Abstract
Energy storage materials are crucial for efficient utilization of electricity in modern electric power supply and renewable energy systems. Film capacitors are promising technologies for electrical energy storage for their high power densities and charge–discharge rate, yet they are limited by their relatively low energy densities. The addition of high-k inorganic particles can lead to high dielectric constant, but at the expense of low breakdown strength and low energy efficiency, which limits their practical applications at high electric fields. In this work, we report all-organic dielectric films based on poly(vinylidene fluoride-co-trifluoroethylene-co-chlorotrifluoroethylene) (PVDF–TrFE–CTFE) terpolymer and aromatic polythiourea (ArPTU) and having enhanced energy density, energy storage efficiency, and breakdown field. Aromatic polythiourea was prepared by a conventional polycondensation method. The ArPTU/PVDF–TrFE–CTFE composite films were fabricated by solution-blending followed by hot pressing. The composite films have lower dielectric loss and higher breakdown field than the PVDF–TrFE–CTFE matrix. More importantly, the blend films also show improved released energy density and reduced loss at high fields. For ArPTU/PVDF–TrFE–CTFE (15/85) composite film, the maximum released energy density is 19.2 J/cm3 at 700 MV/m with an efficiency of 85%. The improved energy density and reduced energy loss are related to the increase in electric resistivity and structural changes. The findings of this research could provide a simple and scalable approach to produce compact and flexible high energy density materials for energy storage devices.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrical energy storage devices can satisfy specific requirements in various fields, such as artificial muscles, capacitors, and smart skins [1,2,3,4,5,6,7]. Among the available electrical energy storage technologies, dielectric capacitors have the highest power density due to their ultra-fast charge–discharge capability [8, 9]. However, their potential application is limited by relatively low energy density. For example, the current state of the art biaxially oriented polypropylene (BOPP) has an energy density of 1.2 J/cm3 in commercial applications, which is much lower than those of batteries and electrochemical supercapacitors [10, 11]. Since more than 25% of the volume and weight is occupied by capacitors in power electronics and pulsed power systems, it is necessary to improve the energy density of capacitors. The stored energy density (U e) is described by U e = ∫EdD, where E is the applied electric field and D is the induced electric displacement. For linear dielectrics, U = 1/2ε 0 ε r E 2, where ε 0 is the vacuum permittivity and ε r is the dielectric constant. Therefore, materials with high dielectric constant and breakdown field can achieve high energy density. It is well known that ceramic materials (e.g., BaTiO3, BaxSr1−xTiO3) have the highest dielectric constant. However, the breakdown field of ceramics is quite low and the preparation process is difficult. Polymers typically have high breakdown field but very small dielectric constant [12, 13]. The introduction of high dielectric constant nanoparticles as dielectric materials into a polymer matrix has been extensively investigated. In many cases, the poor compatibility between the inorganic fillers and the organic polymer matrix results in aggregation and interfacial adhesion, leading to high dielectric loss [14]. Besides, the large difference in dielectric constant between the ceramic fillers and polymer matrix gives rise to a higher local electric field in polymer, which significantly lowers breakdown field of the nanocomposites [15,16,17]. As a result, to further enhance the energy density of capacitors, many efforts have been made to improve the existing polymer dielectrics and design new material structures.
Poly(vinylidene fluoride) (PVDF) and its copolymers have been widely studied for capacitor applications because of their high breakdown field and dielectric constant [18,19,20,21,22,23,24]. The high dipole moment of C–F bonds gives rise to PVDF-based polymers with higher dielectric constant. For example, poly(vinylidene fluoride-co-chlorotrifluoroethylene) (PVDF–CTFE) possesses a dielectric constant of 10 at 103 Hz, and an energy density of 8 J/cm3 at 400 MV/m [25]. Unfortunately, the high remnant polarization and large hysteresis loss of PVDF and its copolymers limit their application as dielectric materials in capacitors. One way to solve this problem is to design relaxor ferroelectric polymers, which have reduced hysteresis, by incorporating structural defects into the PVDF matrix. For example, chlorotrifluoroethylene (CTFE) is introduced into P(VDF-co-trifluoroethylene) (PVDF–TrFE) and narrow hysteresis loops and high dielectric constants are observed [26, 27]. However, PVDF terpolymers display high losses under high fields [28].
Recently, a new class of dielectric polymer based on aromatic polythiourea (ArPTU) has been studied [29, 30]. The ArPTU films display high breakdown strength (>1.0 GV/m) and charge–discharge efficiency (90% at 1.1 GV/m). The thiourea group has a high dipole moment (≈4.89 D), leading to a higher dielectric constant than those of most polymer dielectrics. However, it is still lower than those of PVDF-based polymers. On the other hand, ArPTU polymers can be dissolved only at very low concentrations (generally 1–2% by weight) in polar solvents. Besides, an ArPTU film is brittle due to the rigid aromatic groups, which make it unsuitable for a flexible dielectric film.
To address these issues, all-organic dielectric materials have been investigated, which offer advantages such as easy processing, lighter weight, and possibly lower cost [31,32,33]. In this work, significantly suppressed dielectric loss was obtained by blending a small quantity of ArPTU into the PVDF–TrFE–CTFE matrix while maintaining flexibility of PVDF–TrFE–CTFE. Marked improvements in breakdown field, discharged energy density, and charge–discharge efficiency have been demonstrated in ArPTU/PVDF–TrFE–CTFE composite films. It is demonstrated that the improved dielectric properties are related to the crystalline structures and increased electric resistivity.
Experimental
Materials
The PVDF–TrFE–CTFE 62.2/30.2/7.6 (molar ratio) terpolymer was purchased from Piezotech, Inc./Arkema (Lyon, France). Paraphenylene diisothiocyanate (PDTC), 4,4′-diphenylmethanediamine (MDA), and n-methyl-2-pyrrolidone (NMP, HyDry, with molecular sieves) were purchased from Sigma-Aldrich. N, N-dimethyl formamide (DMF) was purchased from Sinopharm Chemical Reagent Co., Ltd., China.
Polythiourea synthesis and film preparation
The ArPTU was synthesized by the polycondensation method [34]. 0.961 g (5 mmol) of PDTC, 0.991 g (5 mmol) of MDA, and 20 ml of NMP were added to a dry three-neck flask under a N2 atmosphere. After stirred for 6 h at room temperature, the solution was poured into methanol with fiber-like precipitation, followed by washing with methanol and water and dried at 60 °C in vacuum oven for 12 h.
The ArPTU/PVDF–TrFE–CTFE composite films with different ArPTU weight fractions were prepared by a solution blending method. First, the pre-calculated quantities of ArPTU and PVDF–TrFE–CTFE were dissolved in DMF. Then, the mixture was stirred at 60 °C for 2 h. The resulting mixture was precipitated in double-distilled water and was centrifuged to obtain the composites. The obtained ArPTU/PVDF–TrFE–CTFE composites were dried under vacuum at 70 °C for 24 h. The dried composites were hot-pressed at 190 °C under 20 tons pressure to obtain ~20 μm films. It should be noted that ArPTU/PVDF–TrFE–CTFE composite film with 20 wt% of ArPTU is hard to dissolve in solvent, so we did not perform morphology or dielectric characterization.
Instrumentation and characterization
1H NMR spectra were obtained at 400.13 MHz with a Bruker Avance III 400 MHz NMR spectrometer using DMSO-d6 as solvent. The thermal behavior and crystallinity of ArPTU, PVDF–TrFE–CTFE, and the composite films were measured with a TA Instruments Q200 DSC at the heating and cooling rates of 10 °C/min from room temperature to 200 °C under a N2 atmosphere. X-ray diffraction (XRD) was carried out at room temperature using a Bruker D8 Advance diffractometer. Gold electrodes with a thickness of about 15 nm were deposited on both sides of the films by a sputter coater (E-1045, Hitachi, Japan). Electrical resistivity was measured by Hewlett Packard 4140B pA meter/DC voltage source under an electric field of 10 MV/m. Broadband dielectric spectroscopy (BDS) measurements were performed on a Novocontrol Concept 80 broadband dielectric spectrometer (Montabaur, Germany) at room temperature. The breakdown strength measurements were performed by a dielectric breakdown tester at room temperature (CS2671A, China). The electric displacement–electric field (D–E) hysteresis loop measurements were carried out on a Premiere II ferroelectric tester from Radiant Technologies, Inc.
Results and discussion
Polythiourea characterization and thermal properties
Figure 1a shows a representative 1H NMR spectrum for ArPTU. Chemical shifts in the range of 7.20–7.44 and 9.66–9.70 correspond to the protons from the aromatic and the thiourea groups, respectively. The peak at 3.89 ppm is associated with the methylene group. The results of 1H NMR spectroscopy confirm the successful synthesis of ArPTU. A mechanical flexible ArPTU/PVDF–TrFE–CTFE composite film is shown in Fig. 1b and c. The composite film is canary yellow and can be folded. Thermal properties influence the processing of polymer thin films, since a phase transition within the running temperatures of the capacitor will increase the dielectric loss. As shown in Fig. 2, the DSC data display no melting peak, while no crystalline structures are formed during the cooling process, indicating that the ArPTU films have an amorphous structure. It has been reported that isolated or random dipoles in amorphous polar polymers can increase the charge–discharge efficiency because of the weak interactions among these dipoles and between the dipoles and the matrix [35]. Besides, electron–phonon scattering and electron–dipole scattering in amorphous polar polymers can prevent dielectric breakdown [36].
Dielectric properties of polythiourea
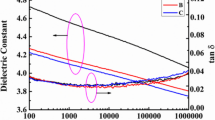
Figure 3a shows the dielectric constant and loss of ArPTU as a function of frequency. Due to the high dipole moment of 4.89 D in the thiourea groups, the dielectric constant of ArPTU is 5.8 at 100 Hz, which is higher than those of most linear polymer dielectric materials [34]. According to the Frohlich model, which is widely used to describe the dielectric constant of polymers, the dielectric constant is proportional to the volumetric dipole density, dipole moment, and correlation factor between the dipoles:
where ε rs and ε r∞ are the dielectric constants at low frequency and optical frequency, respectively; N is the volumetric dipole density; g is the correlation factor; p is the dipole moment; k is the Boltzmann constant; and T is the temperature. The dielectric constant of the ArPTU in this work is higher than that of the aromatic polythiourea (4.5) synthesized by Wu et al., since our ArPTU has a higher volumetric dipole density [29]. The dielectric loss is as low as 0.0067 at 103 Hz and room temperature. Figure 3b shows that the AC conductivity of ArPTU is 10−10 S/m at 100 Hz, suggesting that ArPTU is a good insulating material. The low conductivity indicates that the leakage current will be very small during operation.
Dielectric properties of ArPTU/PVDF–TrFE–CTFE composite films
The influence of ArPTU content of ArPTU/PVDF–TrFE–CTFE composite film on dielectric properties was investigated at room temperature. Figure 4 shows the dielectric constant and loss of ArPTU/PVDF–TrFE–CTFE composite films as a function of frequency. The dielectric constant of PVDF–TrFE–CTFE drops obviously at the frequency of 105 Hz, because of the relaxation of amorphous dipoles in PVDF–TrFE–CTFE [35]. After the incorporation of ArPTU, the frequency dependence becomes weak, probably because thiourea groups in ArPTU interact with the PVDF–TrFE–TrFE matrix, confining the rotation of the amorphous dipoles in PVDF–TrFE–TrFE. The dielectric constant of the ArPTU/PVDF–TrFE–CTFE composite films decreases with increasing ArPTU content. At the frequency of 103 Hz, the dielectric constant of neat PVDF–TrFE–CTFE is 41.9, and the dielectric constant drops to 13.8 with the addition of just 5 wt% of ArPTU. The dielectric constant further decreases to 12.6 and 11.3 with 10 and 15 wt% of ArPTU, respectively. The low dielectric constant of the composite film is mainly attributed to the low dielectric constant of ArPTU. As for the dielectric loss, all samples show significant decrease after the addition of ArPTU, especially at high frequencies. Since the dielectric loss under high frequencies mainly comes from dipolar relaxation, the results again indicate that the thiourea groups in ArPTU may confine dipole relaxation [37]. Generally, the dielectric loss of the ArPTU/PVDF–TrFE–CTFE composite films is lower than those of the all-organic dielectric materials [38, 39]. For example, with 15 wt% of ArPTU, the dielectric loss of the composite film is only 0.01 at 103 Hz.
The breakdown strength of the composite films is another important property of dielectric materials. The breakdown strengths of the composite films with different contents of ArPTU were determined by a two-parameter Weibull statistic distribution method: P(E) = 1−exp[−(E/E 0)β], where P(E) is the cumulative probability for electric failure, E is the experimentally recorded breakdown strength, E 0 is the characteristic breakdown strength at which there is a 63.2% probability for failure, and β is a parameter associated with the scatter of the data. In this work, the breakdown strength is calculated by fitting Weibull failure statistics with 20 specimens per sample. Figure 5a shows the Weibull plots of breakdown strength for PVDF–TrFE, ArPTU, and ArPTU/PVDF–TrFE–CTFE composite films. The breakdown fields calculated by the Weibull distribution formula are 368.2, 746.1, 604.5, 645.7, and 707.2 MV/m for PVDF–TrFE–CTFE, ArPTU, ArPTU/PVDF–TrFE–CTFE (5/95), ArPTU/PVDF–TrFE–CTFE (10/90), and ArPTU/PVDF–TrFE–CTFE (15/85), respectively. For neat ArPTU, the breakdown field is relatively high because of electron–phonon scattering and electron–dipole scattering in the amorphous structure, reducing the conduction current. After the incorporation ArPTU, the breakdown fields of the composite films are significantly improved. With the increasing content of ArPTU in the PVDF–TrFE–CTFE matrix, the breakdown field of the composite film increases, mainly because of the high breakdown field of ArPTU. Figure 5b shows the high-field electrical resistivity of PVDF–TrFE, ArPTU, and the ArPTU/PVDF–TrFE–CTFE composite films. It is clear that with addition of ArPTU, the electrical resistivity of the composite films increases by an order of magnitude over that of the PVDF–TrFE–CTFE matrix. These results indicate that ArPTU can effectively suppress the leakage current and improve the breakdown field. Our ArPTU/PVDF–TrFE–CTFE composite films have much higher breakdown fields than other inorganic filler/polymer dielectric films, since a large electrical mismatch between the matrix and fillers significantly reduces the breakdown field [40].
To explore the dielectric response at high electric fields, we plotted the D–E loops of PVDF–TrFE–CTFE, ArPTU, and ArPTU/PVDF–TrFE–CTFE composite films with increasing applied electric field. Figure 6a–e shows the unipolar D–E loops of PVDF–TrFE–CTFE, ArPTU, and ArPTU/PVDF–TrFE–CTFE composite films. For the PVDF–TrFE–CTFE matrix, relaxor ferroelectric behavior is observed above 150 MV/m, which is similar to other research [41]. The maximum displacement polarization is 0.076 C/m2 among all tested samples. But for ArPTU, the polarization has a nearly linear relationship with the applied field, because of the quick response of the dipoles to the applied field. The maximum displacement polarization of ArPTU is the lowest among all films under all applied fields. The addition of ArPTU into the PVDF–TrFE–CTFE matrix decreases the maximum displacement polarization of the composite films. According to the equation D = ε 0 ε r E, where ε 0 and ε r are the dielectric constants of vacuum and dielectric constant of the composite films, respectively, and the decrease in electric displacement is mainly caused by the decrease of dielectric constant of the composite films. The decreased dielectric constant and maximum displacement polarization sometimes do not necessarily decrease the ultimate energy storage density, but rather avoid early polarization saturation, leading to a higher energy storage density at high fields [42]. After the addition of ArPTU, the composite films no longer show relaxor ferroelectric behavior, illustrating that a small amount of ArPTU blended into PVDF–TrFE–CTFE can change the relaxor ferroelectric properties of PVDF–TrFE–CTFE. Besides, the electrical conduction loss and polarization hysteresis arising from irreversible dipoles can be demonstrated by the remnant displacement. Figure 6f summarizes the remnant displacement of PVDF–TrFE–CTFE and ArPTU/PVDF–TrFE–CTFE composite films. The remnant displacement decreases from 0.015 C/m2 for PVDF–TrFE–CTFE to 0.0014 C/m2 for the ArPTU/PVDF–TrFE–CTFE (15/85) composite film under 300 MV/m. Even at 700 MV/m, the remnant displacement of the ArPTU/PVDF–TrFE–CTFE (15/85) composite film is only 0.0098 C/m2. In other words, the ArPTU/PVDF–TrFE–CTFE composite films show much slimmer loops than PVDF–TrFE–CTFE. This phenomenon is partially attributed to the increased electrical resistivity (illustrated in Fig. 5b), which reduces the electrical conduction loss, leading to lower the remnant displacement.
X-ray diffraction (XRD) was performed to better understand the dielectric behaviors and the structural changes of the composite films. Figure 7a shows the XRD patterns of PVDF–TrFE–CTFE, ArPTU, and the ArPTU/PVDF–TrFE–CTFE composite films. Consistent with the other work, the amorphous ArPTU sample displays no diffraction peaks [34]. For PVDF–TrFE–CTFE, the (110) and (200) reflections are observed at 2θ = 18.4°, which is a single peak typically found in the nonpolar α-phase in the terpolymer [43, 44]. After the incorporation of ArPTU, the peak positions shift to higher angles. According to Bragg’s law, 2dsinθ = n·λ, where d is the interchain distance, λ is the wavelength of X-ray, and θ is the diffraction angle, the calculated interchain distances are 4.80, 4.83, 4.85, and the 4.89 Å for the PVDF–TrFE–CTFE matrix, ArPTU/PVDF–TrFE–CTFE (5/95), ArPTU/PVDF–TrFE–CTFE (10/90), and ArPTU/PVDF–TrFE–CTFE (15/85), respectively. The increased interchain distance provides extra room for the friction-free rotation of dipoles. The width at half maximum increases slightly as the ArPTU content increases. According to the Scherrer equation, D = (K···λ)/(β···cosθ), where D is the mean size of the crystalline domains, K is the Debye–Scherrer constant, λ is the X-ray wavelength (0.15406 nm), β is the width at half maximum, and θ is the diffraction angle, the mean sizes of the crystalline domains are 30.9, 29.7, 28.8, and 28.0 nm for the PVDF–TrFE–CTFE matrix, ArPTU/PVDF–TrFE–CTFE (5/95), ArPTU/PVDF–TrFE–CTFE (10/90), and ArPTU/PVDF–TrFE–CTFE (15/85), respectively. The crystalline domain with reduced size would benefit the rotation of dipoles and thus decrease the hysteresis loss [45]. Besides, the degree of crystallinity of the composite films is higher than that of the PVDF–TrFE–CTFE matrix according to the enthalpy of melting calculated from the differential scanning calorimetry (DSC) heating curves, as shown in Fig. 7b. The degree of crystallinity (χ c) was calculated by χ c = ΔH f/(w∆H °f ) × 100%, where ΔH f is the heat of fusion, ∆H f is the heat of fusion for the 100% crystalline PVDF–TrFE–CTFE (42.0 J/g) [46], and w is the weight fraction. The χ c values are 31.6 wt% for PVDF–TrFE–CTFE, and 33.8, 35.3, and 36.1 wt% for ArPTU/PVDF–TrFE–CTFE (5/95), ArPTU/PVDF–TrFE–CTFE (10/90), and ArPTU/PVDF–TrFE–CTFE (15/85) composite films, respectively. The ArPTU molecules increase the crystallizability of PVDF–TrFE–CTFE, as suggested by the increased crystallization temperature (Tc) of the nanocomposites, as shown in Fig. 7c. For example, the Tc increases from 101.4 °C for PVDF–TrFE–CTFE to 104.5 °C for ArPTU/PVDF–TrFE–CTFE (15/85) composite film. All these results along with the increased electrical resistivity indicate that the incorporation of ArPTU can provide an efficient way to reduce the electrical conduction loss and polarization hysteresis.
The energy storage capability of the ArPTU/PVDF–TrFE–CTFE composite films at high electric fields was investigated. The discharged U e was obtained from the unipolar D–E loop, as shown in Fig. 6, by integration of the area between the ordinate and the discharge curve, namely, U e = ∫EdD [1, 47]. As shown in Fig. 8a, the U e of the ArPTU/PVDF–TrFE–CTFE composite films increases with electric field and ArPTU content. The maximum U e of the ArPTU/PVDF–TrFE–CTFE (15/85) composite film (19.2 J/cm3 at 700 MV/m) is more than three times higher than that of the PVDF–TrFE–CTFE matrix (6.2 J/cm3 at 300 MV/m). The significantly improved U e is mainly attributed to the increase of the breakdown field of the composite films. At the same applied electric field, the ArPTU/PVDF–TrFE–CTFE composite films also have a higher U e than the pure ArPTU film, as a result of the high dielectric constant of ArPTU. To the best of our knowledge, the U e of ArPTU/PVDF–TrFE–CTFE composite film rivals or exceeds those reports for inorganic filler/polymer or all-organic dielectric films [15, 17, 42, 45, 48], and 16 times higher than the energy density of 1.2 J/cm3 at 640 MV/m for commercial BOPP [49]. The thickness of our composite films is about 20 μm, much thicker than those of the commercial dielectric polymer materials (e.g., 6–10 μm), resulting in more defects, which may lead to a lower breakdown field. A further reduction in film thickness would promise higher breakdown field and thus further increase the energy storage density. Besides, the solution blending and hot pressing methods used to prepare our composite films are simpler and less expensive than the uniaxial or biaxial stretching and multi-step extrusion-blowing process commonly used for films.
For practical applications, the charge–discharge efficiency is another important property of dielectric materials for energy storage capacitors, since the energy losses always result in heating and damaging the performance and reliability of the capacitors. Figure 8b shows the efficiency (discharge energy/charge energy) of ArPTU, PVDF–TrFE–CTFE, and the ArPTU/PVDF–TrFE–CTFE composite films at high electric fields. The efficiency of the PVDF–TrFE–CTFE matrix drops from 79% at 100 MV/m to 65% at 350 MV/m, mainly resulting from the ferroelectric hysteresis loss at high fields. The efficiency of the ArPTU film, which is a linear dielectric material, is quite high at high electric fields. Although the efficiency of the ArPTU/PVDF–TrFE–CTFE composite films is not as high as that of the ArPTU film, it is still generally enough for practical applications [50]. The efficiency of the ArPTU/PVDF–TrFE–CTFE (15/85) composite film is above 90% in the range of the electric fields 100–500 MV/m and is 85% even at 700 MV/m because of the decreased conduction loss caused by the increased electric resistivity and reduced hysteresis loss related to the crystalline structures of the polymer matrix. Therefore, we have demonstrated that the use of ArPTU in PVDF-based polymers is a promising industrial route to prepare organic dielectric materials for capacitors.
Conclusions
ArPTU was synthesized by the polycondensation method, and ArPTU/PVDF–TrFE–CTFE composite films were prepared by a solution blending and hot pressing method. The ArPTU/PVDF–TrFE–CTFE composite films have significantly higher dielectric breakdown field, lower energy loss, and higher charge–discharge efficiency than PVDF–TrFE–CTFE. The maximum released energy density of the ArPTU/PVDF–TrFE–CTFE (15/85) composite film is 19.2 J/cm3 at 700 MV/m with an efficiency of 85%. The improved energy density and reduced energy loss of the composite films are related to the crystalline structures and increased electric resistivity. The excellent dielectric performance of the composite films along with the simplicity and scalability of preparation suggests a promising industrial route to prepare compact and flexible high energy density dielectric materials.
References
Chu B, Zhou X, Ren K, Neese B, Lin MR, Wang Q, Bauer F, Zhang QM (2006) A dielectric polymer with high electric energy density and fast discharge speed. Science 313:334–336
Su Y, Sun C, Zhang W, Huang H (2013) Fabrication and dielectric properties of Na0.5Bi0.5Cu3Ti4O12/poly(vinylidene fluoride) composites. J Mater Sci 48:8147–8152. doi:10.1007/s10853-013-7627-8
Marsh RA, Hodgkiss JM, Friend RH (2010) Direct measurement of electric field-assisted charge separation in polymer: fullerene photovoltaic diodes. Adv Mater 22:3672–3676
Zhang QM, Li HF, Poh M, Xu HS, Cheng ZY, Xia F, Huang C (2002) An all-organic composite actuator material with a high dielectric constant. Nature 419:284–287
Zhou W, Yu D (2013) Fabrication, thermal, and dielectric properties of self-passivated Al/epoxy nanocomposites. J Mater Sci 48:7960–7968. doi:10.1007/s10853-013-7606-0
Luo H, Zhou K, Bowen C, Zhang F, Wei A, Wu Z, Chen C, Zhang D (2016) Building hierarchical interfaces using BaSrTiO3 nanocuboid dotted graphene sheets in an optimized percolative nanocomposite with outstanding dielectric properties. Adv Mater Interfaces 3:1600157. doi:10.1002/admi.201600157
Luo H, Wu Z, Chen C, Ma C, Zhou K, Zhang D (2016) Methoxypolyethylene glycol functionalized carbon nanotube composites with high permittivity and low dielectric loss. Compos A 86:57–63
Ducharme S (2009) An inside-out approach to storing electrostatic energy. ACS Nano 3:2447–2450
Li Q, Han K, Gadinski MR, Zhang G, Wang Q (2014) High energy and power density capacitors from solution-processed ternary ferroelectric polymer nanocomposites. Adv Mater 26:6244–6249
El-Kady MF, Strong V, Dubin S, Kaner RB (2012) Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 335:1326–1330
Pikul JH, Zhang HG, Cho J, Braun PV, King WP (2013) High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat Commun 4:1732. doi:10.1038/ncomms2747
Dang ZM, Yuan JK, Zha JW, Zhou T, Li ST, Hu GH (2012) Fundamentals, processes and applications of high-permittivity polymer-matrix composites. Prog Mater Sci 57:660–723
Li JY, Zhang L, Ducharme S (2007) Electric energy density of dielectric nanocomposites. Appl Phys Lett 90:132901. doi:10.1063/1.2716847
Schadler L (2007) Nanocomposites: model interfaces. Nat Mater 6:257–258
Tang H, Lin Y, Sodano HA (2013) Synthesis of high aspect ratio BaTiO3 nanowires for high energy density nanocomposite capacitors. Adv Energy Mater 3:451–456
Wu W, Huang XY, Li ST, Jiang PK, Toshikatsu T (2012) Novel three-dimensional zinc oxide superstructures for high dielectric constant polymer composites capable of withstanding high electric field. J Phys Chem C 116:24887–24895
Li J, Claude J, Norena-Franco L, Seok S, Wang Q (2008) Electrical energy storage in ferroelectric polymer nanocomposites containing surface-functionalized BaTiO3 nanoparticles. Chem Mater. 20:6304–6306
Mendes SF, Costa CM, Caparros C, Sencadas V, Lanceros-Mendez S (2012) Effect of filler size and concentration on the structure and properties of poly(vinylidene fluoride)/BaTiO3 nanocomposites. J Mater Sci 47:1378–1388. doi:10.1007/s10853-011-5916-7
Ranjan V, Yu L, Nardelli M, Bernholc J (2007) Phase equilibria in high energy density PVDF-based polymers. Phys Rev Lett 99:047801. doi:10.1103/PhysRevLett.99.047801
Claude J, Lu Y, Li K, Wang Q (2008) Electrical storage in poly (vinylidene fluoride) based ferroelectric polymers: correlating polymer structure to electrical breakdown strength. Chem Mater 20:2078–2080
Carabineiro SAC, Pereira MFR, Nunes-Pereira J, Silva J, Caparros C, Sencadas V, Lanceros-Mendez S (2012) The effect of nanotube surface oxidation on the electrical properties of multiwall carbon nanotube/poly(vinylidene fluoride) composites. J Mater Sci 47:8103–8111. doi:10.1007/s10853-012-6705-7
Vacche SD, Oliveira F, Leterrier Y, Michaud V, Damjanovic D, Manson JAE (2014) Effect of silane coupling agent on the morphology, structure, and properties of poly(vinylidene fluoride–trifluoroethylene)/BaTiO3 composites. J Mater Sci 49:4552–4564. doi:10.1007/s10853-014-8155-x
Luo H, Zhang D, Jiang C, Yuan X, Chen C, Zhou K (2015) Improved dielectric properties and energy storage density of poly(vinylidene fluoride-co-hexafluoropropylene) nanocomposite with hydantoin epoxy resin coated BaTiO3. ACS Appl Mater Interfaces 7:8061–8069
Luo H, Zhang D, Wang L, Chen C, Zhou J, Zhou K (2015) Highly enhanced dielectric strength and energy storage density in hydantoin@BaTiO3–P(VDF-HFP) composites with a sandwich-structure. RSC Adv 5:52809–52816
Zhang Z, Meng Q, Chung TCM (2009) Energy storage study of ferroelectric poly (vinylidene fluoride-trifluoroethylene-chlorotrifluoroethylene) terpolymers. Polymer 50:707–715
Chung TCM, Petchsuk A (2002) Synthesis and properties of ferroelectric fluoroterpolymers with Curie transition at ambient temperature. Macromolecules 35:7678–7684
Lu YY, Claude J, Neese B, Zhang QM, Wang Q (2006) A modular approach to ferroelectric polymers with chemically tunable curie temperatures and dielectric constants. J Am Chem Soc 128:812–8121
Zhang S, Neese B, Ren K, Chu B, Zhang Q (2006) Microstructure and electromechanical responses in semicrystalline ferroelectric relaxor polymer blends. J Appl Phys 100:044113. doi:10.1063/1.2335778
Thakur Y, Lin M, Wu S, Cheng Z, Jeong DY, Zhang QM (2015) Tailoring the dipole properties in dielectric polymers to realize high energy density with high breakdown strength and low dielectric loss. J Appl Phys 117:114104. doi:10.1063/1.4915942
Chen Q, Shen Y, Zhang S, Zhang QM (2015) Polymer-based dielectrics with high energy storage density. Annu Rev Mater Res 45:433–458
Shehzad K, Ul-Haq A, Ahmad S, Mumtaz M, Hussain T, Mujahid A, Shah AT, Choudhry MY, Khokhar I, Ul-Hassan S, Nawaz F, Rahman F, Butt Y, Pervaiz M (2013) All-organic PANI–DBSA/PVDF dielectric composites with unique electrical properties. J Mater Sci 48:3737–3744. doi:10.1007/s10853-013-7172-5
Li R, Xiong C, Kuang D, Dong L, Lei Y, Yao J, Jiang M, Li L (2008) Polyamide 11/poly (vinylidene fluoride) blends as novel flexible materials for capacitors. Macromol Rapid Commun 29:1449–1454
Chen XZ, Li X, Qian XS, Wu S, Lu SG, Gu HM, Lin M, Shen QD, Zhang QM (2013) A polymer blend approach to tailor the ferroelectric responses in P(VDF–TrFE) based copolymers. Polymer 54:2373–2381
Wu S, Li W, Lin M, Burlingame Q, Chen Q, Payzant A, Xiao K, Zhang QM (2013) Aromatic polythiourea dielectrics with ultrahigh breakdown field strength, low dielectric loss, and high electric energy density. Adv Mater 25:1734–1738
Zhu L (2014) Exploring strategies for high dielectric constant and low loss polymer dielectrics. J Phys Chem Lett 5:3677–3687
Sun Y, Boggs SA, Ramprasad R (2015) The effect of dipole scattering on intrinsic breakdown strength of polymers. IEEE Trans Dielectr Electr Insulation 22:495–502
Feng Y, Li WL, Hou YF, Yu Y, Cao WP, Zhang TD, Fei WD (2015) Enhanced dielectric properties of PVDF-HFP/BaTiO3-nanowire composites induced by interfacial polarization and wire-shape. J Mater Chem C 3:1250–1260
Yang J, Yang X, Pu Z, Chen L, Liu X (2013) Controllable high dielectric permittivity of poly (arylene ether nitriles)/copper phthalocyanine functional nanohybrid films via chemical interaction. Mater Lett 93:199–202
Yu S, Qin F, Wang G (2016) Improving the dielectric properties of poly (vinylidene fluoride) composites by using poly (vinyl pyrrolidone)-encapsulated polyaniline nanorods. J Mater Chem C 4:1504–1510
Liu S, Zhai J, Wang J, Xue S, Zhang W (2014) Enhanced energy storage density in poly (vinylidene fluoride) nanocomposites by a small loading of suface-hydroxylated Ba0.6Sr0.4TiO3 nanofibers. ACS Appl Mater Interfaces 6:1533–1540
Yang L, Tyburski BA, Domingues Dos Santos F, Endoh MK, Tadanori K, Huang D, Wang Y, Zhu L (2014) Relaxor ferroelectric behavior from strong physical pinning in a poly (vinylidene fluoride-co-trifluoroethylene-co-chlorotrifluoroethylene) random terpolymer. Macromolecules 47:8119–8125
Li Q, Zhang G, Liu F, Han K, Gadinski MR, Xiong C, Wang Q (2015) Solution-processed ferroelectric terpolymer nanocomposites with high breakdown strength and energy density utilizing boron nitride nanosheets. Energy Environ Sci 8:922–931
Lovinger AJ, Davis DD, Cais RE, Kometani JM (1987) The role of molecular defects on the structure and phase transitions of poly(vinylidene fluoride). Polymer 28:617–626
Hasegawa R, Takahash Y, Tadokoro H, Chatani Y (1972) Crystal structures of three crystalline forms of poly (vinylidene fluoride). Polym J 3:600–610
Li J, Seok SI, Chu BJ, Dogan F, Zhang QM, Wang Q (2009) Nanocomposites of ferroelectric polymers with TiO2 nanoparticles exhibiting significantly enhanced electrical energy density. Adv Mater 21:217–221
Klein RJ, Runt J, Zhang QM (2003) Influence of crystallization conditions on the cicrostructure and electromechanical properties of poly (vinylidene fluoride-trifluoroethylene-chlorofluoroethylene) terpolymers. Macromolecules 36:7220–7226
Zhu M, Huang XY, Yang K, Zhai X, Zhang J, He JL, Jiang PK (2014) Energy storage in ferroelectric polymer nanocomposites filled with core-shell structured polymer@BaTiO3 nanoparticles: understanding the role of polymer shells in the interfacial regions. ACS Appl Mater Interfaces 6:19644–19654
Li W, Jiang L, Zhang X, Shen Y, Nan CW (2014) High-energy-density dielectric films based on polyvinylidene fluoride and aromatic polythiourea for capacitors. J Mater Chem A 2:15803–15807
Rabuffi M, Picci G (2002) Status quo and future prospects for metallized polypropylene energy storage capacitors. IEEE Trans Plasma Sci 5:1939–1942
Kumar PS, Sundaramurthy J, Subramanian S, Babu VJ, Singh G, Allakhverdiev SI, Ramakrishna S (2014) Hierarchical electrospun nanofibers for energy harvesting, production and environmental remediation. Energy Environ Sci 7:3192–3222
Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No. 21376022), the International S&T Cooperation Program of China (No. 2013DFA51860), and the Fundamental Research Funds for the Central Universities (JC1504).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, H., Liu, Z. & Wang, F. Improved dielectric properties and energy storage density of poly(vinylidene fluoride-co-trifluoroethylene-co-chlorotrifluoroethylene) composite films with aromatic polythiourea. J Mater Sci 52, 5048–5059 (2017). https://doi.org/10.1007/s10853-016-0742-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0742-6