Abstract
ABS is an engineering plastic that has butadiene part uniformly distributed over the acrylonitrile-styrene matrix. It possesses excellent toughness, good dimensional stability, easy processing ability, chemical resistance, and cheapness. However, it suffers from inherent shortcomings in terms of mechanical strength and vulnerability to environmental conditions. Furthermore, it is non-conducting and easily fretted. Plating on ABS can serve to enhance the strength and structural integrity as well as to improve durability and thermal resistance resulting in metallic properties on the ABS material. ABS is described as the most suitable candidate for plating because it is possible to deposit an adherent metal coating on it by only the use of chemical pretreatment process and without the use of any mechanical abrasion. This article aims to review the history of ABS plastics, properties of ABS, processes and mechanisms of plating, and studies of plating on ABS involving mainly eco-friendly methods of plating by discussing the literature published in recent years. The details of electroplating of ABS carried out in the authors’ laboratory are also presented.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plastic substances are usually plated in order to bring about merits of the metals to the polymer substrate [1–6]. Metallization on plastic imparts reflectivity, abrasion resistance, electrical conductivity, and a variety of decorative lusters, high wear and corrosion resistances, electromagnetic shielding, weight reduction, formability enhancements, high impact resistance and weatherproofing, lower cost, flexibility in parts design, and reduced weight compared to its metal counterparts [7–17]. Metallized plastics become useful in electronic industry, petroleum industry, national defense field, toys manufacturing industry, automotive and computer body parts, electronic housings, wheel covers, lamp housings, ventilation, air conditioning parts, pipes and fittings, and many more things [18–31].
Among the plastics such as polypropylene, polysulfone, polyethersulfone, polyetherimide, Teflon®, polyarylether, polycarbonate, polyphenylene oxide (modified), polyacetal, urea formaldehyde, diallyl phthalate, mineral-reinforced nylon (MRN), and phenolic that are available today, ABS becomes the obvious choice for electroplating because of the uniqueness of this thermoplastic that it has uniform distribution of butadiene part over the acrylonitrile-styrene matrix, qualifying it for best adhesion of the metal to the substrate, cost-effectiveness, low coefficient of thermal expansion, ease of molding, and good appearance after plating [32–36].
ABS and ABS-like substrates are gaining importance as they can be produced by the modern additive manufacturing (AM) processes and plating can be applied. AM technology encompasses processes such as stereolithography, selective laser sintering, fused deposition modeling, laminated object manufacturing, solid ground curing, and solid creation system.
Plating process can be sub-divided into two parts, namely, electroless plating and electroplating. Electroless plating evenly deposits an electrically conductive metallic layer on the insulating ABS substrate and also prepares the surface for further adherent coating of electroplated layer. Electroplating step with the aid of current builds additional thickness of metals like copper, nickel, or chrome just as and when required by the part or for finishing purposes [18, 33, 37–51].
The present article summarizes different methods of AM processes associated with ABS, history and properties of ABS, mechanisms of plating, eco-friendly methods of ABS plating and evaluation of mechanical properties of plated ABS. A brief account of electroplating work conducted in authors’ laboratory is also presented.
Historical aspects of ABS plastic
The origins of ABS plastic can be traced back to mid-1940s. Acrylonitrile–styrene copolymers were in use since 1940s and the drawbacks of these copolymers led to the incorporation of third monomer butadiene rubber. Butadiene imparted higher strength and impact resistance character to the plastic. High molecular mass butadiene–acrylonitrile copolymers and styrene–acrylonitrile copolymers were utilized to produce bulletproof polymer sheets during the last years of World War II. The copolymer systems possessed large impact strength because of their low thermoplastic flow properties. Molded parts such as sheets, profiles, and pipes were the first products to be made out of ABS. The development of injection molding and graft polymerization techniques paved the way for the revolutionization of applications of ABS plastics. ABS was introduced in 1950 for use in textiles, fashion, toys, and domestic applications. Between late 1950s and early 1960s, the Lego Group R & D lab in Billund switched from process development of cellulose acetate (CA) to ABS. They found that ABS was more stable, tough, and colorfast than CA. By 1970, ABS replaced CA fully in both Europe and North America [52–54]. The advent of AM techniques especially fused deposition modeling in 1990 and various 3D printers post the year of 1993 led to the increased use of ABS. Open source desktop 3D printers such as RepRap, capable of printing the majority of its own replicate parts, were introduced in the market in 2008 [55, 56].
Properties of ABS
Typically, ABS is a product of systematic polymerization of monomers, namely, acrylonitrile, butadiene, and styrene as shown in Fig. 1. There exist two phases of the ABS terpolymer: a continuous phase of styrene–acrylonitrile (SAN) and a dispersed phase of polybutadiene as shown in Fig. 2a, b. Commercially available grades of ABS possess medium to high impact, low to high surface gloss, and high heat distortion properties [34].
ABS polymers exhibit high toughness (even in cold conditions), adequate rigidity, good thermal stability, and high resistance to chemical attack and environmental stress cracking. Other significant properties of ABS include cheapness, durability, and low coefficient of thermal expansion. The ease of molding allows the fabrication of dimensionally stable ABS parts with superior surface quality. No other thermoplastic material displays such a wonderful combination of technically important properties. Molecular and morphological factors are paramount in deciding the properties of ABS. The matrix composition and molecular mass, the type of rubber, the volume ratio of the rubber to the continuous phase, the rubber particle size, the grafted rubber structure, and the additive content also play a vital role. In fact, optimized impact strength of ABS can be achieved by controlling the rubber particle size, distribution, and microstructure. Greater toughness is obtained by increasing the butadiene rubber content and molecular weight of un-grafted SAN phase. Surface gloss values of up to 95 % can be obtained depending on the specific grade and the mold or polishing roll surface. ABS may be utilized in high performance ABS-polycarbonate alloys (ABS-PC) so as to strike a balance between higher toughness and thermal properties. Hence, it is possible to manufacture a wide range of products bearing in mind the requirements of the users.
The continuous styrene–acrylonitrile or SAN phase is a determinant of chemical properties of product ABS. It is generally not affected by acids, alkalis, or aqueous solutions of salts. But, ABS is hydrophobic and the contact angle between ABS and water is 81.0 ± 0.6°. The polymer shows an absorption amount up to 1.5 % of water upon storage in aqueous media owing to the presence of residual emulsifier and the polarity of the nitrile side groups. ABS polymers are insoluble in paraffinic hydrocarbons. However, on account of nature and quantity of the rubber phase, some amount of weight gain may be observed. They are also resistant to animal and vegetable fats and to a range of cosmetic creams. The SAN phase of ABS is soluble in halogenated hydrocarbons, aromatics, esters, and ketones. Oxidizing agents, especially inorganic acids, break up the chains and thus cause degradation of the polymer. Environmental stress cracking of ABS is low and can be further lowered by increasing the acrylonitrile content and the molecular mass of the SAN phase. When subjected to heat, light, and weathering conditions, yellowing and graying of the surface as well as reduction in toughness characteristics is observed as a result of the presence of double bonds in the butadiene rubber phase. Oxidation also causes the loss of toughness. Rubber phase crumbles when working temperatures above 280 °C are employed as it loses its toughness property. Polymer chain decomposes at temperatures above 300 °C. Severe processing measures applied can also destroy the rubber and matrix phases [15, 52, 57–61]. General properties of ABS are shown in Table 1 [62].
Manufacturing processes of ABS plastic
ABS or ABS-like parts can be manufactured by employing AM technologies. The nomenclature “ABS-like” is followed due to the reason that they exhibit mechanical properties similar to common ABS but different chemical properties [63]. AM has emerged to be a main-stream manufacturing technology over few decades since its inception [64]. AM is considered as a feasible technological option by the product manufacturing industry as it addresses the major challenges such as significant reduction of product development time and cost of tooling, human intervention, thereby resulting in rapid product development and manufacturing cycle necessary for the evaluation of form, fit and functionality of a design as well as creation of complicated parts [65–68].
The idea of AM was first conceived in 1980s by Charles Hull. He invented this technology while trying to demonstrate an alternate process for conventional tool-making and molding by fabricating UV-curable polymers for Ultra Violet Products, California. This technique called ‘stereolithography’ enabled quick production of plastic prototypes [69]. He and 3D Systems Company together created .STL file format which assisted the computer-aided design (CAD) to communicate with the rapid prototyping (RP) machine so as to transmit files for printing 3D objects [70]. The successive arrival of fused deposition modeling (FDM) process developed by Scott Crump heralded the revolution of manufacturing industry [71]. A special apparatus known as “3D printer” capable of printing plastic, metal, and ceramic parts was fabricated in 1993 by Michael Cima and Emanuel Sachs of MIT [72]. Companies like DTM Corporation, Z Corporation, Solidscape Geometries, Objet Geometries, Helisys, and Organovo devised 3D printers for commercial applications that are available in the market today [73].
The type of AM processes/machines that are currently in use is Stereolithography Apparatus (SLA), Selective Laser Sintering (SLS), Solid ground curing (SGC), Laminated Object Manufacturing (LOM), 3-Dimensional Printing (3DP), Fused Deposition Modeling (FDM), Solid Creation System (SCS), Solid Object Ultraviolet-laser Plotter (SOUP), Selective Adhesive and Hot Press (SAHP), Multi-Jet Modeling system (MJM), Direct Shell Production Casting (DSPC), Multiphase Jet Solidification (MJS), and Ballistic Particle Manufacturing (BPM) [74].
ABS and ABS-like parts developed by AM processes are being explored for the plating applications [36, 53, 75–79]. Electroplating is adopted to overcome the limitations in terms of mechanical properties inherently present in the rapid prototypes [80]. Also, there is increased attention towards the development of metal–polymer combination materials for the manufacturing of functional prototypes [81].
Plating process on ABS plastic
A surge of activities that took place in the area of resin development and production technology resulted in the replacement of plating of metals by plastics. Plating on plastics (POP) became a popular alternate for metal finishing in the early years of 1960s. Introduced first in Europe and North America, POP was a success on industrial level due to the advancements of chemical processing of ABS plastics. It became possible to obtain highly decorative and functional-plated ABS parts by the prior chemical etching of ABS surface using chromic acid-based mixtures.
ABS is the most usually electroplated plastic and it has found the widest acceptance in the plating industry. Today ABS can be plated with metals which can retain their luster and shape for several years. The current market for plating of ABS is great and a significant amount of research is being carried out across the world on ABS-like materials with regard to surface preparation and deposition of metal or metal composites [33, 34, 39, 41, 53, 82–100]. As represented in the flowchart (Fig. 3), conventional electroplating on ABS process can be divided into two important steps: surface preparation and metal plating.
Surface treatment stage involves chemical process steps like etching, neutralization, activation, and acceleration. Etching with strong oxidizing solutions such as chromium trioxide and sulfuric acid mixture at elevated temperatures (65–75 °C) roughens the surface for good mechanical adhesion, enhances the surface area of the thermoplastic, converts it from hydrophobic material to hydrophilic material and creates micro-pores which act as bonding sites between the substrate and the metal. The outcome of etching step is owed to redox reaction between butadiene and hexavalent chromium. After thorough rinsing material is put in a neutralizing solution like sodium bisulfite which can remove the excess etchant by chemical reduction resulting in avoidance of formation of ‘skip plates’ during later part of coating process due to un-reduced Cr6+ ions. The neutralizer or chrome reducer is a ready source of electrons to reduce contaminating and detrimental hexavalent chromium to trivalent chromium. Activating agents consist of precious metals such as palladium, platinum or gold, and stannous chloride/hydrochloric acid solution. Activation step serves to produce catalytic surface required for effective plating. Colloidal palladium is deposited into the porous surface thus preparing the ground for the subsequent formation of thin metallic layer through electroless step. The role of accelerator is to eliminate the excess of stannous hydroxide surrounding the palladium metal and maintaining the palladium sites intact for electroless plating.
The electroless plating bath composition includes a semi-stable solution containing a metal salt, a reducer, a complexing agent for the metal, a stabilizer, and a buffer system. When the palladium-activated part is put in the bath, metal gets reduced on the palladium sites and the autocatalytic reaction continues till the part is removed from the solution. Continuous metal layers are created in this step. The basic reactions that take place in the copper and nickel electroless plating steps respectively are
After electroless plating, electrolytic plating of acid copper is carried out to build up higher layer thickness and give a bright conductive surface. This copper coating acts as a buffer layer between the base material and the final metal plate. It also contributes to the stability of the final plate. Electrolytic nickel coating is done following the electroplating copper plating step in order to fulfill the corrosion and abrasion resistance requirements. It sets up a barrier between the copper deposit and the corrosive environment. The finishing of the coated part can be done using chrome flash, brass, gold, silver, etc. [32–34, 101–127].
Mechanism of plating on ABS plastic
Among various theories proposed for the formation of adherent metallic films on ABS upon plating process, ‘anchor effect’ or ‘press stud theory’ is regarded as a binding explanation. According to this theory, the holes or micro-pores which are alluded to ‘anchor points’ or ‘press studs’ are formed in the surface conditioning step by etching the butadiene out of the matrix. These holes get filled subsequently in the metallizing step and demonstrate an illustration of lock-and-key theory as schematically shown in Fig. 4 [32]. Favorable adhesion between the metal layer and ABS surface can be attributed to anchorage effect [13, 14].
Schematic illustration of etched ABS showing anchor points of butadiene polymer removed (Reproduced with permission from Ref. [32])
In the conventional method, when ABS surface is etched with strongly oxidizing chemicals such as chromic acid, butadiene part of ABS is dissolved and cavities of nano- or micro-dimensions are formed. As a result of strong oxidation of the polymer, surface is rendered hydrophilic and rough [128, 129]. The reaction that takes place is shown in Fig. 5.
Few studies using traditional surface treatment methods have exhibited results confirming the role of ‘anchoring effect’ for the formation of adherent metal films. In one of the studies, electroless Ni–P alloy/MWCNT composite films were deposited on ABS resins to provide several properties of CNTs or metal/CNT composites to the resins. The resin was subjected to a series of surface conditioning steps including etching in chromic acid solution (4 M CrO3 +4 M H2SO4) at 70 °C for 10 min and activation in 5.6 × 10−4 M PdCl2 + 0.12 M HCl solution at 35 °C for 5 min. The Ni–P alloy/MWCNT composite was produced on ABS surface from the electroless plating bath consisting of NiSO4·6H2O, C6H5NaO7, NaH2PO2·H2O, (NH4)2SO4, trimethyl stearyl ammonium chloride and MWCNT. Scanning electron microscopy (SEM) analysis of the so fabricated composite film revealed that initially, the hollows that were formed due to the selective dissolution of butadiene part of ABS during roughening process get filled up by Ni–P alloy. As the electroless deposition proceeds, pores are completely filled and some MWCNTs are incorporated in the Ni–P alloy matrix. As the electrolysis time is increased, a thicker film of Ni–P/MWCNT composite forms on ABS surface. Peel strength examination using adhesion tensile tester showed that the adhesion strength values were higher than 1300 N/cm2 because of anchor effect. Also coefficient of friction of the composite film was of lower order than that of Ni–P alloys. So, incorporation of MWCNTs in the regular electroless plating bath proved to be advantageous to be deposited not only on conducting materials but also on insulators such as resins [8]. Similar study was undertaken in the year of 2014 so as to form composite films of multiwalled carbon nanotubes with electroless copper (Cu/MWCNTs) on the ABS resin surface in order to arrive at increased electrical conductivity. After traditional process of surface preparation which gives rise to a roughened surface of ABS base, Cu/MWNTs composite was deposited from the plating bath consisting of 0.06 M CuSO4·5H2O as a copper source, 0.03 M EDTA as complexing agent, 0.1 M glyoxylic acid as a reducing agent, and 1 g/dm3 sodium lauryl sulfate (SLS) and 1 g/dm3 hydroxypropyl cellulose (HPC) as dispersing agents. Plating was done at 60 °C for 120 min. It was found from SEM that the pores formed during the etching stage got almost filled up with copper, and a few MWCNTs were present in the copper matrix. The cross-sectional SEM analysis suggested that the dispersing agents bring down the copper deposition rate resulting in lower thickness of the composite film. Adhesion strength value determined using tensile adhesion tester (Phototechnica, Romulus) was about 2500 N/cm2 due to anchor effect. This value is similar to electrolessly deposited copper on ABS. The composite also showed lower friction coefficients. Hence, deposition of Cu/MWNTs on ABS serves to reduce the friction coefficient and contribute increased electrical conductivity to ABS [130].
The roughened surface favors a number of hollows on it. These hollows give rise to large surface area and more and more of anchor sites. If the hollows happen to have complex shapes, plating film gets hooked or anchored firmly when brought in contact. Thus, excellent adhesion is observed making it difficult to be peeled off [8].
The cavities formed act as mechanical inter-locking sites or anchor points for the successive adsorption of activating agents such as palladium followed by electroless nickel or copper. The anchoring of palladium ensures the penetration of metal deep into the hollows and result in stronger bonding between ABS base and metal layer. The strength of adhesiveness between the substrate surface and plated metal depends on how effective the etching step is [12, 18, 129].
Another theory pointed out that the reason for strong bonding between the plated metal and the ABS plastic was chemical forces such as Van der Waal’s attraction or valence bonds of the metal and charged plastic surface which is a result of etching. It was also claimed that deposition of adherent metallic coating on ABS took place due to a wetting mechanism which is based on the surface energies of the metal film and the base polymer material. The continuous supply of metal ions or ‘wetting’ of the ABS surface by aqueous electrolyte solution is the cause of effective deposition of metal. The electrostatic theory explained the formation of adherent metal coating on ABS as due to the generation of electrical double layer on the ABS surface dipped in the electrolyte solution [27].
Studies of plating on ABS
Currently, electroplating on ABS plastics is accomplished by employing two feasible technologies, viz. electroplating following the electroless plating process and direct electroplating. Surface treatment, whether by chemical or mechanical means, remains to be a critical factor whatever may be the plating process used. There is a quote that is popular among the electroplaters, “One can make a poor coating perform with excellent pre-treatment, but one cannot make an excellent coating perform with poor pre-treatment.” This highlights the need of suitable pre-treatments before metallization [42, 131].
As mentioned previously in “Plating process on ABS plastic” section, electroless plating route consists of three major steps of surface preparation such as etching, activation, and metal deposition. Conventional surface conditioning methods use chromium-based chemicals as etching solutions, palladium, and tin-based solutions as activating agents. A number of research works including the patented ones published in 1960s and 1970s utilized chromic–sulfuric acid mixture as an effective etchant [12, 89, 90, 93, 98, 105, 106, 110, 112, 113, 116–118, 132–134]. Chromium-based baths are commonly used till date even though surface modifications using novel methods are highly researched on in the past couple of decades. The dependency of electroplating industry on conventional etching method is justified because of the fact that no surface pre-treatment method has been able to deliver as good an adhesion as the former for the reasons stated in “Eco-friendly methods of plating on ABS” section. However, the replacement of chromium-based etchants is the need of the hour today. Cr6+ in chromic acid is identified as a hazardous air pollutant. It is a known carcinogen. It can cause damage to respiratory organs, stomach and intestine, and male reproductive system when inhaled or ingested. There are also problems associated with handling of hot and highly corrosive chromic acid during plating process. There will be rapid loss of efficiency of the bath requiring replacement with costly etching baths. The neutralization of chrome is a big challenge. If chrome is not removed completely, it will poison the catalyst that to be deposited subsequently in the activation step. As a result, ‘skip plates’ appear upon metallization [7, 33, 34, 37, 51, 94, 129, 135–140]. Stringent regulations imposed by the governments towards the possible future ban of chromic acid utilization limit its use [11, 38, 141–145]. Palladium has been employed as a “universal catalyst” during the activation step of the electroless process [27, 89, 98, 106, 111, 116–118, 132]. But, its ever increasing demand as a hydrogenation and reduction catalyst presents shortage of supply. In addition to high-priced catalyst and the noble metal wastage issue and toxicity of tin adds to the problem [7, 10, 19, 21, 37, 38, 129, 139, 146–149]. Overall, the electroless plating method preceding the electroplating process suffers from serious drawbacks since it requires a multi-stage operation, longer deposition times, expensive catalyst, and complex and environmentally hazardous solutions. Also it is not possible to achieve fair control over film thickness and uniformity. This calls for alternate procedure development for surface preparation [1, 7, 9, 37, 38, 41, 42, 88, 131, 148–150].
The first attempt of direct electroplating was made in a patented work published in 1972 [116]. This was started with a purpose of elimination of poisonous chromium baths and reduction of surface pre-treatment steps so that severe problems related to electroless route could be overcome. The pre-requisite prior to coating is the seeding of the surface with a catalyst to make it electrically conducting. Complete metallization is achieved with subsequent electroplating. The disadvantages of the process include unavoidable steps of etching and neutralization in most cases, and the need of an expensive catalyst [10, 36, 37, 42, 79, 106, 120, 131, 139, 143, 148, 150, 151].
Eco-friendly methods of plating on ABS
Studies on economical and eco-friendly routes of plating processes have been the subject of interest for researchers over many years. A summary of eco-friendly and simple methods of pre-treatments is presented in this section. Their advantages compared to conventional plating techniques are highlighted. As is known, discrepancies still exist, mostly with regard to the adhesion of the plated film. A pertinent account of the distance from the beneficial characters of classical methods of plating is also provided.
One of the strategies employed is wet chemical methods involving strong acids as a replacement for chromium. Teixeira and Santini studied the effects of etching of ABS with different combinations of sulfuric acid, with hydrogen peroxide and/or nitric acid in a quest of less aggressive or non-polluting etching solutions. The samples were subsequently coated with electroless nickel, and subjected to visual examination of uniformity and test of adhesion using “Scotch Tape Test” as adopted by the industry. The results revealed that the quality of nickel coating applied on ABS was adequate in terms of uniformity and adherence even though the etching performance was not as intense as that of conventional chromic acid conditioning [18]. Tang et al. carried out a cost-efficient and environment-friendly process of surface activation on ABS. ABS foils were first etched with 1:4 H2O2:H2SO4 mix solution. Chitosan biopolymer film was utilized to fix palladium on the substrate as catalytic sites by a chemical process as opposed to physical sorption in conventional sensitization-activation method. A tight, dense and continuous Ni–P plating layer was formed when electroless nickel plating was done. The characterizations confirmed the enhancement of adhesion of plating layer and ABS substrate [21]. The same group of researchers successfully eliminated the use of palladium in the continuation of their work. They immobilized nickel nanoparticles in place of palladium as catalyst on the faintly etched ABS surface treated with chitosan. A glossy and smooth Ni–P layer resulted upon electroless nickel coating [148]. They also reported Ni/Au coating on ABS subjected to surface pre-treatment process similar to that used in the latter. Gold was effectively coated on the immobilized nickel layers which acted as auto-catalysts. By employing thiosulfate and sulfite ligands along with sodium hypophosphite as reducing agent, and sodium citrate as a stabilizer, the need of toxic cyanide bath was eliminated [37]. Another set of experiments conducted investigated the etching system comprising of H2SO4–MnO2 colloid as an eco-friendly substitute. The average adhesion strength between the ABS surface and successively plated copper film was found to be 1.19 kN/m whereas the classical CrO3–H2SO4 etching system used as a reference presented a value of 1.42 kN/m. The average surface roughness (R a) and maximum roughness (R max) values were found to be 320 and 408 nm, respectively, which were comparable to that determined for reference CrO3–H2SO4 colloid (R a = 420 and R max = 510 nm respectively) [141]. FTIR and XPS characterizations carried out in a complementary study revealed the formation of –COOH, –OH as result of oxidation of C=C groups of butadiene and –CONH2 due to the conversion of C≡N groups of acrylonitrile by the action of etchant. These groups caused the surface to be hydrophilic and decreased the surface contact angle thus enhancing the adherence of the plated film on ABS surface [11]. Another work examined the effects H2SO4–MnO2 etchant on the topography of electrolessly copper-modified ABS resin with varying concentrations of H2SO4 and sonication times. Formation of more uniform and smaller cavities compared to chromic acid etching leading to reduced average surface roughness (278 nm) and maximum roughness (285 nm) values, enhanced average adhesion strength comparable to chrome etch (1.39 kN/m), and lower surface roughness were the result of this iteration [143]. In a modification of the experiments, phosphoric acid was introduced in the etching chemicals mixture for the determination of etching performance of ABS-Polycarbonate (PC/ABS). The presence of H3PO4 in MnO2–H3PO4–H2SO4 influences the “soluble” MnO2 content to increase. Aqueous solution containing tetramethylammonium hydroxide and 1-methyl-2-pyrrolidinone as ‘swelling agents’ was employed prior to etching in order to obtain efficient etching performance. It was observed that the surface contact angle of PC/ABS diminished from 95.71° to 28.31° and adhesion strength attained the value of 1.04 KN/m. FT-IR and XPS characterizations proved the formation of –COOH and –OH groups on PC/ABS surface because of swelling and etching steps. Increase in adhesion strength of PC/ABS and electroless copper deposit was attributed to the effectiveness of these two processes [144]. Note that a proprietary chemical (CATAPOSIT44, purchased from Rohm and Haas Company) was used in the activation step of the above experiments. A major improvement to the above process was reported by Shu and Wang. The need for use of expensive precious metals as activators could be avoided using the pre-treatment route proposed in their research. Following etching by H2SO4–MnO2 mixture, copper particles from copper sulfate solution as auto-catalysts were deposited. This step eliminates the Pd-based activation process. The reduction of copper particles on ABS is made possible by dimethylamineborane which is a powerful reducing agent. Subsequent electroless copper coating is carried out. Adherence tests indicate that the average adhesion strength of about 1.31 K/Nm which is near 1.19 K/Nm shown by SnCl2/PdCl2 colloid containing activator is obtained [19].
In another attempt to solve the environmental problems arising because of the increasing usage of strongly oxidizing hazardous chemicals such as chromic acid, hydrofluoric acid and hydrochloric acid as etchants, an environment-friendly etching process comprising a dispersant which is a mixture of proprietary chemical foaming agent and solvent was designed. The dispersant was made by mixing chemical foaming agent MS140D received from Dongjinsemichem Corporation (Seoul, Korea) with methanol solvent. The dispersant was applied uniformly on ABS. Methanol helped distribute chemical foaming agent evenly beneath ABS surface. Upon infra-red heat-treatment, chemical foaming agent created fine pores in ABS. Ultrasonic agitation produced a number of holes which could increase the number of anchor points that enhance the adhesion strength of the material. It was found that surface activation using this process was effective thus becoming a promising alternate for the conventional processes. Fair adhesion strengths reported by the cross-cutting method inspired the authors to arrive at the above conclusions [134]. An illustration of industrial electroless plating process is shown schematically in Fig. 6.
Common industrial electroless plating process divided in three main steps (Reproduced with permission from Ref. [38])
In an environmental-friendly approach, nickel was deposited electrolessly using supercritical carbon dioxide (scCO2) for impregnating with Pd catalytic agent on the hydrophilically modified ABS. First poly(ether-ester-amide)s (PEEA) was dry-blended on ABS to impart hydrophilicity. Pd(II)-hexafluoroacetylacetonate, Pd(hfa)2 was then infused into the substrate at high-pressure conditions using scCO2. Pd was reduced into the polymer matrix by a heat treatment. After evacuation of the adsorbed CO2, electroless coating reaction was carried out. Nickel got deposited around the impregnated Pd nanoparticles resulting in semi-continuous structure over the ABS surface. This structure due to anchoring effect contributed to higher adhesiveness between metal layer and the polymer [9].
Direct metal electroplating is a popular choice because it can replace conventional coating processes that involve strong acidic oxidizing chemicals as etching solutions that suffer from drawbacks like high-temperature operations, high costs associated with the use of activators as well as difficulty of monitoring electroless baths. In addition, a large number of steps needed for electroless plating are avoided. Nickel electroplating has been achieved as the top coating on ABS in one of the novel works. This is accomplished by prior two stages comprising chemical deposition of polypyrrole (PPy) to make the surface conductive, and copper electroplating. First step of PPy pre-treatment comprises two sub-steps of immersing ABS in 0.3 M pyrrole solution and oxidation using 0.9 M FeCl3. The stirring with pyrrole causes the swelling and melting of ABS to give it a fish scale-like texture. FeCl3 is used as a polymerizing agent. A black, homogeneous and adherent PPy film is deposited at the end of this step. Following the polymerization step, copper is metallized on ABS electrolytically. The mechanical bonding between PPy/ABS and copper is found to be 100 %. With the increase in deposition time, copper thickness is increased. Compact cubic grains of copper are observed after longer electrolysis duration. Nickel electroplating step is carried out with ease on surface uniformly coated with copper. The standard industrial nickel bath containing nickel sulfate (270 g/L), nickel chloride (90 g/L), boric acid (45 g/L), and proprietary additives and brighteners is used with pH 5 and current density of 20 mA/cm2 at ambient temperature. SEM images exhibit homogeneous nickel deposits on Cu/PPy/ABS. Adhesion testing from standard sellotape test shows 100 % adherence. Plating times of 15, 30, and 45 min deposit 4, 9, and 10 µm thickness of copper, respectively. Metallic bilayer on PPy-treated ABS is depicted as shown in Fig. 7 [139].
Scheme of metallic bilayer coating of PPy/ABS (Reproduced with permission from Ref. [20])
In a direct electroplating approach which can serve as an alternate approach of plating on plastics (POP), conductive ABS surfaces were formed by coating either aluminum or aluminum-carbon black containing enamel pastes onto ABS and subsequently plating it by electroless copper from a bath containing 15 % copper sulfate (CuSO4) and 5 % hydrofluoric acid (HF). Copper gets reduced onto Al seeds and deposits in about 10 min as a conductive layer. Incorporation of carbons particles decreases the time required to reach the plateau conductivity. All the Al-seeded specimens failed the standard tape test but Al–C coated ABS samples passed the test at longer deposition times as no noticeable damage was observed upon the peel-off [42]. The expansion of the work included the testing in four different baths, namely, sulfuric acid, phosphoric acid, nitric acid, and acetic acid. Electroless copper plating was successfully achieved in all the four baths. All baths developed good coverage but only nitric acid-based baths failed to show conducting ability due to corrosion of copper or over-etching of Al seeds by HNO3. Samples coated from these baths showed excellent adhesion according to standard adhesion tests (ASTM D 3359-02). Although, the electrical performance of the baths was not as good as that obtained for HF baths [131]. In an advanced work, ABS parts produced by FDM process were Al-seeded by using aluminum powder, activated charcoal, enamel, and distilled water. The electroless copper baths explored were HF, H2SO4, H3PO4, and CH3COOH. Geometries of the parts used consisted cone, pyramid, cuboidal, and cylindrical. The results indicated that copper deposition was effective in HF and cuboidal samples presented better electrical conductivities than other geometries used [41]. Optimized HF containing electroless Cu bath was used for further study and it was noted that Al seeded parts gave better electrical performance compared to chrome-etched or H2SO4–MnO2 samples [88].
Electroless plating of nickel and copper is also done on the poly(acrylic acid) (PAA) grafted ABS. This pollutant chromium-free and palladium-free process takes advantage of the ion-exchange properties of PAA to result in adherent metallic layer on the ABS which is comparable to commercial process involving chromium etching. The grafted PAA film was responsible for excellent Cu2+ immobilization. All these complexed copper ions get reduced within the film after immersing in an alkaline solution of sodium borohydrure NaBH4. This was followed by the electroless plating of copper or nickel. Adherent metallic layers were obtained which are attributed to the presence of copper particles inside the PAA layer. They exert anchoring effect and cause high values of adhesion strength between the ABS and electroless metal. The scheme of direct metallization is shown in Fig. 8 [38].
Surface preparation of ABS substrates for electroless metallization by a the usual industrial process b authors’ Cr- and Pd-free process (Reproduced with permission from Ref. [38])
The same authors extended the work on direct method of copper electroplating on the PPy-activated surface of ABS. This method avoids the use of hazardous chrome and precious metal baths and causes electrical conductivity on the substrate in only one step. Effectiveness of chemical deposition of PPy holds the key for the success of the electrolytic plating of copper on PPy/ABS. Homogeneous and adherent copper coating is achieved through this process [20].
Photocatalytic surface activation method was pursued prior to copper electroless plating as another possible alternate for complex surface treatments involving chromium baths and precious metal activators. ABS was soaked in a TiO2-dispersed solution and irradiated with UV light. A mercury short arc lamp was used as the light source. Subsequent electroless copper plating was carried out. Adhesion strength analysis using cross-cut tape test (ISO 2409) showed ABS pre-treated in 0.01 g/L of TiO2 dispersed solution contained suitable morphology for plating. Because of photocatalytic reaction, main carbon rings in ABS get destroyed and react quickly with oxygen. C=O and C–O groups that get activated cause the increase in the wettability from polar groups. This leads to close contact between ABS and electroless copper. This way photocatalytic pre-treatment potentially increases the adhesion between the ABS base and electroless metal layer. However, adhesion strength is only about 1.0 kN/m [12]. This type of re-treatment that precedes the electrolytic route is not as effective as dynamic chemical process (DCP) [94]. In a separate study, adhesive strength of 1.25 kN/m which is higher than that of optimal H2SO4–MnO2 colloid was observed for ABS coated with electroless copper which was subjected to photocatalytic pre-treatment. This investigation was undertaken with the intention of evaluating the effects of the TiO2 content, irradiation time, and UV power upon the surface topography as well as surface characterization. It was found that the surface hydrophilicity of ABS and adhesion strength between the plated copper and ABS surface improved with an increase in the UV power and an increase in irradiation time. The high contents of C=O and –COOH were the attributes for such a behavior. Photocatalytic surface preparation was proposed as a replacement for wet chemical methods discussed before [135].
Mechanical studies of plated ABS plastic
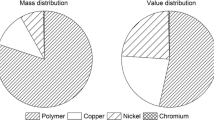
Herein, we report some studies on changes in mechanical properties of electroplated ABS plastics subjected to different pre-treatments. It has to be noted that not much literature is available for the mechanical behavior studies of plated studies. Table 2 shows the type of plating and mechanical properties of plated ABS/ABS-like polymers. The maximum adhesion strength is obtained for the Ni–P alloy/MWCNT- and Cu/MWCNT-coated ABS samples.
In an attempt to determine the enhancement of strength and toughness characteristics of ABS materials produced by fused deposition modeling upon electroplating, Kannan et al. characterized the plated and non-plated specimens for tensile and flexural tests. Tensile strength tests were carried out as per ASTM E-837 standards. In this test, the force necessary to pull the specimen apart till the point of breaking is measured. The term tensile modulus which is equal to the ratio of stress to strain below the proportional limit of the specimen is significant. The tensile test results indicated that the copper and nickel depositions increased the tensile strength of FDM-ABS sample by 47 % compared to the bare sample. Flexure test of the specimens was conducted to determine the force required to bend a beam under three points of loading conditions. The material is made to lie on a support span and load is put in the center producing three points bending at known rate. For ASTM D790 samples, the analysis gets completed when the specimen reaches 5 % deflection or breaks before 5 %. Flexural modulus is the term that describes material’s stiffness when flexed. The results in Table 3 showed that flexural strength of the plated specimen doubled to that of normal specimen [77]. In a similar study, surface roughness in accordance with is 3073-1967 and RA-2006 standards, and UTS value measurements as per IS 1608-2005 standards are done. It is observed in Table 3 that with the increase of plating thickness, roughness is reduced and UTS values are increased [81].
In another study, investigation of the effect of deposition of thin metallic foils on mechanical properties like impact resistance and hardness of ABS polymers developed by fused deposition modeling was carried out. First, electrodeposition was carried out on hardness and impact specimens produced using Stratasys FDM vantage SE machine. Surface treatment of ABS followed by electroless plating, electrolytic acid copper plating, electrolytic acid nickel plating, and electrolytic chrome plating is performed. Coating thickness of 60, 70, and 80 μm was achieved on the test specimens. Un-coated samples were used as reference samples.
Drop weight impact tests were conducted by directly dropping the impact testor on normal and electrodeposited samples with different drop weights of 0.89g, 1.395, and 2.33 kg, and drop height of 400 mm. It was observed that 60, 70, and 80 µm specimens, respectively, exhibited impact resistance in the increasing order compared to the non-plated samples. The higher impact resistance of the 80 µm specimens was due to the rapid absorption of energy by the nickel layer and immediate transfer to copper layer and then to the base material.
Hardness of the coated and uncoated samples was evaluated by Rockwell Hardness test as per ASTM D785 standards. Readings were obtained from dial indicator of the Rockwell hardness tester by applying the minor and major loads. Enhancement of hardness property was seen in the electroplated samples compared to the normal samples. Owing to the ductility, hard nature, and corrosion resistance property of nickel, plated samples exhibited superior hardness. By this way, the inferior strength characteristics were overcome using the electroplating approach as shown in Table 4 [75].
Assessment of mechanical properties such as tensile strength (ASTM D638) and compressive strength (ASTM D695) of the injection molded (IM) ABS was carried out under wet conditions. Electroplated ABS samples showed a slight decrease of tensile strength compared to bare ABS samples. This behavior is attributed to the pores inherently present in the sample. It was found that the compressive strength values of the ABS parts increased significantly upon electroplating [80].
The role of electroless copper coating to produce electrically conductive FDM-ABS parts for electroforming applications was evaluated. It was found that the simplified electroless copper plating route involving pre-activation with SnCl2 and HCl and activation with AgNO3 and HCHO followed by electroless plating was a promising alternative to silver painting in terms of precision and reproducibility [76].
Details of electroplating work carried out in-house are given in Fig. 9. Figures 10 and 11 show process sequence followed and the pictures of coated samples, respectively. Table 5 shows the results of tests conducted on nickel-plated ABS samples.
Conclusion of plastic plating
Plastic electroplating is a typical of the modern processing technologies for new materials. Compared with metal parts, electroplated plastic products can not only achieve good metal texture but also can reduce product weight, and effectively improve the appearance and decorative requirements, mechanical strength of the surface as well as provide improved performance in the applications pertaining to electricity, heat, corrosion, etc., However, plating processing route has to be selected considering the material properties, mechanical properties, material costs, the cost of plating, electroplating, and ease of dimensional accuracy and other factors. With the rapid development of industry, plastic plating has found increasingly widespread applications as plastic products have become an important means of surface decoration. Although polypropylene, polysulfone, polycarbonate, nylon, phenolic glass fiber-reinforced plastic, and polystyrene are generally plated, ABS is found to be the most widely plated plastic for electroplating purpose.
Conclusions
This review highlights the history and development of ABS plastics, evolution of ABS-like materials by AM, and their general properties and, effects of plating on ABS and fused deposition modeled ABS. The processes and mechanisms involved in plating are effectively described. Plating on the ABS material is shown to increase the mechanical adhesion, impart esthetic appearance and provide corrosion resistance to the sample. The major breakthrough in the plating process is seen in the replacement of conventional surface conditioning methods by environment-friendly processes.
References
Wang W, Ji S, Lee I (2013) A facile method of nickel electroless deposition on various neutral hydrophobic polymer surfaces. Appl Surf Sci 283:309–320. doi:10.1016/j.apsusc.2013.06.108
Tran V, Massardier V, Guyot A, Nguyen T (1993) Interactions in metal-polymer-metal interfaces. Polymer 34(15):3179–3183
Grundmeier G, Stratmann M (2005) Adhesion and de-adhesion mechanisms at polymer/metal interfaces: mechanistic understanding based on in situ studies of buried interfaces. Annu Rev Mater Res 35:571–615
Holubar P, Jilek M, Sima M (2000) Present and possible future applications of superhard nanocomposite coatings. Surf Coat Technol 133:145–151
Long DP, Blackburn JM, Watkins JJ (2000) Chemical fluid deposition: a hybrid technique for low-temperature metallization. Adv Mater 12(12):913–915
Cheng K, Yang MH, Chiu WW, Huang CY, Chang J, Ying TF, Yang Y (2005) Ink-jet printing, self-assembled polyelectrolytes, and electroless plating: low cost fabrication of circuits on a flexible substrate at room temperature. Macromol Rapid Comm 26(4):247–264
Garcia A (2011) Ligand induced electroless plating of polymers. PhD Dissertation, École Polytech
Arai S, Sato T, Endo M (2011) Fabrication of various electroless Ni–P alloy/multiwalled carbon nanotube composite films on an acrylonitrile butadiene styrene resin. Surf Coat Technol 205(10):3175–3181
Tengsuwan S, Ohshima M (2014) Environmentally benign electroless nickel plating using supercritical carbon-dioxide on hydrophilically modified acrylonitrile–butadiene–styrene. Appl Surf Sci 311:189–200
Dechasit P, Trakarnpruk W (2011) Ni electroless plating of ABS polymer by palladium and tin-free process. J Met Mater Min 21(2):19–27
Wang Z, Li Z, He Y, Wang Z (2011) Study of an environmentally friendly surface etching system of ABS for improving adhesion of electroless Cu film. J Electrochem Soc 158(11):D664–D670
Kim GG, Kang JA, Kim JH, K-y Lee, Kim SJ, Kim S-J (2007) Photocatalytic pretreatment of acrylonitrile–butadiene–styrene polymer for electroless plating. Scripta Mater 56(5):349–351
Di L, Liu B, Song J, Shan D, Yang D-A (2011) Effect of chemical etching on the Cu/Ni metallization of poly (ether ether ketone)/carbon fiber composites. Appl Surf Sci 257(9):4272–4277
Ferreira ODS, Stevens A, Schrauwen C (2009) Quantitative comparison of adhesion in metal-to-plastic systems. Thin Solid Films 517(10):3070–3074
Ma Z, Tan K, Kang E (2000) Electroless plating of palladium and copper on polyaniline films. Synth Met 114(1):17–25
Kulkarni MV, Elangovan K, Hemachandra RK (2013) Effects of electroplating on the mechanical properties of injection molded thermoplastics. Int J Plast Technol. doi:10.1007/s12588-013-9056-6
Vander Togt RT (2011) Bumper and process to bond ionomers to plastic. US Patent 4,999,227
Teixeira LAC, Santini MC (2005) Surface conditioning of ABS for metallization without the use of chromium baths. J Mater Process Technol 170(1):37–41
Shu Z, Wang X (2012) Environment-friendly Pd free surface activation technics for ABS surface. Appl Surf Sci 258(14):5328–5331
Bazzaoui M, Martins J, Bazzaoui E, Albourine A (2012) Environmentally friendly process for nickel electroplating of ABS. Appl Surf Sci 258(20):7968–7975
Tang X, Cao M, Bi C, Yan L, Zhang B (2008) Research on a new surface activation process for electroless plating on ABS plastic. Mater Lett 62(6):1089–1091
Skelly J (2008) Decorative plating processes for common plastic resins: resin selection as well as plastic part design is critical to matching the right finishing method with the intended application. Met Finish 106(6):61–65
Haba B, Sugai K, Morishige Y, Kishida S (1994) Large scale two-step selective aluminum CVD on laser patterned palladium lines. Appl Surf Sci 79:381–384
Chen Y, Reisman A, Turlik I, Temple D (1995) Cu CVD from copper (II) hexafluoroacetylacetonate II. Laser-assisted selective area deposition. J Electrochem Soc 142(11):3911–3918
Seeböck R, Esrom H, Charbonnier M, Romand M, Kogelschatz U (2001) Surface modification of polyimide using dielectric barrier discharge treatment. Surf Coat Technol 142:455–459
Tóth Z, Szörényi T, Toth A (1993) Ar+ laser-induced forward transfer (LIFT): a novel method for micrometer-size surface patterning. Appl Surf Sci 69(1):317–320
McCaskie JE, Tsiamis C (1985) Metal plating on plastics. US Patent 4,520,046
Yang C-Y (2012) Method for preparing electronic component-mounting device. US Patent App. 13/471,108
Hooke R (2014) Method of making shaped charges and explosively formed projectiles. US Patent 8,813,651
Hoyt R, Wrobel J, Cushing J, Jaster M, Voronka N, Frank S, Slostad J, Paritsky L (2014) Structures with internal microstructures to provide multifunctional capabilities. US Patent 14/462,541
Nath J, Shen Y, Nealis E (2014) Systems and methods for manufacturing passive waveguide components. US Patent App. 14/453,563
McCaskie JE (2006) Plating on plastics: a survey of mechanisms for adhering metal films to plastic surfaces. Met Finish 104(5):31–39
Mallory GO, Hajdu JB (1990) Electroless plating: fundamentals and applications. William Andrew, New York
Arnold JR (2003) High quality copper-nickel-chromium plating on plastics: a continuous process and its challenges. Plat Surf Finish 91:355–379
Kulkarni MV, Elangovan K, Hemachandra Reddy K (2012) Improvements in impact resistance property of metal plated abs and Nylon6 thermoplastics. FS J Eng Res 1(1):31–33
Equbal AAKS (2014) Metallization on FDM parts using the chemical deposition technique. Coatings 4:574–586. doi:10.3390/coatings4030574
Tang X, Wang J, Wang C, Shen B (2011) A novel surface activation method for Ni/Au electroless plating of acrylonitrile–butadiene–styrene. Surf Coat Technol 206(6):1382–1388
Garcia A, Berthelot T, Viel P, Mesnage A, Jégou P, Nekelson F, Roussel S, Palacin S (2010) ABS polymer electroless plating through a one-step poly(acrylic acid) covalent grafting. ACS Appl Mater Interfaces 2(4):1177–1183
Kuzmik JJ (1990) Electroless plating: fundamentals and applications (Reprint Edition). In: Mallory GO, Hajdu JB (eds). The American Electroplaters and Surface Finishers Society, Orlanda, FL, pp 377–399
Hanna F, Hamid ZA, Aal AA (2004) Controlling factors affecting the stability and rate of electroless copper plating. Mater Lett 58(1):104–109
Equbal A, Sood AK (2014) Electroless plating of copper on different shaped ABS parts: a comparison. Int J Adv Mater Manuf Charact 4(1):32–41. doi:10.11127/ijammc.2014.03.05
Li D, Goodwin K, Yang C-L (2008) Electroless copper deposition on aluminum-seeded ABS plastics. J Mater Sci 43(22):7121–7131. doi:10.1007/s10853-008-3031-1
Liu H-P, Li N, Bi S-F, Li D-Y, Zou Z-L (2008) Effect of organic additives on the corrosion resistance properties of electroless nickel deposits. Thin Solid Films 516(8):1883–1889
Lu G, Li X, Jiang H (1996) Electrical and shielding properties of ABS resin filled with nickel-coated carbon fibers. Compos Sci Technol 56(2):193–200
Walsh F, De León CP, Kerr C, Court S, Barker B (2008) Electrochemical characterisation of the porosity and corrosion resistance of electrochemically deposited metal coatings. Surf Coat Technol 202(21):5092–5102
Agarwala RC, Agarwala V, Sharma R (2006) Electroless ni-p based nanocoating technology—a review. Synth React Inorg Met-Org Nano-Met Chem 36(6):493–515
Balaraju J, Narayanan TS, Seshadri S (2003) Electroless Ni–P composite coatings. J Appl Electrochem 33(9):807–816
Tzeng S-S, Chang F-Y (2001) EMI shielding effectiveness of metal-coated carbon fiber-reinforced ABS composites. Mater Sci Eng A 302(2):258–267
Charbonnier M, Romand M, Goepfert Y (2006) Ni direct electroless metallization of polymers by a new palladium-free process. Surf Coat Technol 200(16):5028–5036
Sahoo P, Das SK (2011) Tribology of electroless nickel coatings—a review. Mater Des 32(4):1760–1775
Sudagar J, Lian J, Sha W (2013) Electroless nickel, alloy, composite and nano coatings—a critical review. J Alloys Compd 571:183–204
Agarwala R, Agarwala V (2003) Electroless alloy/composite coatings: a review. Sadhana 28(3–4):475–493
Equbal A, Dixit NK, Sood AK (2013) Electroless plating on plastic. IJSER 4
Maul J, Frushour BG, Kontoff JR, Eichenauer H, Ott K-H, Schade C (2000) Polystyrene and styrene copolymers. In: Ullmann’s encyclopedia of industrial chemistry. Wiley, New York. doi:10.1002/14356007.a21_615.pub2
Federation BP (2015) A history of plastics. British Plastics Federation. http://www.bpf.co.uk/plastipedia/plastics_history/Default.aspx
Brickipedia (2015) Acrylonitrile butadiene styrene. Brickipedia. http://lego.wikia.com/wiki/Brickipedia:Brickipedians
Truss R, Chadwick G (1976) Tensile deformation behaviour of ABS polymers. J Mater Sci 11(1):111–117. doi:10.1007/BF00541081
Truss R, Chadwick G (1977) The fracture behaviour of ABS polymers. J Mater Sci 12:503–508. doi:10.1007/BF00540274
Bucknall C, Clayton D, Keast WE (1972) Rubber-toughening of plastics- Part 2 Creep mechanisms in HIPS/PPO blends. J Mater Sci 7(12):1443–1453. doi:10.1007/BF00574936
Kulich DM, Gaggar S, Lowry V, Stepien R (2004) Acrylonitrile–butadiene–styrene (ABS) polymers. Kirk-Othmer Encyclopedia of Chemical Technology
Moore J (1973) Acrylonitrile–butadiene–styrene (ABS)-a review. Composites 4(3):118–130
ABS material data sheet. teststandard.com/data_sheets/ABS_Data_sheet.pdf
Lifton VA, Lifton G, Simon S (2014) Options for additive rapid prototyping methods (3D printing) in MEMS technology. Rapid Prototyp J 20(5):403–412
Huang SH, Liu P, Mokasdar A, Hou L (2013) Additive manufacturing and its societal impact: a literature review. Int J Adv Manuf Technol 67:1191–1203. doi:10.1007/s00170-012-4558-5
Yuan L (2008) A preliminary research on development of a fiber-composite, curved FDM system. National University of Singapore
Wong KV, Hernandez A (2012) A review of additive manufacturing. ISRN Mech Eng. doi:10.5402/2012/208760
Yan XG, Gu PENG (1996) A review of rapid prototyping technologies and systems. Comput Aided Des 28(4):307–318
Diegel O, Reay S, Singamneni S, Withell A (2010) Tools for sustainable product design: additive manufacturing. J Sustain Dev 3(3):68–75
Stereolithography; S-PCHPi, http://spie.org/x91418.xml. Accessed 27 Sept 2015
3DSystems. 30 years of innovation. http://www.3dsystems.com/30-years-innovation. Accessed 27 Sept 2015
Crump SS (1992) Apparatus and method for creating three-dimensional objects. US Patent 5,121,329
Sachs EM, Haggerty JS, Cima MJ, Williams PA (1993) Three-dimensional printing techniques. US Patent 5,204,055
Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM (2014) Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. doi:10.1021/ac403397r
Chen S, Patel N, Schaffer D, Maharbiz M (2011) Trap and corral: a two-step approach for constructing and constraining dynamic cell contact events in differentiating progenitor cell populations. J Micromech Microeng 21(5):054027
Kannan S, Senthilkumaran D (2014) Assessment of mechanical properties of Ni-coated abs plastics using FDM process. IJMME-IJENS 14(3):30–35
Monzon MD, Diaz N, Benitez AN, Marrero MD, Hernandez PM (2010) Advantages of fused deposition modeling for making electrically conductive plastic. Int Conf Manuf Autom. doi:10.1109/ICMA.2010.36
Kannan S, Senthilkumaran D, Elangovan, K. (2013) Development of composite materials by rapid prototyping technology using FDM method. IEEE, 281–283
Chandrasekhar U, Venkatesh K, Elangovan K, Rangaswamy T (2011) Integrated use of rapid prototyping and metal plating techniques for development of micro air vehicles. IJEST 3(1):188–193
Equbal A, Sood AK (2015) Investigations on metallization in FDM build ABS part using electroless deposition method. J Manuf Process 19:22–31
Saleh N, Hopkinson N, Hague RJ, Wise S (2004) Effects of electroplating on the mechanical properties of stereolithography and laser sintered parts. Rapid Prototyp J 10(5):305–315
Kannan S, Senthilkumaran D (2014) Investigating the influence of electroplating layer thickness on the tensile strength for fused deposition processed ABS thermoplastics. IJET 6(2):1047–1052
Lurie KL, Smith GT, Khan SA, Liao JC, Ellerbee AK (2014) Three-dimensional, distendable bladder phantom for optical coherence tomography and white light cystoscopy. J Biomed Opt 19(3):036009
Stremsdoerfer G, Ghanem F, Saikali Y, Fares-Karam A (2003) Copper deposition by dynamic chemical plating. J Mater Sci 38(15):3285–3291. doi:10.1023/A:1025190022069
Weng D, Landau U (1995) Direct electroplating on nonconductors. J Electrochem Soc 142(8):2598–2604
Krishnan KH, John S, Srinivasan K, Praveen J, Ganesan M, Kavimani P (2006) An overall aspect of electroless Ni–P depositions—a review article. Metall Mater Trans A 37(6):1917–1926
Feldstein N (1981) Dispersions for activating non-conductors for electroless plating. US Patent 4,282,271
Feldstein N (1982) Electroless plating. US Patent 4,321,285
Equbal A, Equbal A, Sood AK (2014) Metallization on FDM processed parts using electroless procedure. Proc Mater Sci 6:1197–1206. doi:10.1016/j.mspro.2014.07.193
Kirman T (1972) Electroless nickel plating process for nonconductors. US Patent 3,684,572
Feldstein N (1976) Electroless plating process employing non-noble metal hydrous oxide catalyst. US Patent 3,993,799
Elmore G, Davis K (1969) Mechanism of bonding electroless metal to organic substrates. J Electrochem Soc 116(10):1455–1458
Abu-Isa IA (1973) Metal plating of polymeric surfaces. Polym-Plast Technol Eng 2(1):29–65
Metal plating of thermoplastics (1971). US Patent 3,620,804
Magallón-Cacho L, Pérez-Bueno J, Meas-Vong Y, Stremsdoerfer G, Espinoza-Beltrán F (2011) Surface modification of acrylonitrile-butadiene-styrene (ABS) with heterogeneous photocatalysis (TiO2) for the substitution of the etching stage in the electroless process. Surf Coat Technol 206(6):1410–1415
Krulik GA (1980) Method for electroless plating on nonconductive substrates using palladium/tin catalyst in aqueous solution containing a hydroxy substituted organic acid. US Patent 4,182,784
Daneshmand S, Aghanajafi C, Nadooshan AA (2010) The effect of chromium coating in RP technology for airfoil manufacturing. Sadhana 35(5):569–584
Gabe D (1977) Corrosion and protection of sintered metal parts. Powder Metall 20(4):227–231
Conlan W, Dutkewych O (1973) Pre-etch treatment of acrylonitrile-butadiene-styrene resins for electroless plating. US Patent 3,769,061
Weakley-Bollin SC (2010) The effect of injection molding conditions on the near-surface rubber morphology, surface chemistry and adhesion performance of semi-crystalline and amorphous polymers. University of Michigan, Ann Arbor
Beacom SE, Wedel RG (1970) Scanning electron microscopy of plastic surfaces as related to metal deposition. J Colloid Interface Sci 34(3):375–386
Kanani N (2004) Electroplating: basic principles, processes and practice. Elsevier, Oxford
Kato K (1968) Electron microscope studies on the etching of ABS mouldings for electroplating. Polymer 9:419–424
Dennis JK, Such TE (1993) Nickel and chromium plating. Elsevier, Amsterdam
Donovan LP, Timmer RJ (2002) Decorative chrome electroplate on plastics. US Patent 6,468,672
Hurley JL (1975) Decorative electroplates for plastics. US Patent 3,868,229
Narcus H (1979) Bright electroless plating process and plated articles produced thereby. US Patent 4,169,171
Gutzeit G, Talmey P (1958) Chemical nickel plating processes and baths therefor. US Patent 2,935,425
Jones RD (2005) Decoratively finished thermoplastic product and method for manufacturing same. US Patent App. 10/649,190
Metal plating of plastics (1970). US Patent 3,501,332
Marie OJ (1966) Method of electrodepositing a corrosion resistant nickel-chromium coating and products thereof. US Patent 3,282,810
Omura Y (1997) Process for catalyzation in electroless plating. US Patent 5,660,883
Odekerken JM (1972) Process for coating an object with a bright nickel/chromium coatin. US Patent 3,644,183
Eileen M (1972) Process for electroless plating of abs resins. US Patent 3,689,303
Paul T, Crehan WJ (1953) Process of chemical nickel plating. US Patent 2,658,839
Patel GN, Bolikal D, Patel HH (1992) Chromic acid free etching of polymers for electroless plating. US Patent 07/488,256
Brown SD, Ottavio ED, Kuzmik JJ, Grunwald JJ (1972) Method of treating plastic substrates and process for plating thereon. US Patent 3682786
Kuzmik J (1974) Preparation of plastic substrates for electroless plating and solutions therefor. US Patent 3,790,400
Roubal J, Korpiun J (1977) Pretreatment of plastic materials for metal plating. US Patent 06/701,585
Amelio WJ, Jung DY, Markovich V, Sambucetti CJ (1986) Process for manufacturing a concentrate of a palladium-tin colloidal catalyst. US Patent 09/831,008
Naruskevicius L, Rozovskis G, Vinkevicius J, Baranauskas M, Mobius A, Pies P (2004) Etching plastic surface under mild conditions, treating with solution containing cobalt, silver, tin, and/or lead salts having ph from about 7.5 to about 12.5, treating with sulfide solution, metallizing. US Patent US 6712948 B1
De De Minjer CH, vd Boom PFJ (1973) The nucleation with SnCl2–PdCl2 solutions of glass before electroless plating. J Electrochem Soc 120(12):1644–1650
Lindsay JH, La Sala J, Ghorashi HM (1983) Etching and bonding oxygen to acrylonitrile-butadiene-styrene terpolymer or polyphenylene ethers, vacuum deposition, followed by electrodeposition of metal films. US Patent 4,395,313
Cohen R, Meek R (1976) The chemistry of palladium–tin colloid sensitizing processes. J Colloid Interface Sci 55(1):156–162
Lindsay DJ, Feldstein N (1989) Activators for colloidal catalysts in electroless plating processes. US Patent 4,820,547
Comparison of sealing methods for FDM materials (2011). Stratasys
McCarthy DL, Williams CB (2012) Creating complex hollow metal geometries using additive manufacturing and electroforming. In: International Solid Freeform Fabrication Symposium, pp 108–120
Wang Y, Bian C, Jing X (2013) Adhesion improvement of electroless copper plating on phenolic resin matrix composite through a tin-free sensitization process. Appl Surf Sci 271:303–310
Wang G, Li N, Li D (2007) Effect of Pd ions in the chemical etching solution. J Univ Sci Technol Beijing Miner Metall Mater 14(3):286–289
Inagaki N, Kimura H (2009) Electroless copper plating on acrylonitrile butadiene styrene material surfaces without chromic acid etching and a palladium catalyst. J Appl Polym Sci 111(2):1034–1044
Arai S, Kanazawa T (2014) Electroless deposition and evaluation of Cu/multiwalled carbon nanotube composite films on acrylonitrile butadiene styrene resin. Surf Coat Technol 254:224–229
Li D, Yang C-L (2009) Acidic electroless copper deposition on aluminum-seeded ABS plastics. Surf Coat Technol 203(23):3559–3568
Helmut K, Eugen M-S (1972) Process for the pretreatment of plastic for the purpose of adhesive metallization. US Patent 3,702,285
Isa A, Vincent W (1974) Plating pre-treatment in acrylonitrile butadiene styrene. US Patent 3,847,648
Kang DH, Choi JC, Choi JM, Kim TW (2010) An environment-friendly surface pretreatment of ABS plastic for electroless plating using chemical foaming agents. Trans Electr Electron Mater 11(4):174–177
Zhao W, Ma Q, Li L, Li X, Wang Z (2014) Surface modification of ABS by photocatalytic treatment for electroless copper plating. J Adhes Sci Technol 28(5):499–511
Kim GG, Kang JA, Kim SJ, Kim SJ, Shin SH (2007) Improved adhesion to ABS plastics by a photocatalytic reaction. In: Materials science forum. Trans Tech Publication, Dürnten, pp 75–78
European Parliament and the Council, 2002/95/EC, 2003
WHO regional publications (2000), Air quality guidelines for Europe. 2nd edn. Copenhagen
Bazzaoui M, Martins J, Bazzaoui E, Albourine A, Wang R, Hong P-D (2013) A simple method for acrylonitrile butadiene styrene metallization. Surf Coat Technol 224:71–76
Parkinson R, Hart T, Electroplating on plasticts. NiDi Technical Series, Nickel Development Institute
Li Z, Li N, Yin L, He Y, Wang Z (2009) An environment-friendly surface pretreatment of ABS resin prior to electroless plating. Electrochem Solid-State Lett 12(12):D92–D95
European Parliament and the Council, 2005/90/EC, 2006
Yang Z, He Y, Li Z, Li N, Wang Z (2011) Adhesion improvement of ABS resin to electroless copper by H2SO4–MnO2 colloid with ultrasound-assisted treatment. J Adhes Sci Technol 25(11):1211–1221
Ma Q, Zhao W, Li X, Li L, Wang Z (2013) Study of an environment-friendly surface pretreatment of ABS-polycarbonate surface for adhesion improvement. Int J Adhes Adhes 44:243–249
Brandes M (2007) Advances in methods for plating on plastics tout advantages over conventional technologies: alternative plating procedures negate the need for chromic sulfuric acid, resulting in a more environmentally friendly process for electroplaters. Met Finish 105(3):35–40
Brocherieux A, Dessaux O, Goudmand P, Gengembre L, Grimblot J, Brunel M, Lazzaroni R (1995) Characterization of nickel films deposited by cold remote nitrogen plasma on acrylonitrile–butadiene–styrene copolymer App. Surf Sci 90:47–58
Tsuru Y, Mochinaga K, Ooyagi Y, Foulkes FR (2003) Application of vapor-deposited carbon and zinc as a substitute for palladium catalyst in the electroless plating of nickel. Surf Coat Technol 169:116–119
Tang X, Bi C, Han C, Zhang B (2009) A new palladium-free surface activation process for Ni electroless plating on ABS plastic. Mater Lett 63(11):840–842
Yen P-C (1995) Improved ABS plastic activating treatment for electroless copper plating. Polymer 36(17):3399–3400
Ono S, Naitoh K, Osaka T (1999) Initial propagation stage of direct copper plating on non-conducting substrates. Electrochim Acta 44(21):3697–3705
Gui-Xiang W, Ning L, Hui-Li H, Yuan-Chun Y (2006) Process of direct copper plating on ABS plastics. Appl Surf Sci 253(2):480–484
Acknowledgements
This research was supported by the Space Application Centre, ISRO, Government of India with the project ID ‘OGP 103.’
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Olivera, S., Muralidhara, H.B., Venkatesh, K. et al. Plating on acrylonitrile–butadiene–styrene (ABS) plastic: a review. J Mater Sci 51, 3657–3674 (2016). https://doi.org/10.1007/s10853-015-9668-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9668-7