can be considered as promising candidate for sensors measuring traces of acetone in many practical applications.
Figure 8 plots linear fits for all types of sensors toward acetone with concentration range from 100 to 5 ppm. An empirical equation is often used to relate the response of a metal oxide semiconductor and the target gas concentration: S = 1+a[C]b [38, 39], where a and b are constants, C the target gas concentration. The equation can be rewritten in a linear form: log (S–1) = blog (C) + log (a). The measured results demonstrated that both pure and Fe-doped SnO2 gas sensors show good linear relationships. In particular, the correlation coefficient (R2) of the 1.0 mol% Fe-doped SnO2 sensor is about 0.98954, which exhibits the best linear relationship for acetone detection.
In practical applications, it is required that acetone sensors should have quick response and recovery time. Therefore, the response/recovery characteristics of the acetone sensors based on pure and Fe-doped SnO2 3D microstructures for 100 ppm acetone were investigated at the optimum working temperature. Figure 9a illustrates the dynamic response–recovery characteristics of 1.0 mol% Fe-doped SnO2 sensor at 200 °C. The measured results showed that the response and recovery times for 1.0 mol% Fe-doped SnO2 sensor were about 1 and 58 s, respectively. In addition, Fig. 9b depicts the response and recovery time to 100 ppm acetone of all acetone sensors based on Fe-doped SnO2 with different concentration of the dopant. These measurements show that response/recovery times are similar to the most of the sensors with exception to the sensor based on 3.0 mol% Fe-doped SnO2. The longer recovery time for this sensor may be attributed to the adsorption/desorption speed of acetone molecules on the surface of sensing material [40].
Meanwhile, the current–voltage (I-V) characteristics were conducted on the 1.0 mol% Fe-SnO2 sensor using semiconductor device analyzer, as shown in Fig. 10. When the as-fabricated gas sensor was exposed into air (without acetone gas), the measured current is about 0.16 µA under the applied voltage of 4 V. However, after injection into 100 ppm acetone gas, the measured current can reach to 21.5 µA for 1.0 mol% Fe-doped SnO2 sensor. The enhancement of conductance for the sensor can be attributed to the increases in the charge carriers after exposure into acetone gas. The similar results have been reported in previous work [41].
As the selectivity is also one of the main characteristics of gas sensors, it was also tested. All sensors were exposed to 100 ppm of various gases at 200 °C, such as acetone (CH3COCH3), ethanol (C2H5OH), ammonia (NH3), hydrogen (H2), carbon monoxide (CO), methanol (CH3OH) and methane (CH4). Figure 11 shows the selectivity of the pure and Fe-doped SnO2 microstructures sensors to different gases. It can be clearly observed that all sensors showed very low responses upon exposure to gases CH4, CO, H2, NH3, while the major response was detected for the measuring acetone. It is also clear that the 1.0 mol% Fe-doped SnO2 demonstrated the highest response to acetone among the other gases.
The reversibility and long-term stability are also very important requirements for the practical application of these sensors. To further investigate the reversibility, cycle sensing experiments were performed on 1.0 mol% Fe-doped SnO2 acetone sensor as shown in Fig. 12a. After seven cycles between exposure to acetone and fresh air, both the response and resistance can recover to their initial values after removal of acetone, exhibiting an excellent reversibility. Figure 12b shows the long-term stability results measured for 100 ppm acetone every ten days within two months. The measured maximal deviations of the response for 1.0 mol% Fe-doped SnO2 gas sensor to acetone were <7% over a period of 50 days testing, which illustrated good long-term stability of the developed sensing material for practical applications.
The overall sensing capabilities of the sensor based on the developed 1.0 mol% Fe-doped 3D SnO2 gas sensing materials are compared with the best previous reports for the SnO2–based sensors in Table 1. The results indicate that the 1.0 mol% Fe-doped 3D SnO2 acetone sensor has lowest detection limit (~100 ppb) and operational temperature of 200 °C, high response toward acetone, which clearly demonstrates excellent sensing capabilities for acetone.
Gas sensing mechanism
It is well known that the gas sensing mechanism of an oxide semiconductor is based on the resistance change through the thickness of the depletion layer, which varies according to the amount of oxygen absorbed on the surface [50]. Figure 13 presents schematic illustration of the gas sensing mechanism for pure SnO2 and Fe-doped SnO2 3D microstructures to acetone. When the pure SnO2 microstructures are exposed to air, oxygen molecules can be adsorbed on the SnO2 surface and ionized to O2 −, O−, O2− ions by trapping electrons from the conduction band of SnO2 (Fig. 13a) [51]. As a result, a charge depletion layer is generated at the surface of SnO2, which leads to the increase in its resistance [52]. When material is exposed to the reducing gas (acetone, in this instance), the adsorbed oxygen species react with the acetone and release electrons into the conduction band, which will decline the resistance of the sensor (Fig. 13c). The main reaction on the SnO2 surface is speculated to be as follows:
For the Fe-doped SnO2 microstructures, the enhanced sensing properties are likely attributed to the introducing of Fe element on the surface of 3D SnO2 microstructures, which leads to a larger lattice distortion, which ultimately enhances oxygen adsorption and promotes the dissociation process of the molecular oxygen resulting in the greater and faster degree of electron depletion from the SnO2 microstructures (Fig. 13b) [53]. On the other hand, the heterojunction constituted at the surface between Fe2O3 and SnO2 also enlarges the space charge layer. When the Fe-doped SnO2-based sensors are exposed to acetone vapor atmosphere, the depleted layer was fading away fast, and the trapped electrons were released back to the conduction band (Fig. 13d). However, when the SnO2 is over-doped by Fe the response decreases due to the reducing of the available adsorption sites. Therefore, we attribute 1.0 mol% Fe-doped SnO2 3D microstructure as the optimal doped 3D structure providing maxim sensibility and selectivity toward acetone sensing on the ppb level.
Conclusions
Enhancement of the acetone sensing toward the ppb-level detection was achieved by modification of the sensing material representing 1.0 mol% Fe-doped 3D SnO2 hierarchical microstructures. These new 3D microstructures were successfully synthesized and fabricated via one-step hydrothermal method. As-prepared Fe-doped SnO2 samples consist of 3D flower-like hierarchical microstructures and have no other impurities, which was subsequently confirmed by the various materials characterization methods. The sensors based on these newly developed 3D microstructures have demonstrated that the Fe-doped of 3D SnO2 can significantly enhance both response and selectivity of the material toward the acetone measurement at ppb level compared with the sensor based on pure SnO2. It was also experimentally found that the 1.0 mol% Fe-doped SnO2 microstructures exhibit the highest response, fast response/recovery time, lowest detection limit and good selectivity to acetone at the optimum working temperature of 200 °C. The excellent sensing capabilities are attributed to the particular unique hierarchical morphology and incorporation of Fe2O3 to form the heterojunction interface. The obtained results evidently demonstrated that the effective integration of the metal oxide nanoparticles at the right concentration will be very helpful approach for development of new semiconductor sensing materials for various devices.
References
Liu S, Zhang F, Li H, Chen T, Wang Y (2012) Acetone detection properties of single crystalline tungsten oxide plates synthesized by hydrothermal method using cetyltrimethyl ammonium bromide supermolecular template. Sens Actuators, B 162:259–268
Liu X, Hu J, Cheng B, Qin H, Jiang M (2008) Acetone gas sensing properties of SmFe1-xMgxO3 perovskite oxides. Sens Actuators, B 134:483–487
Righettoni M, Tricoli A, Pratsinis SE (2010) Si:WO3 Sensors for highly selective detection of acetone for easy diagnosis of diabetes by breath analysis. Anal Chem 82:3581–3587
Kumar S, Huang J, Abbassi-Ghadi N, ŠPaněL P, Smith D, Hanna GB (2013) Selected ion flow tube mass spectrometry analysis of exhaled breath for volatile organic compound profiling of esophago-gastric cancer. Anal Chem 85:6121–6128
Kamat PC, Roller CB, Namjou K, Jeffers JD, Faramarzalian A, Salas R, Mccann PJ (2007) Measurement of acetaldehyde in exhaled breath using a laser absorption spectrometer. Appl Optics 46:3969–3975
Choi SJ, Lee I, Jang BH, Youn DY, Ryu WH, Park CO, Kim ID (2013) Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal Chem 85:1792–1796
Tang W, Wang J, Qiao Q, Liu Z, Li X (2015) Mechanism for acetone sensing property of Pd-loaded SnO2 nanofibers prepared by electrospinning: fermi-level effects. J Mater Sci 50:2605–2615. doi:10.1007/s10853-015-8836-0
Kumar A, Sanger A, Kumar A, Chandra R (2016) Highly sensitive and selective CO gas sensor based on a hydrophobic SnO2/CuO bilayer. RSC Adv 6:47178–47184
Jain S, Sanger A, Chauhan S, Chandra R (2014) Hydrogen sensing properties of nanostructured Pd/WO3 thin films: role of hydrophobicity during recovery process. Mater Res Express 1:035046
Sun Y, Chen L, Wang Y, Zhao Z, Li P, Zhang W, Leprince-Wang Y, Hu J (2017) Synthesis of MoO3/WO3 composite nanostructures for highly sensitive ethanol and acetone detection. J Mater Sci 52:1561–1572. doi:10.1007/s10853-016-0450-2
Zhu BL, Xie CS, Zeng DW, Wang AH, Song WL, Zhao XZ (2005) New method of synthesizing In2O3 nanoparticles for application in volatile organic compounds (VOCs) gas sensors. J Mater Sci 40:5783–5785. doi:10.1007/s10853-005-4562-3
Song P, Wang Q, Yang Z (2012) Acetone sensing characteristics of ZnO hollow spheres prepared by one-pot hydrothermal reaction. Mater Lett 86:168–170
Wang C, Liu J, Yang Q, Sun P, Gao Y, Liu F, Zheng J, Lu G (2015) Ultrasensitive and low detection limit of acetone gas sensor based on W-doped NiO hierarchical nanostructure. Sens Actuators, B 220:59–67
Sanger A, Kumar A, Kumar A, Chandra R (2016) Highly sensitive and selective hydrogen gas sensor using sputtered grown Pd decorated MnO2 nanowalls. Sens Actuators, B 234:8–14
Sanger A, Kumar A, Kumar A, Jaiswal J, Chandra R (2016) A fast response/recovery of hydrophobic Pd/V2O5 thin films for hydrogen gas sensing. Sens Actuators, B 236:16–26
Liu Y, Jiao Y, Zhang Z, Qu F, Umar A, Wu X (2014) Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor, and supercapacitor applications. Appl Mater Interfaces 6:2174–2184
Lee JH, Katoch A, Choi SW, Kim JH, Kim HW, Kim SS (2015) Extraordinary improvement of gas-sensing performances in SnO2 nanofibers due to creation of local p–n heterojunctions by loading reduced graphene oxide nanosheets. Appl Mater Interfaces 7:3101–3109
Li L, He S, Liu M, Zhang C, Chen W (2015) Three-dimensional mesoporous graphene aerogel-supported SnO2 nanocrystals for high-performance NO2 gas sensing at low temperature. Anal Chem 87:1638–1645
Wu J, Zeng D, Tian S, Xu K, Li D, Xie C (2015) Competitive influence of surface area and mesopore size on gas-sensing properties of SnO2 hollow fibers. J Mater Sci 50:7725–7734. doi:10.1007/s10853-015-9339-8
Yu YT, Dutta P (2011) Examination of Au/SnO2 core-shell architecture nanoparticle for low temperature gas sensing applications. Sens Actuators, B 157:444–449
Gao F, Qin G, Li Y, Jiang Q, Luo L, Zhao K, Liu Y, Zhao H (2016) One-pot synthesis of La-doped SnO2 layered nanoarrays with enhanced gas-sensing performance toward acetone. RSC Adv 6:10298–10310
Zhao Q, Ju D, Deng X, Huang J, Cao B, Xu X (2015) Morphology-modulation of SnO2 hierarchical architectures by Zn doping for glycol gas sensing and photocatalytic applications. Sci Rep 5:7874
Lou Z, Wang L, Fei T, Zhang T (2012) Enhanced ethanol sensing properties of NiO-doped SnO2 polyhedra. New J Chem 36:1003–1007
Sun P, Zhou X, Wang C, Wang B, Xu X, Lu G (2014) One-step synthesis and gas sensing properties of hierarchical Cd-doped SnO2 nanostructures. Sens Actuators, B 190:32–39
Wang Z, Liu L (2009) Synthesis and ethanol sensing properties of Fe-doped SnO2 nanofibers. Mater Lett 63:917–919
Li X, Zhou X, Guo H, Wang C, Liu J, Sun P, Liu F, Lu G (2014) Design of Au@ ZnO yolk–shell nanospheres with enhanced gas sensing properties. Appl Mater Interfaces 6:18661–18667
Qin G, Gao F, Jiang Q, Li Y, Liu Y, Luo L, Zhao K, Zhao H (2016) Well-aligned Nd-doped SnO2 nanorods layered array: preparation, characterization and enhanced alcohol-gas sensing performance. Phys Chem Chem Phys 18:5537–5549
Li W, Ma S, Li Y, Li X, Wang C, Yang X, Cheng L, Mao Y, Luo J, Gengzang D (2014) Preparation of Pr-doped SnO2 hollow nanofibers by electrospinning method and their gas sensing properties. J Alloy Compd 605:80–88
Kim HJ, Choi KI, Pan A, Kim ID, Kim HR, Kim KM, Na CW, Cao G, Lee JH (2011) Template-free solvothermal synthesis of hollow hematite spheres and their applications in gas sensors and Li-ion batteries. J Mater Chem 21:6549–6555
Grosvenor AP, Kobe BA, Biesinger MC, Mcintyre NS (2004) Investigation of multiplet splitting of Fe2p XPS spectra and bonding in iron compounds. Surf Interface Anal 36:1564–1574
Bai S, Hu J, Li D, Luo R, Chena A, Liu CC (2011) Quantum-sized ZnO nanoparticles: synthesis, characterization and sensing properties for NO2. J Mater Chem 21:12288–12294
Wei S, Zhou M, Du W (2011) Improved acetone sensing properties of ZnO hollow nanofibers by single capillary electrospinning. Sens Actuators, B 160:753–759
Sahay PP, Nath RK (2008) Al-doped ZnO thin films as methanol sensors. Sens Actuators, B 134:654–659
Li X, Zhou X, Guo H, Wang C, Liu J, Sun P, Liu F, Lu G (2014) Design of Au@ZnO yolk-shell nanospheres with enhanced gas sensing properties. Appl Mater Interfaces 6:18661–18667
Chen D, Xu J, Xie Z, Shen G (2011) Nanowires assembled SnO2 nanopolyhedrons with enhanced gas sensing properties. Appl Mater Interfaces 3:2112–2117
Liu C, Zhao L, Wang B, Sun P, Wang Q, Gao Y, Liang X, Zhang T, Lu G (2017) Acetone gas sensor based on NiO/ZnO hollow spheres: fast response and recovery, and low (ppb) detection limit. J Colloid Interface Sci 495:207–215
Choi KI, Kim HJ, Kang YC, Lee JH (2014) Ultraselective and ultrasensitive detection of H2S in highly humid atmosphere using CuO-loaded SnO2 hollow spheres for real-time diagnosis of halitosis. Sens Actuators, B 194:371–376
Zhang L, Zhao J, Lu H, Li L, Zheng J, Li H, Zhu Z (2012) Facile synthesis and ultrahigh ethanol response of hierarchically porous ZnO nanosheets. Sens Actuators, B 161:209–215
Wang H, Qu Y, Chen H, Lin Z, Dai K (2014) Highly selective n-butanol gas sensor based on mesoporous SnO2 prepared with hydrothermal treatment. Sens Actuators, B 201:153–159
Zhou X, Liu J, Wang C, Sun P, Hu X, Li X, Shimanoe K, Yamazoe N, Lu G (2015) Highly sensitive acetone gas sensor based on porous ZnFe2O4 nanospheres. Sens Actuators, B 206:577–583
Zhang Z, Zhu L, Wen Z, Ye Z (2017) Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens Actuators, B 238:1052–1059
Li L, Lin H, Qu F (2013) Synthesis of mesoporous SnO2 nanomaterials with selective gas-sensing properties. J Sol-Gel Sci Techn 67:545–555
Li J, Tang P, Zhang J, Feng Y, Luo R, Chen A, Li D (2016) Facile synthesis and acetone sensing performance of hierarchical SnO2 hollow microspheres with controllable size and shell thickness. Ind Eng Chem Res 55:3588–3595
Chen D, Xu J, Xie Z, Shen G (2011) Nanowires assembled SnO2 nanopolyhedrons with enhanced gas sensing properties. Appl Mater Interfaces 3:2112–2117
Song P, Wang Q, Yang Z (2012) Preparation, characterization and acetone sensing properties of Ce-doped SnO2 hollow spheres. Sens Actuators, B 173:839–846
Sun P, Cai Y, Du S, Xu X, You L, Ma J, Liu F, Liang X, Sun Y, Lu G (2013) Hierarchical α-Fe2O3/SnO2 semiconductor composites: hydrothermal synthesis and gas sensing properties. Sens Actuators, B 182:336–343
Kim KW, Cho PS, Kim SJ, Lee JH, Kang CY, Kim JS, Yoon SJ (2007) The selective detection of C2H5OH using SnO2–ZnO thin film gas sensors prepared by combinatorial solution deposition. Sens Actuators, B 123:318–324
Huang J, Yu K, Gu C, Zhai M, Wu Y, Yang M, Liu J (2010) Preparation of porous flower-shaped SnO2 nanostructures and their gas-sensing property. Sens Actuators, B 147:467–474
Zhang S, Ren F, Wu W, Zhou J, Xiao X, Sun L, Liu Y, Jiang C (2013) Controllable synthesis of recyclable core–shell γ-Fe2O3@SnO2 hollow nanoparticles with enhanced photocatalytic and gas sensing properties. Phys Chem Chem Phys Pccp 15:8228–8236
Wen W, Wu JM, Wang YD (2012) Large-size porous ZnO flakes with superior gas-sensing performance. Appl Phys Lett 100:262111–262114
Xu J, Chen Y, Chen D, Shen J (2006) Hydrothermal synthesis and gas sensing characters of ZnO nanorods. Sens Actuators, B 113:526–531
Kaneti YV, Yue J, Jiang X, Yu A (2013) Controllable synthesis of ZnO nanoflakes with exposed (1010) for enhanced gas sensing performance. J Phys Chem C 117:13153–13162
Zhang T, Chen M, Gu F, Han D, Wang Z, Guo G (2012) Alcohol sensing properties of Er-doped In2O3 hollow spheres. Integr Ferroelectr 138:117–122
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51205274), Higher school science and technology innovation Project of Shanxi (2016137), Natural Science of Shanxi Province (2016011039), Talent Project of Shanxi Province (201605D211036), Science and Technology Major Project of the Shan Xi Science and Technology Department (20121101004), Key Disciplines Construction in Colleges and Universities of Shanxi ([2012]45).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hu, J., Wang, Y., Wang, W. et al. Enhancement of the acetone sensing capabilities to ppb detection level by Fe-doped three-dimensional SnO2 hierarchical microstructures fabricated via a hydrothermal method. J Mater Sci 52, 11554–11568 (2017). https://doi.org/10.1007/s10853-017-1319-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1319-8