Abstract
In this paper, different contents of molybdenum oxide/tungsten oxide (MoO3/WO3) composite nanostructures were synthesized by hydrothermal method. Field emission scanning electron microscopy images revealed that the morphologies of WO3 nanostructures were significantly influenced by the Mo amount. Furthermore, the introduction strategy of MoO3 into WO3 could effectively improve the gas sensing properties. Especially, the sensor based on the 4 mol% MoO3/WO3 composite nanostructures exhibited enhanced gas sensing performance, giving a low limit of detection (500 ppb). It shows high responses of 28.5 and 18.2–100 ppm ethanol and acetone at the operating temperature of 320 °C, which were about 2.3 and 1.7 times higher than those of the pure WO3, respectively. The enhanced sensing properties of MoO3/WO3 gas sensor can be attributed to the addition of MoO3, which has been discussed in relation to the gas sensing mechanism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Volatile organic compounds (VOCs; e.g., acetone, toluene, and formaldehyde) have high vapor pressure and fairly low boiling points, which make them evaporate easily at room temperature [1, 2]. VOCs have been recognized as the principal cause of many diseases including headaches, throat irritation, kidney disease, and even damage to the liver, lung, and the central nervous system [3–5]. Therefore, it is necessary to develop an efficient and convenient sensing technology for VOC detection [6, 7]. Compared with traditional gas sensing methods, semiconductor metal oxide gas sensors based on TiO2, SnO2, In2O3, WO3, ZnO, Nb2O5, and MoO3 have attracted great interest because of their high sensitivity, easy preparation, low consumption, and high integrability properties [8–12].
Among these metal oxide semiconductor materials, WO3 is considered to be the most promising material due to its remarkable performance and practical application in gas detection [13, 14]. Recently, great efforts have been made to further improve the gas sensing performance of WO3 gas sensor using various methods such as improving the specific surface area [15], decreasing the grain size [16], doping with noble metal [17], and forming the heterojunction [18]. For example, Chi et al. synthesized WO3 nanotubes using electrospinning technique, and the as-prepared WO3 gas sensor showed low limit of detection (2.5–0.5 ppm) and quick response/recovery time (5/22 s) to acetone [19]. Yang et al. fabricated Au-functionalized WO3 gas sensor under mild conditions, and the measured response could reach 63.6 for 10 ppm n-butanol, which is about 60 times higher than that of the pure one [20]. Zhao et al. prepared Co3O4–WO3 heterojunction gas sensor for acetone detection, and the response was about two times that of pure WO3 sensor [21]. According to the previous works, it has been demonstrated that introducing extrinsic dopants is one of the most facile and effective ways to improve the gas sensing performance of WO3 gas sensor toward the target gases. However, to the best of our knowledge, few studies have been focused on MoO3/WO3 composite nanostructures for ethanol and acetone detection.
Herein, the pure WO3 and different MoO3/WO3 composite nanostructures have been successfully synthesized by a one-step hydrothermal method. The obtained results show that the morphology and gas sensing performance of MoO3/WO3 nanostructures are critically dependent on the Mo content. Especially, the 4 mol% MoO3/WO3 gas sensor exhibits the highest response, low limit of detection, excellent selectivity, and good stability. Meanwhile, to further understand the enhanced gas sensing performance, the mechanisms of the MoO3/WO3 composite were also studied.
Experimental
Chemicals
Tungsten hexachloride (WCl6 ≥ 99.9 %) and ammonium heptamolybdate tetrahydrate ((NH4)6Mo7O24·4H2O ≥ 99.0 %) were purchased from Sigma-Aldrich. Absolute ethanol was obtained from Sinopharm Chemical Reagent Company. All reagents were of analytical grade and were used as received without further purification.
Sample preparation
In a typical process, 1.51 mmol of WCl6 and a definite amount (0, 2, 4, 6, and 8 mol %) of (NH4)6Mo7O24·4H2O were added into 60 mL of absolute ethanol. After stirring vigorously for 30 min, the homogeneous solution was transferred into a Teflon-lined stainless steel autoclave and heated at 160 °C for 24 h. After cooling to room temperature naturally, the precipitate was collected and dried at 80 °C for 10 h. Finally, the as-prepared product was calcined at 500 °C for 2 h. For convenience, pure WO3 and MoO3/WO3 samples were labeled as WM0 and WMx (x = 2, 4, 6, and 8), which corresponds to the mole ratios of Mo and W.
Characterization
X-ray powder diffraction (XRD) analysis was performed using a DRIGC-Y 2000A X-ray diffractometer with Cu-Kα1 radiation (λ = 1.5406 Å) and the scanning speed was 6° min−1. Field emission scanning electron microscope (FESEM) images were recorded on a JEOL JSM-7001F microscope operating at 15 kV which is equipped with a QUANTAX 200 energy-dispersive spectrometer (EDS) for elemental analyses. X-ray photoelectron spectroscopy (XPS) analysis was carried out using an ESCALAB 250Xi. The specific surface areas and the cumulative volume of pores of the sample were determined using Tristar 3020 Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods.
Gas sensing measurements
The fabrication process of gas sensor can be described as follows: firstly, the as-calcined sample was mixed with deionized water to form a paste and coated on a ceramic tube (4 mm in length, 1.2 mm in external diameter, and 0.8 mm in internal diameter) attached with a pair of Au electrodes and Pt wires. After that, the element was sintered at 500 °C for 2 h after drying in the shade. Then, a Ni–Cr alloy coil heating wire was inserted into the tube as a heater to keep the sensor working at a given temperature. The measurements of the gas sensing properties were carried out using static test method with CGS-4TP intelligent analysis system (Elite, Beijing, China). The relative humidity in the measurement atmosphere was maintained at about 30 %. We denote by R a and R g the resistances in air and in the target gas, respectively. Then the response (R) of the gas sensor to the target gas was defined as the ratio of R a to R g. The response and recovery times were defined as the time for 90 % of the total resistance change and the time for 90 % recovery of the resistance change, respectively.
Results and discussion
Structure analysis
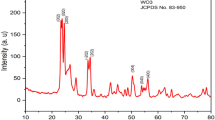
The crystal structures of the pure WO3 and MoO3/WO3 composite were investigated using XRD. Figure 1 shows the XRD patterns of the as-obtained samples after annealing at 500 °C. Distinct characteristic peaks ((002), (020), and (200)) were clearly observed. The main diffraction peaks of all samples correspond to the monoclinic phase [space group P21/n(14)] of WO3 with the lattice parameters a = 7.297 Å, b = 7.539 Å, c = 7.688 Å, and β = 90.91°, which is well consistent with the standard JCPDS card No. 43-1035. Moreover, no diffraction peak shifts or obvious diffraction peak of MoO x was observed, which is probably due to the relatively low amount of Mo in the samples. However, when the content of Mo in WO3 was increased to 20 mol%, the diffraction peaks of MoO3 were obtained (Supporting Information Fig. S1).
Morphological analysis
The morphologies of the as-prepared WO3 samples were observed using FESEM. Figure 2a presents the pure WO3 sample, exhibiting a flower-like structure with diameters ranging from 1.5 to 2 μm and composed of intersecting nanoflakes. For MoO3/WO3 samples, it seems that the introduction of Mo element can significantly influence the morphology. Figure 2b, c shows the MoO3/WO3 samples WM2 and WM4, which were composed of two-tier structures with lots of fluffs on the surface. With further increasing the content of Mo, the nanoflake structures were replaced by nanoparticles (Fig. 2d, e), and the sizes of the samples decrease from 230 to 180 nm. The measured results show that the presence and the content of Mo element have significant influence on the morphology of MoO3/WO3 composite nanostructures. Similar results have been reported in many previous works regarding this phenomenon [22, 23]. To confirm the existence of Mo, EDS was conducted on sample WM8 as shown in Fig. 2f. The peaks correspond to W, O, and Mo elements. No other impurity element was observed, indicating the high purity of the samples. Meanwhile, the elemental mapping experiment was also conducted on WM8, which further confirms the uniform distribution of Mo (Fig. S2 in Supporting Information).
The BET surface areas and porous structures were investigated by nitrogen adsorption–desorption using BET and BJH methods. Figure 3 shows the nitrogen adsorption–desorption isotherms of all the as-prepared samples. According to the IUPAC classification, it can be found that both pure WO3 and MoO3/WO3 nanostructures have isotherms of type II with a type H3 hysteresis loop, which implies nonporous structures. The inset table shows the corresponding BET surface areas of the pure WO3 and MoO3/WO3 composite nanostructures. It can be clearly observed that the surface area of the as-prepared samples increased at the initial stage, reached the maximum value for WM6 (18.811 m2/g), and decreased with a further increase in the concentration of Mo. The larger surface area of sensing materials normally results in more active sites on the surface for chemical or physical interactions, thereby giving rise to better sensing performance [24].
XPS results
To further determine the elemental compositions and chemical oxidation valance state, XPS measurements were conducted on WM4. The binding energies were calibrated by referencing the C 1s peak (284.6 eV) to reduce the sample charging effect. Figure 4a exhibits the XPS survey spectrum of WM4 with a binding energy ranging from 0 to 1200 eV, and the main constituent elements were tungsten, molybdenum, and oxygen. Figure 4b shows the high-resolution scan of W 4f. The doublets of W 4f 7/2 and W 4f 5/2 peaks, located at 35.6 and 37.8 eV, correspond to W–O bond, which is well consistent with previous works [25–27]. Figure 4c illustrates the high-resolution spectrum of O 1s, which can be fitted into three symmetrical Gaussian peaks. The corresponding peaks located at 529.9 and 530.6 eV can be ascribed to lattice oxygen in WO3. The third peak at 531.6 eV might be due to the chemically adsorbed oxygen [16]. Figure 4d displays the Mo 3d spectrum, which can be deconvoluted into two major peaks with binding energies at 233.2 and 236.3 eV, corresponding to Mo 3d 5/2 and Mo 3d 3/2, respectively. The measured results indicate that Mo element exists mainly in the Mo6+ chemical state in MoO3, which is in accordance with the results of XRD [28].
Gas sensing characteristics
It is well known that the working temperature plays an important role in the gas sensing performance of a gas sensor [20, 29]. To investigate the gas sensing property, the temperature dependence behavior of all the as-fabricated gas sensors to 100 ppm ethanol and acetone was evaluated under different temperatures, as shown in Fig. 5a, b. The results reveal that the responses of all sensors increased with the operating temperature and achieved their maximum values. Further increase in the operating temperature results in the decrease of responses. Moreover, the measured results show that the WM4 exhibits the maximum response values of 28.5 and 18.2 toward 100 ppm ethanol and acetone at 320 °C, respectively, which is much higher than that of pure WO3 sensor (12.5 and 10.9 for ethanol and acetone). Consequently, an optimum working temperature of 320 °C was chosen for the remainder of the experiments on WM4 gas sensor. Table 1 presents the detailed information on the optimum operating temperatures for all the as-prepared gas sensors, and it seems that the optimum operating temperature decreases with the increase of the Mo amount.
Figure 6a, b shows the dynamic sensing transients of the pure WO3 and MoO3/WO3 composite nanostructures to 0.5–1000 ppm of ethanol and acetone under optimum operating temperatures. When the gas sensors were exposed to target gases, the response of all the as-prepared gas sensors shows a clear increase with the increase of gas concentration. The results reveal that the gas sensors based on WM2, WM4, and WM6 exhibit higher sensibility at each concentration than WM0. In particular, the WM4 sensor exhibits the highest responses for target gases, and the sensitivities are about 2.3 and 1.7 times higher than those of WM0 sensor for 100 ppm ethanol and acetone, respectively, while the WM8 sensor even displays slightly lower response than WM0, which indicates that the excessive MoO3 may suppress the gas sensing properties of sensor. Furthermore, when the ethanol/acetone concentration is as low as 500 ppb, the measured sensitivities of WM4 sensor could still reach 5/2.3, which demonstrates that the sensor could detect the concentrations of ethanol and acetone down to ppb level. Therefore, the WM4 gas sensor is potentially used in monitoring trace ethanol and acetone.
Figure 6c, d illustrates the response versus concentration curves of all samples toward ethanol and acetone. The measured results show that all the as-prepared gas sensors show a rapidly increasing response with increasing target gas concentration (0.5–3000 ppm). Especially, the WM4 sensor shows the best gas sensing performance to ethanol and acetone under different concentrations. The responses of WM4 sensor to 1000 ppm ethanol (152.7) and acetone (108.7) are remarkably higher than those of WM0 (95 and 40.2), indicating the enhanced gas sensing properties. However, the gas response values begin to plateau as the concentration of target gas increases further, which suggests that the sensors gradually tend to saturate. The saturation effect can be explained by the competition between the adsorption sites and the concentration of the target gas [30]. The surface reaction is linearly dependent on the tested gas concentration as long as the adsorption sites are enough. In the low gas concentration of ethanol and acetone, the available adsorption sites on the surface of WMx can be regarded as infinite, and the rate-determining step might be the surface reaction between target gas molecules and WMx surface. However, at a high concentration of target gas, the gas molecules have to compete for adsorption sites due to the insufficiency of available adsorption sites on the surface of WMx, which becomes the rate-determining step.
Figure 7a, b shows the response/recovery curves of all the as-fabricated gas sensors to 100 ppm ethanol and acetone at their optimum operating temperatures. The gas sensors’ responses reached the certain values or returned to the initial values quickly when they were exposed to or released from the target gases. Figure 7c shows the response/recovery times of as-fabricated gas sensors to ethanol, and most of gas sensors exhibit quick response/recovery behaviors within 3/4 s. Figure 7d displays the response/recovery times of sensors to acetone, the corresponding response/recovery times are within 3/4 s except the pure WO3 sensor. The relatively fast response and recovery times compared to bulk WO3 can be associated to the special hierarchical structures. The hierarchical structures composed of nanosheets/nanowires would form network pores, grain boundaries, and junction barriers, which are beneficial for the gas diffusion, oxygen diffusion, and re-adsorption [31–33].
Selectivity is another important parameter of gas sensors for their practical application. The selectivity was also investigated on pure WO3 and MoO3/WO3 gas sensors. Figure 8 illustrates the bar graph of the as-prepared sensors to 100 ppm of variety of VOCs, such as ethanol, acetone, methyl alcohol, formaldehyde, ammonium hydroxide, and toluene. All gas sensors exhibit higher responses to ethanol than the other gases, which suggest that the as-fabricated gas sensors exhibit outstanding selectivity to ethanol. At the same time, the long-term stability experiments were conducted on WM4 gas sensor every 10 days for 3 months (Fig. 9). The measured results show that the responses of WM4 sensor toward ethanol and acetone are fluctuating slightly around 28.5 and 18.2, respectively, which confirms the excellent long-term stability of WM4 composite nanostructures. In addition, a comparison between the sensing performances of WM4 sensor and literature reports is summarized in Table 2. It is noteworthy that the WM4 sensor exhibits higher response compared with other nanostructured WO3 sensors reported in previous works.
Gas sensing mechanism
For pure WO3, when the gas sensor was exposed to air, the thickness of electron depletion layer will increase due to the ionization of oxygen molecules. Therefore, the resistance of WO3 will increase, which result from the lower concentration of free electrons in the conduction band (Fig. 10a). When the gas sensor was exposed to reducing gases, the oxygen species will react with the target gas and release electrons into the conduction band. Thus, the thickness of the depletion layer at the interfaces will decrease, which will lead to the decrease of the measured resistance of the WO3 gas sensor (Fig. 10b).
Compared with pure WO3, the enhanced gas sensing properties of MoO3/WO3 gas sensor can be attributed to the synergetic effect and the heterojunction of WO3 and MoO3. Firstly, both MoO3 and WO3 are important sensing materials. There is a synergetic effect of different gas sensing materials, which has been observed in the other hierarchical composites [41–43]. Secondly, it can be ascribed to the heterojunction, which formed at the interface between MoO3 and WO3. The different work functions will lead to the negatively charged carries moving from WO3 to MoO3 until their Fermi levels align, creating a thicker electron depletion layer at the interface (Fig. 10c, d) [20, 44]. As a result, it exhibits enhanced sensing property to ethanol and acetone than pure WO3. The sensing mechanism controlled by the change of the electron depletion layer thickness has also been found in other composites [45, 46]. However, the excessive content of Mo element will suppress the sensing properties of the samples, because excess dopant would reduce the available adsorption sites between WO3 and the target gas, which is in agreement with the previous report [47].
Conclusions
In summary, pure WO3 and MoO3/WO3 composite nanostructures were successfully synthesized and characterized. The SEM results show that the morphology of WO3 can be greatly affected by the content of Mo. The gas sensing properties were measured on pure WO3 and MoO3/WO3 gas sensors and the results suggest that the moderate introduction of MoO3 could significantly improve the gas sensing properties. Moreover, the WM4 gas sensor exhibits enhanced responses to ethanol and acetone, which are about 2.3 and 1.7 times higher than those of pure WO3 gas sensor, respectively. The low limit of detection of WM4 can be down to 500 ppb. The enhanced gas sensing performance of the MoO3/WO3 may be attributed to the synergetic effects of MoO3 and WO3. This study will be beneficial for the construction of WO3-based composite gas sensors with higher response, fast response/recovery time, and good selectivity and stability for trace ethanol and acetone detection.
References
Kim YS, Ha SC, Yang H, Kim YT (2007) Gas sensor measurement system capable of sampling volatile organic compounds (VOCs) in wide concentration range. Sens Actuator B 122:211–218
Dar GN, Umar A, Zaidi SA, Ibrahim AA, Abaker M, Baskoutas S (2012) Ce-doped ZnO nanorods for the detection of hazardous chemical. Sens Actuators B 173:72–78
Rakshit T, Santra S, Manna I, Ray SK (2014) Enhanced sensitivity and selectivity of brush-like SnO2 nanowire/ZnO nanorod heterostructure based sensors for volatile organic compounds. RSC Adv 4:36749–36756
Rai P, Majhi SM, Yu Y-T, Lee J-H (2015) Synthesis of plasmonic Ag@SnO2 core–shell nanoreactors for xylene detection. RSC Adv 5:17653–17659
Jia QQ, Ji HM, Zhang Y, Chen YL, Sun XH, Jin ZF (2014) Rapid and selective detection of acetone using hierarchical ZnO gas sensor for hazardous odor markers application. J Hazard Mater 276:262–270
Shin J, Choi SJ, Youn DY, Kim D (2012) Exhaled VOCs sensing properties of WO3 nanofibers functionalized by Pt and IrO2 nanoparticles for diagnosis of diabetes and halitosis. J Electroceram 29:106–116
Zeng Y, Zhang T, Wang LJ, Kang MH, Fan HT, Wang R, He Y (2009) Enhanced toluene sensing characteristics of TiO2-doped flowerlike ZnO nanostructures. Sens Actuators B 140:73–78
Rani RA, Zoolfakar AS, Ou JZ, Field MR, Austin M, Kalantar-zhadeh K (2013) Nanoporous Nb2O5 hydrogen gas sensor. Sens Actuators B 176:149–156
Mao YZ, Ma S, Li XB, Wang CY, Li FM (2014) Effect of Mn doping on the microstructures and sensing properties of ZnO nanofibers. Appl Surf Sci 298:109–115
Dong CJ, Xing XX, Chen N, Liu X, Wang YD (2016) Biomorphic synthesis of hollow CuO fibers for low-ppm-level n-propanol detection via a facile solution combustion method. Sens Actuators B 230:1–8
Sun P, Wang C, Liu JY, Zhou X, Li XW, Hu XL, Lu GY (2015) Hierarchical assembly of α-Fe2O3 nanosheets on SnO2 hollow nanospheres with enhanced ethanol sensing properties. ACS Appl Mater Interfaces 7:19119–19125
Vuong NM, Hieu NM, Kim D, Choi B, Kim M (2014) Ni2O3 decoration of In2O3 nanostructures for catalytically enhanced methane sensing. Appl Surf Sci 317:765–770
Wang ZY, Sun P, Yang TL, Gao Y, Li XW, Lu GY, Du Y (2013) Flower-like WO3 architectures synthesized via a microwave-assisted method and their gas sensing properties. Sens Actuator B 186:734–740
Wang C, Sun R, Li X, Sun YF, Sun P, Liu FM, Lu GY (2014) Hierarchical flower-like WO3 nanostructures and their gas sensing properties. Sens Actuator B 204:224–230
Zeng J, Hu M, Wang WD, Chen HQ, Qin YX (2012) NO2-sensing properties of porous WO3 gas sensor based on anodized sputtered tungsten thin film. Sens Actuator B 161:447–452
Zhang YD, He WW, Zhao HX, Li PJ (2013) Template-free to fabricate highly sensitive and selective acetone gas sensor based on WO3 microspheres. Vacuum 95:30–34
Xu LJ, Yin ML, Liu SZ (2015) Superior sensor performance from Ag@WO3 core–shell nanostructure. J Alloys Compd 623:127–131
Kida T, Nishiyama A, Hua ZQ, Suematsu K, Yuasa M (2014) WO3 nanolamella gas sensor: porosity control using SnO2 nanoparticles for enhanced NO2 sensing. Langmuir 30:2571–2579
Chi X, Liu CB, Li Y, Wang ZJ, Bo XQ, Liu LL, Su C (2014) Tungsten trioxide nanotubes with high sensitive and selective properties to acetone. Sens Actuator B 194:33–37
Yang XJ, Salles V, Kaneti Y, Liu MS, Maillard M, Journet C, Jiang XC, Brioude A (2015) Fabrication of highly sensitive gas sensor based on Au functionalized WO3 composite nanofibers by electrospinning. Sens Actuator B 220:1112–1119
Zhao XD, Ji HM, Jia QQ, Wang MJ (2015) A nanoscale Co3O4–WO3 p–n junction sensor with enhanced acetone responsivity. J Mater Sci 26:8217–8223. doi:10.1007/s10854-015-3484-3
Wang CY, Ma SY, Sun A, Qin R, Yang FC, Li XB (2014) Characterization of electrospun Pr-doped ZnO nanostructure for acetic acid sensor. Sens Actuator B 193:326–333
Sun P, Zhou X, Wang C, Wang B, Xu XM, Lu GY (2014) One-step synthesis and gas sensing properties of hierarchical Cd-doped SnO2 nanostructures. Sens Actuators B 190:32–39
Lu YY, Zhan WW, He Y, Wang YT, Kong XJ, Kuang Q, Xie ZX, Zheng LS (2014) MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Appl Mater Interface 6:4186–4195
Zhou D, Shi F, Xie D, Wang DH, Xia XH, Wang XL, Gu CD, Tu JP (2016) Bi-functional Mo-doped WO3 nanowire array electrochromism-plus electrochemical energy storage. J Colloid Interface Sci 465:112–120
Zhou D, Xie D, Shi F, Wang DH, Ge X, Xia XH, Wang XL, Gu CD, Tu JP (2015) Crystalline/amorphous tungsten oxide core/shell hierarchical structures and their synergistic effect for optical modulation. J Colloid Interface Sci 460:200–208
Cai GF, Tu JP, Zhou D, Wang XL, Gu CD (2014) Growth of vertically aligned hierarchical WO3 nano-architecture arrays on transparent conducting substrates with outstanding electrochromic performance. Sol Energy Mater Sol Cells 124:103–110
Swiatowska-Mrowiecka J, de Diesbach S, Maurice V, Zanna S, Klein L, Briand E, Vickridge I, Marcus P (2008) Li-ion intercalation in thermal oxide thin films of MoO3 as studied by XPS, RBS, and NRA. J Phys Chem C 112:11050–11058
Kaneti YV, Zakaria QMD, Zhang Z, Chen C, Yue J, Liu M, Jiang XC, Yu A (2014) Solvothermal synthesis of ZnO-decorated α-Fe2O3 nanorods with highly enhanced gas-sensing performance toward n-butanol. J Mater Chem A 2:13283–13292
Ahn MW, Park KS, Heo JH, Kim DW, Choi KJ, Park JG (2009) On-chip fabrication of ZnO-nanowire gas sensor with high gas sensitivity. Sens Actuator B 138:168–173
Huang JR, Xu XJ, Gu CP, Yang M, Yang M, Liu JH (2011) Large-scale synthesis of hydrated tungsten oxide 3D architectures by a simple chemical solution route and their gas-sensing properties. J Mater Chem 21:12283–13289
Sun P, Wang WN, Liu YP, Sun YF, Ma J, Lu GY (2012) Hydrothermal synthesis of 3D urchin-like α-Fe2O3 nanostructure for gas sensor. Sens Actuator B 173:52–57
Alenezi MR, Henley SJ, Emerson NG, Silva RP (2014) From 1D and 2D ZnO nanostructures to 3D hierarchical structures with enhanced gas sensing properties. Nanoscale 6:235–247
Jia QQ, Ji HM, Gao P, Bai X, Jin ZG (2015) Control of the acetone sensitive and selective properties of WO3 nanofibers by doping Co ions: effect of crystal symmetric structure on the responsivity of gas–solid boundaries for gas sensor. J Mater Sci 26:5792–5802. doi:10.1007/s10854-015-3138-5
Gao P, Ji HM, Zhou YG, Li XL (2012) Selective acetone gas sensors using porous WO3–Cr2O3 thin films prepared by sol–gel method. Thin Solid Film 520:3100–3106
Xiao JK, Song CW, Dong W, Li C, Yin YY, Zhang XN, Song MY (2015) Synthesis, characterization, and gas sensing applications of WO3 nanobricks. J Mater Eng Perform 24:3026–3031
Yao Y, Ji FX, Yin ML, Ren XP, Ma Q, Yan JQ (2016) Ag nanoparticle-sensitized WO3 hollow nanosphere for localized surface plasmon enhanced gas sensors. ACS Appl Mater Interface 8:18165–18172
Li XX, Zhang GY, Cheng FY, Guo B, Chen J (2006) Synthesis, characterization, and gas-sensor application of WO3 nanocuboids. J Electrochem Soc 153:H133–H137
Labidi A, Gillet E, Delamare R, Maaref M, Aguir K (2006) Ethanol and ozone sensing characteristics of WO3 based sensors activated by Au and Pd. Sens Actuator B 120:338–345
Ahsan M, Ahmad MZ, Tesfamichael T, Bell J, Wlodarski W, Motta N (2012) Low temperature response of nanostructured tungsten oxide thin films toward hydrogen and ethanol. Sens Actuator B 173:789–796
Chen YJ, Zhu CL, Shi XL, Cao MS, Jin HB (2008) The synthesis and selective gas sensing characteristics of SnO2/α-Fe2O3 hierarchical nanostructures. Nanotechnology 19:205603
Zhu CL, Chen YJ, Wang RX, Wang LJ, Cao MS, Shi XL (2009) Synthesis and enhanced ethanol sensing properties of α-Fe2O3/ZnO heteronanostructures. Sens Actuator B 140:185–189
Sun P, Cai YX, Du SS, Xu XM, You L, Ma J, Liu FM, Liang XS, Sun YF, Lu GY (2013) Hierarchical α-Fe2O3/SnO2 semiconductor composites: hydrothermal synthesis and gas sensing properties. Sens Actuator B 182:336–343
Majhi Bertouluzzi L, Tietwyk KJ, Ginsburg A, Keller DA, Yaro PL, Anderson AY, Bisquert J, Zaban A (2016) Combinatorial investigation and modelling of MoO3 hole-selective contact in TiO2/Co3O/MoO3 all-oxide solar cells. Adv Mater Interface 3:1–7
Lundstrom KI, Shivaraman MS, Syensson CM (1975) A hydrogen-sensitive Pd-gate MOS transistor. J Appl Phys 46:3876–3881
Yu J, Wlodarski W, Li YX, Kalantar-zadeh K (2010) Nanorod based Schottky contact gas sensors in reversed bias condition. Nanotechnology 21:265502
Liu CB, Shan H, Liu L, Li SC, Li HY (2014) High sensing properties of Ce-doped α-Fe2O3 nanotubes to acetone. Ceram Int 40:2395–2399
Acknowledgements
We appreciate the support of the National Natural Science Foundation of China (51205274), Shanxi Province Science Foundation (2016011039), Shanxi Province Special Talent Fund (201605D211020), University of Science and Technology innovation Research Project of Shanxi Province (2016137), Graduate Education Innovation Fund (02100738), Science and Technology Major Project of the Shan Xi Science and Technology Department (20121101004), and Key Disciplines Construction in Colleges and Universities of Shanxi [(2012)45].
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, Y., Chen, L., Wang, Y. et al. Synthesis of MoO3/WO3 composite nanostructures for highly sensitive ethanol and acetone detection. J Mater Sci 52, 1561–1572 (2017). https://doi.org/10.1007/s10853-016-0450-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0450-2