Abstract
The use of coatings in biomaterials has been fundamental on the applicability of many medical devices and has helped improve mechanical properties such as wear and fatigue and biological properties such as biocompatibility and bioactivity of implant prosthesis, thus, in essence, ameliorating human quality life. The aim of the present paper is to give a review on cold spray (CS) coating systems that are emerging in orthopedics industry (internal fixation systems and prosthesis) as well as those for antibacterial purposes (in body and touch external surfaces). These studies are very new, the oldest dating from the half of last decade and most deal with the improvement of biocompatibility and bioactivity of hard tissue replacement; therefore, research on biocoatings is in constant development with the aim to produce implant surfaces that provide a balance between cell adhesion and low cytotoxicity, mechanical properties, and functionalization. CS offers many advantages over conventional high-temperature processes and seems to be able to become competitive in front of the low-temperature techniques. It is mainly cost effective, appropriate for oxygen-sensitive materials, and environmentally green. It basically involves the use of feedstock material in powder form, which is supersonically sprayed onto the appropriate substrate but without any melting as it occurs in conventional thermal spray processes. Biocompatible metallic materials and polymers have been successfully deposited by this method because it is based on the plasticity of the coating material; pure ceramic deposits, for example of hydroxyapatite, are still a challenge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The massive increase in human aging has affected different areas such as economical, social, and health, especially the last one, with the increase of chronic diseases. It is anticipated that elderly people (+65 years) will reach up to 20 % of European population in 2050, compared with the 10 % of nowadays [1]. For centuries, a diseased tissue was removed to improve marginally the quality of life. With the scientific advances in biomedical field, however, it has led to an increase in human survivability aversely to the quality of tissues, thus the arising need to replace tissues [2]. In terms of orthopedics, more and more patients will require the use of prostheses in order to replace critical parts of the skeletal system.
Current patients’ complaint about conventional prostheses includes (i) socket-related problems of discomfort, sores, rashes, and pain, (ii) the difficulties on donning the prosthesis, (iii) the unreliability of prosthesis being securely suspended, and (iv) mobility difficulties. While innovation on new materials with better mechanical and biological properties is day by day carried out through the collaboration of many scientific disciplines, the osteointegration by the surface modification of conventional prosthetic materials can still offer many possibilities for the improvement of bone resorption decreasing the allergenic response. In addition, in many cases, the surgery for prosthesis replacement is extremely aggressive and the cost is high; therefore, whatever solution that can extend the prosthesis life will be very welcomed by clinical community.
The biomaterials field is always under development and has experienced considerable progress especially over the last 60–70 years [3, 4]. The definition of a biomaterial, currently proposed as “a non-viable material, used in a medical device, intended to interact with biological systems,” was definitely established by William in 1987 [5]. The development of biomaterials for medical applications has evolved through three generations, each with a distinct objective (Fig. 1). Specific familiar terms such as bioinert, bioactive, or biodegradable allow their classification according to their characteristics within the body.
Bioactive materials are an intermediate between resorbable and bioinert (Fig. 2) [6]. The first denoted bioactive material was Bioglass®, also known as 45S5 bioactive glass in the late 1960s by Larry Hench [7], and the concept of using synthetic resorbable ceramics as bone substitutes was introduced in 1969 [8]; then, hydroxyapatite (HA) as well as some other glass–ceramics appeared within the market circa 1985s [9, 10]. Bioactive materials are classified in two categories: (i) osteoproductive materials, that are recognized by the intracellular and extracellular responses elicited at their interfaces (e.g., Bioglasses), whereas (ii) osteoconductive materials only elicit an extracellular response at their interfaces (e.g., HA). Bioglasses induce integration between bone and implant in the form of a continuous interfacial layer, while osteoconductivity only induces bone growth directly at the implant surface and often results in a fibrous capsule between the implant surface and bone.
Bioactivity spectrum for various bioceramic implants: a relative rate of bioactivity and b time dependence of formation of bone bonding at an implant interface [A 45S5 Bioglass®, B Mina13 Ceravital®, C 55S4.3 Bioglass®, D A/W glass–ceramic, E HA, F KGy213 Ceravital®] [6]
The two primary issues in biomaterials are biocompatibility and structural compatibility [11]. Considering the biocompatibility as “the ability of a material to perform with an appropriate host response in a specific application” [5], it implicitly refers two terms: biosafety and biostability, where the material does not have to provoke chronic inflammation/infection that may cause cell death or produce a disfunction in cellular and tissue matrix [12]. Structural compatibility refers to mechanical properties and becomes especially important for prosthesis biomaterials.
Surface characteristics such as roughness [13] and porosity [14] influence cell attachment and promote bone ingrowth fixation between implant and host issues due to its structure and free surface. The concept of bioactivity is actually highly related with those characteristics, which will be many times addressed along this paper.
Surface treatments and biocompatible coatings: current status
Surface engineering has helped biomedical science to provide better understanding of implant–tissue interactions; the surface modification methods include both the chemical modification and surface roughness as well. The atoms on the surface are more prompt to undergo phase transformations, crystallization, or corrosion (dissolution) processes; this higher energy and higher reactivity are particularly important in view of adsorbates from the biological system. Cellular activity, protein adsorption, or tissue response has been specially induced in titanium-based alloys by surface roughening, acid treatment, anodization, and coating techniques, i.e., thermal spraying, methods that produce surface topography changes mainly at the microscale level [15]. Other attempts to improve osteoblast activity include the promotion of surface roughness with combined micrometer and nanometer structures such as photo, electron beam, and colloidal lithography or electrochemical anodisation [12, 16].
Concerning metal coatings, vacuum plasma spraying (VPS) is, for example, widely used to prepare rough and porous titanium coatings [17–21]. Yang et al. [17] obtained titanium (Ti) coatings on Ti substrates consisting of an outer layer full of macropores with a surface roughness of approximately Ra = 100 μm (such macropores are reported to be beneficial for tissue ingrowth into the coating), a middle layer consisting of a mixture of micropores and macropores and an inner dense layer. By contrast, however, Borsari et al. [19] used the same technique to produce rough but dense VPS-Ti coatings with the purpose of avoiding as much the reduction in bone density, also known as “stress shielding,” as possible, and thus prolong the prosthesis lifespan. The aim of that study was to investigate the in vitro effect of high roughness (Ra = 73.75 μm) and dense Ti surface in comparison with medium (Ra = 18.42 μm) and high roughness (Ra = 39.64 μm) and open porous coatings. Such new ultra-high rough and dense VPS coating provided a good biological response; at least in vitro, it behaved similarly to the coatings already used in orthopedics. The effect of the coating stability and ultra-high roughness level after surgical implantation and during dynamic bone healing and remodeling has yet to be established. Other titanium coatings for medical devices may include open porosity [17].
Other thermal spray (TS) metal coating attempts include tantalum (Ta) and silver (Ag). Tantalum coatings have an excellent corrosion, good formability, low coefficient of expansion, excellent wear resistance, and excellent biocompatibility and radio-opacity for biomedical applications. Recent in vitro, in vivo, and clinical studies demonstrated that tantalum is a promising bioactive metal [22, 23]. Since tantalum applications in biomedical devices have been limited by processing challenges rather than biological performance, Ta coatings have been achieved via plasma spray (APS) and high-velocity oxy-fuel (HVOF). Optimizing spraying parameters leads to minimum porosity and oxide content but without good corrosion protection [22, 23]; in addition, there are still some drawbacks such the high cost and the high reactivity at temperatures above 500 °C where oxidation causes loss of ductility and cracking of the surface material. Other coating methods by which tantalum has been deposited include LENS™ (Laser Engineered Net Shaping) [24], sputter deposition [25], chemical vapor deposition (CVD), and electrodeposition.
From another hand, silver has been highlight since ancient times for its antibacterial, antifungal, and antiviral properties; also its compounds, such as silver nitrate and silver sulfadiazine, have been used for the treatment of burns, wounds, and several bacterial infections [26–28]. Pure silver coatings by different methodologies have been tested with very good results, especially in catheters [29–34]. Using thermal spray methods, however, silver has been co-deposited with many other materials [35–39]. For example, HA/silver composite coatings obtained via VPS proved to combine antibacterial and bioactivity properties; it was found non-cytotoxicity for the coatings and they were covered by bone-like apatite layer after immersed in simulated body fluids (SBF), suggesting that their bioactivity was not affected obviously by the addition of silver in the coatings [40].
Concerning ceramic coatings, plasma-sprayed alumina and zirconia are being used clinically, mostly due to their higher wear resistance than titania. However, alumina and zirconia coatings cannot bind directly to bone tissues due to their bioinert nature, thus limiting their use in hard tissue applications. Moreover, there is a controversy on the binding strength and on particle release from plasma-sprayed coatings into the host tissue, caused by either dissolution or fretting. Therefore, the use of bioactive HA coatings produced by plasma spraying (APS) [41–44], high-velocity oxy-fuel (HVOF) [45–47], and flame spray as well [48] was a very successful achievement; in HVOF, particles reach lower temperatures and higher velocities that minimize the time of residence of the particles within the spray beam and therefore its thermal decomposition [1, 49]. HA-coated prosthesis maximizes fixation and decreases the migration of microparticles along the prostheses [50]; they are a good alternative to cemented prosthesis, which have high rates of loosening. In addition, Chern et al. [51] compared the coating–substrate bonding strength of HA with those of other bioactive coatings such as bioglass and bioglass-HA and found that bonding strength was 33.0 ± 4.3, 39.1 ± 5.0, and 52 ± 11.7 MPa for bioglass, bioglass-HA, and HA coatings, respectively. It was demonstrated that after 4 weeks bone ingrowth was significantly higher in bioglass and HA coatings but after 16 weeks only bioglass maintained its high percentages of bone ingrowth while in HA decreased with time [52]. Despite having excellent bioactivity, the mechanical properties of bioactive glasses are worse than those of bioactive HA; this problem can be solved by combining those bioactive materials with metals or polymers to produce a composite coating surface [53]. Cai et al. [54] developed a sintered Co–Cr–Mo/Bioglass composite coating for medical implant application in order to be compared to plasma-sprayed coatings. Those coatings show a more porous structure than plasma spray but less wear resistance. Nevertheless, an adequate bonding between Co–Cr–Mo/Bioglass composite coating was achieved and furthermore an apatite layer on top of the coating performed bioactivity. Moreover, more processes are used to fabricate composite bioglass coatings, such as sol–gel [55], electrophoretic deposition [56], and pulsed laser deposition [57].
Even enhanced biocompatibilities are achieved using nanocrystalline ceramics [58, 59]. HA particles’ shape has a high influence in cell performance (e.g., needle-shaped particles promote inflammatory reaction, spherical-shaped particles show increased inhibition with time and concentration of those in U2-OS cancer cells, and irregular-shaped particles produce a greater response than spherical-shaped particles).
However, there is still some concern about the uniformity, the adherence of the coatings, and the dissolution rates due to crystalline issues affecting long-term stability. The lack of uniformity is related to the uncontrollable crystallinity within HA plasma coatings, leading to many different phases such as alpha tricalcium phosphate (α-TCP), beta tricalcium phosphate (β-TCP), tetracalcium phosphate (TTCP), oxyapatite (OHA), and amorphous phases (ACP), whereas the concern on the adherence is attributed to the presence of amorphous phases at the coating–substrate interface. Ceramic bond coats based on zirconia and titania have been plasma sprayed in order to be employed to act as a chemical barrier against in vivo release of metal ions from the implant and improve the adhesive bond [60]. Table 1 includes the requirements of HA coatings for implants for surgery specified by different ISO and ASTM standards [61–65].
Other coating technologies have also been employed for the production of HA coatings but are less cost effective when compared to thermal spray processes (TS). Table 2 shows some advantages and disadvantages of TS compared to such other possibilities.
Some other variations of these types of coatings have been performed using HA–TiO2 mixtures to improve mechanical properties, i.e., bond strength, fracture toughness, and wear resistance [70–72], fluorapatite–HA mixtures given that fluorapatite offers the potential for lower mineral ion release by dissolution [73], yttria-stabilized zirconia-reinforced HA/Ti–6Al–4V composites which leads to significantly higher mechanical properties than pure HA coatings (even after immersion in SBF solution) [74], Ta/HA layers to improve the corrosion resistance and biocompatibility [75], Ag–HA mixtures to reduce bacterial adhesion [76–78], or using carbon-nanotube reinforcement imparting strength and toughness to brittle HA bioceramic coating [79].
All these surface modifications have been developed over the past decades to improve the bioactivity mostly of Ti-based implants and their bonding strength to the host tissue. However, although many research groups are still working on this topic, there has been more recently an upgrade on the study of polymer composite materials as an alternative choice to overcome the shortcomings of metals and ceramics [4]. The poly(ether ether ketone) (PEEK), for example, highlights for its biocompatibility, bioinertness, and similar elastic modulus to the bone and their good mechanical properties for hard tissue applications (hip and knee replacements) [80]; other polymers such as poly(lactic acid) (PLA)—bone plates, tendons, and ligaments—and poly-l-lactide (PLLA)—bone plates—stand out for their fully resorbable property; polyurethane (PU) and silicone rubber (SR) get distinguished because of their flexibility in catheters. Some of the attempts to improve the bioactivity of these polymers include coating with tantalum [81], gold, titanium dioxide (TiO2), diamond-like carbon (DLC), and tert-butoxides [82].
The bioactivity requirement, depending on the component application, can be also pursued by proper manufacturing routes [83]. In this direction, some researchers have developed human hip joint prosthesis made of fiber-reinforced poly(ether ether ketone) (CF/PEEK) and coated the stem with vacuum plasma-sprayed (VPS) Ti/HA coatings [84]; the mechanical tests of the prosthesis produced by Riner et al. indicated good long-term stability of the bone-prosthesis system, while the in vitro and in vivo tests proved no cytotoxicity and necrotic effects in rabbits. Apart from plasma spraying, HA coatings have been also produced on PEEK substrates by other processes such as RF magnetron sputtering and aerosol technique.
Biocoatings market for orthopedic implant with focus on thermal spray
As shown in Fig. 1, after the first generation of load-bearing implants (cortical bone substitution) using bioinert materials [stainless steel 316L, cobalt chromium alloy, titanium, or titanium alloy (Ti–6 %Al–4 %V)], the second generation involving surface treatments emerged prominently in 1985 with the first HA-coated femoral prosthesis (Furlong®, JRI, London, UK) [85]. In general, in vitro and in vivo studies indicate that bioactive biomaterials’ application in biomedical field increases the long-term durability of prostheses. Since first clinically reported trials of HA coatings on femoral stems, HA coatings were extensively used in dental and orthopedic prosthesis [86]. HA coatings are currently being used in total hip [87, 88] and knee [89] replacement implants, ankle and shoulder implants, and screws and pins in bone plates for fixing bone fractures. Medical studies were undergone in acetabular cups for total hip arthroplasty [90] and tibial component for total knee arthroplasty [91] at a minimum duration of follow-up of 5 years comparing different fixations like HA coatings, porous surfaces, and cemented fixations. HA coating surfaces stabilized after an initial period of early migration, whereas cemented components showed an initially lower but over time continuously increasing migration. Some of the biomaterials used in skeletal system applications are listed in Table 3 together with medical market.
At the moment, some of the successful bioactive coatings for implants have been produced by electrodeposition and plasma spraying: Peri-apatite™, Biomet’s Osteocoat®, and Corail®, among the most important. Other successful approaches have been (i) the development of a macro-sized interconnected porosity in the range of 100–500 µm within a metal coating with the aim to promote proper bone ingrowth, i.e., Regenerex™, Trabecular Metal™, and Arcam AB Trabecular Structures™ and (ii) nanoscale topographies, i.e., OsseoSpeed™ and Nanotite™. Table 4 presents some of the characteristics of the current commercial orthopedic implants [92].
Thermal spray processes
Conventional technologies
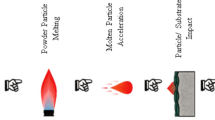
TS is a group of techniques to produce metallic and non-metallic coatings where the feedstock is sprayed in molten or semi-molten state onto a prepared substrate. Their basic principle is to impart sufficient kinetic and thermal energy to the raw material (in powder, wire, or rod form) to create a confined high-energy particle stream and propel the energetic particles toward the substrate. Through the solidification of the droplets on impact with the substrate, they create cohesive bonds with each other and adhesive bonds with the substrate; many different spraying parameters need to be optimized to produce suitable coatings for the desired applications (Fig. 3).
The particles are heated by electrical (air plasma or arc wire spraying) or chemical (detonation gun, flame spraying, or high-velocity oxy-fuel) means. Droplets impact and start bonding onto the substrate due to high cooling rate, typically excess of 106 K/s for metals [93–95]. Coating properties directly depend on particle temperatures and speeds, which produce thin layers of lamellas, often called “Splats” that finally build up the deposit. There are three types of bonding mechanisms at interface substrate–coating, being predominant the mechanical bonding followed by metallurgical ones [95]:
-
Mechanical bonding, particles, molten or semi-molten, impact onto surface substrate (previously grit-blasted), and remaining adhered due to its roughness.
-
Metallurgical bonding, given by the occurrence of interdiffusion processes between substrate–coating and even though the formation of one new compound such as intermetallic phases.
-
Physical bonding, reached by Van deer Waals forces between substrate–coating.
Thermal spraying techniques are divided into three subgroups according to the energy source (Fig. 4), and the selection of the appropriate spraying method will be determined by coating material characteristics, coating performance requirements, economics, and part size and portability.
Advantages of conventional technologies
A big advantage of thermal spray processes is the ability to deposit an extremely wide range of materials. Virtually, any material that has a stable molten phase can be deposited, and even some materials that do not melt, such as graphite and many carbide or boride ceramics, can often be co-deposited with another sprayable material to create a composite coating material. Another one is that the range of suitable substrate materials is even greater that the range of sprayable materials. In addition to metals, ceramics, glasses, and polymers, thermal spray coatings have been successfully applied to many other substrate materials including wood. Conventional thermal spray also offers the advantage of high deposition rates, which are orders of magnitude higher than those of most alternative coating technologies, such as electroplating or vapor deposition, where deposition occurs at the atomic or molecular level. When the objects to be coated are very large or difficult to move, the ability to apply coating in situ is also an advantage. Furthermore, coatings can be applied without significant heat input and it is possible to strip off and recoat worn or damaged coatings without changing part properties or dimensions. Further advantages of thermal spraying include its rapid coating deposition, low cost, high efficiency, and rapid execution process.
Although all the techniques exposed in Table 2 are suitable to produce bioactive HA layers, only thermal spraying, in particular plasma spraying, is the commercially accepted method by Food and Drug Administration (FDA), the USA for producing HA coatings [96].
Limitations of conventional technologies
TS deposition features depend on the used technique, and the thermal and kinetic applied energy will be different. High temperatures cause oxide inclusions (overcoat metallic materials) as well as decomposition/degradation in oxygen-sensitive materials such titanium or HA, respectively. Oxide inclusions improve mechanical properties like wear resistance and hardness, but an excessive presence at intersplat regions leads to cohesive failure and wear debris [97]. Processes that minimize heating of the spray material, such as HVOF and D-Gun, typically result in lower oxide concentration and minimal changes in alloy chemistry. Also, the controlled inert atmosphere of VPS creates very little or no oxide during the deposition process; however, some changes in the alloy chemistry may still occur due to relatively high temperatures in the plasma jet. High porosity could be beneficial in some applications like in the case of prosthesis to promote a good bone ingrowth; conversely, excessive porosity can also be a problem if the coating is intended to protect the underlying substrate from species that can cause corrosion or other problems. Porosity depends moreover on size particle distribution and spray distance, producing “unmelted” particles according to their inertia when are fed into the plume. Also, low-velocity processes tend to have higher level of porosity in the range of 5–15 % volume, and higher velocity processes origin coatings with less porosity (3–8 % volume). Another limitation is the introduction of residual stresses that limit the maximum thickness due to the solidification of droplets when they cool down [98]. Finally, the deposition is limited to surfaces in a direct line of sight of the spray gun.
Cold spray (CS), a novel spray technique in the late 1980s, mainly arises from the limitations of some coating types of thermal spray that seem to be overcome for some materials. CS is a low-temperature process based on the plastic deformation of the spraying material, and it is suitable for the deposition of oxygen-sensitive materials or for temperature-sensitive materials like nanostructured and amorphous powders [97–100]. Moreover, it should also be noticed that, compared to the conventional thermal spray technologies and other coating alternatives like painting and electrodeposition, CS is an environmentally friendly approach since its effluents are easy to control and dispose and it is a non-combustion process.
Cold spray technique
CS is the newest recent spray technology from the thermal spray family from 1980s; it is based on the kinetics energy and stands above conventional spray techniques for its low temperature rates. Small particles (5–50 μm) are accelerated by a pre-heated gas temperature (25–1100 °C) lower than the melting point of the material and propelled toward a prepared substrate at supersonic velocities (300–1200 m/s). Supersonic flows from gas dynamics are obtained within nozzle with the principal purpose to maximize the thrust and obtain a better coating quality. Nozzle design influences on particle velocity, depending basically on the type of nozzle and its geometry. From the three basic nozzles, convergent-barrel (CB nozzle), convergent-divergent (CD nozzle), and convergent-divergent-barrel (CDB nozzle), the one which achieves a higher particle velocity is CD nozzle, known also as Laval nozzle with its conical geometry.
Particle binding is made by kinetics energy when particle impacts onto a surface causing plastically deformation [98], becoming particle velocity an important parameter. Due to high kinetics, CS is able to produce quality dense coatings. However, depending on the spraying conditions, it is possible to obtain porous coatings if the application requires it. Figure 5 shows the schema of CS technique.
Instantly, the feedstock located in the feeder is propelled by gas (normally N2) and pre-heated gas (N2, Air or helium) at determinate temperature and pressure, into the spray nozzle, to propel particles at high velocities to build up the coating. Stages of coating formation are shown in Fig. 6 [101].
Stages of coating formation in the cold spray process [97]
Advantages of CS technology
The main advantage of the CS process is that it is a solid-state process, which results in many unique coating characteristics. High deposition efficiency values have been achieved with metals, alloys, and composites; high deposition rates can produce a thick coating in a single pass (1–2 mm) due to its typical spray beam of about 10 mm diameter [98].
CS can be viewed as a triplex process (grit blast, spray coat, and shot peen), as expected to be caused by the velocity Gaussian distribution across the spray beam; flexibility in substrate–coating selection is good to produce coatings that could lead to unacceptable interfaces in APS or HVOF, i.e., intermetallic phases between Cu–Al with APS; minimum thermal input to the substrate facilitates the use of temperature- and oxygen-sensitive materials such magnesium, titanium, and polymers. Moreover, residual tensile thermal stresses remain in a TS coating produced by a conventional process, whereas CS induces stresses mostly in compressive nature across the entire coating thickness, which improves mechanical properties such as fatigue. However, some investigations confirm that in specific cases neutral and tensile stresses may appear to be depending on substrate/coating combination and surface treatment. Suhoen et al. [102] deposited Al, Ti, and Cu onto carbon steel (CS), SS, and Al substrates with different surface treatments. It has been shown that compressive residual stresses predominate in Cu deposition onto the majority of the specimens; also Ti coatings may show compressive, neutral, or tensile residual stresses depending on the substrate; by contrast, Al coatings exhibited tensile residual stresses onto all the substrates.
Furthermore, compressive residual stresses may be detrimental if relatively thick coatings are sprayed onto thin substrates and they produce their deformation; also, they have to be taken into account in the case that they promote tensile stresses to the substrate. Therefore, residual stresses should be considered in any application where structures are required to carry load.
It has been demonstrated that metals, polymers, ceramics, and composite materials are able to be applied with CS technology in a wide range of applications that are in constant development, such as those involving corrosion protection, repairing structures, catalyst deposition, electromagnet transition, and electronic and medical devices [103, 104].
Depending on spraying materials’ properties, grid blasting could be a good option to improve particle attachment onto substrate if a mechanical anchoring effect contributes in the bonding mechanism [105–107]. However, it might be detrimental if this induces hardening of the substrate surface since it would change the mechanical surface characteristics [108–110].
Process parameters of CS technology
The actual mechanism by which the solid particles deform and bond during CS is still not well understood. Particles undergo an extensive plastic deformation when impact onto the substrate, which results in the creation of jets, known as adiabatic shear bands (ASB), in the case of ductile materials such metals. It is believed that the contact of substrate surface and particle with the high pressures is necessary for particle bonding. Actually, for metals which are the mostly deposited materials by CS, the resulting microstructure resembles that of a cold worked material, with elongated grains and even recrystallized areas at particle interfaces where a higher temperature is reached, a result of adiabatic shearing [111]; such microstructures have been well compared to those of powder-compacted and explosive-welded materials. A wide range of ductile materials (metallic and polymeric) have been successfully deposited by CS, whereas non-ductile materials such as ceramics are able to deposit onto ductile substrates where particle could be embedded.
Generally, for each material, there is a critical velocity (V c) for its successful deposition onto a certain substrate. Only those particles that exceed this critical velocity (V p > V c) will be successfully deposited to build up a coating, but higher impact velocities may result in erosion of surface substrate.
This critical velocity depends, from one side, on the intrinsic characteristics of the spraying material, i.e., the physical and mechanical properties such as density, melting point, and ultimate strength, and from another side on the particle size, morphology, temperature, and substrate; in addition, the particle velocity (V p) also depends on the spray gun parameters, i.e., gas composition, gas preheat temperature, gas pressure, and nozzle geometry. An optimization of all these parameters is many times critical for a good deposition [100].
Metal, cermet, and polymeric coatings have been successfully produced onto different substrates, but ceramic coatings are still a challenge due to their intrinsic brittleness. Blends of metal–ceramic feedstock powders have been sprayed by CS leading to improved coating properties such as wear and hardness [112]; as it will be later discussed, this alternative has been successfully used to produce titanium–HA coatings.
Biocoatings via CS
Metal biocoatings
Biocompatible metals were the first family of materials to be sprayed via CS within this field due to their high plasticities and thus the feasibility to produce coatings with good efficiencies. The first metal coatings that were used for biomedical applications were of stainless steel (SS) and titanium. In analogy to porous plasma-sprayed titanium coatings, these have been also produced by CS with the aim to allow bone ingrowth.
By changing the spraying conditions, it is possible to reach different porosity levels. Li et al. [113] presented the microstructure of cold-sprayed Ti and Ti6Al4V coatings onto Ti6Al4V substrates and the effect of heat treatment on coating microstructure. These authors achieved an average porosity of 5.4 ± 2.4 and 22.3 ± 4.7 % before the heat treatment; after the heat treatment, the porosity increased to 21.6 ± 4.6 and 29.7 ± 5.1 % probably by the healing of the incomplete interfaces through the atom diffusion during annealing treatment. In addition, Wong et al. is another example of how the authors achieve different degrees of porosity by a wide range of modification of the spraying conditions (Fig. 7) [114]. It might be also worth noting that the density of the microstructure can be influenced by the tamping effect; this is the successive impact of following particles, therefore leading to more porous structures on the top rather than near the interface with the substrate [115].
Porous Ti coatings by CS from less to high energetic conditions (a–d) [122]
On the other hand, some authors have used materials such as magnesium or aluminum to produce porosity. Sun et al. [116] produced porous titanium coatings spraying Mg + Ti powders onto titanium, where the magnesium behaved as a space holder and is eliminated by vacuum sintering. Plasma-sprayed porous titanium coatings usually exhibit irregular porosity distribution and the pores are not well interconnected, while other methods such as sintering titanium beads or fibers have relatively low porosity (<37 %) and low cohesive and/or bond strength. By contrast, CS coatings by Mg + Ti resulted in an average porosity of 48.6 % and pore sizes in the range of 70–150 μm. Bending modulus and compressive modulus of porous titanium coating were close to the bone and thus may be beneficial to reducing stress shielding. Qiu et al. used aluminum as a porogen to form porous titanium coatings [117], which was removed after spraying by alkaline leaching. Considering all tests, the average pore size was between 74 and 91 μm and the pore percentage between 48 and 66 %. Figure 8 shows the porous morphologies and cross section of both studies with pore sizes of 50–150 and 70–150 µm, respectively.
Furthermore, well-adhered, thick, and homogeneous titanium coatings have been also produced onto PEEK biopolymer without its degradation, with the aim to enhance PEEKs biocompatibility for implant applications [118]. This responds to the new emerging use of PEEK as a novel alternative within the biomedical field. Table 5 shows the CS spray conditions of metal coatings used for biocoatings. Spraying onto UHMWPE has also been produced with the aim to avoid having the polyethylene liner and the acetabular cup as two separate components. In such a way, the rough titanium shell and the polymer contacting the femoral head can be achieved within the same component; this was obtained through proper surface activation before spraying [119].
As the microstructure of feedstock powders is maintained in the coatings via CS, it is possible to obtain fined–grain coatings, which might be beneficial in biomedical field. For example, Al-Mangour et al. [120] performed mechanical and corrosion properties in stents coated by fine grain powders; they used a mixture of L605 cobalt–chromium (Co–Cr) alloy and 316-L SS onto mild steel, where it was observed that the addition of cobalt powders helped obtain dense coatings. A heat treatment improved then the densification and porosity reduction as well as a significant increase of ductility; although in vivo and in vitro tests are still pending, the Co–Cr alloy showed a lower corrosion rate than pure SS, making it suitable for the development of a new class of metallic biomaterials.
Other attempts were done with Tantalum (Ta). CS, as it works with low temperatures, is being studied to produce Ta coatings where it is observed good interface adhesion, low porosity, and increase of hardness [121].
Finally, metal coatings have also been produced by CS for bone fracture fixation systems in order to prevent bonding or one or more types of corrosion between the metallic fastener and the metallic bone plate [122]. Where the components of an internal fixation device subsequently bond together, the surgeon may have extreme difficultly in disengaging one component from the other, such as disengaging a bone screw from within an opening in a bone plate. The bonding may prevent the separation of the components, and therefore it can result in injuries due to the prevention of the components being removed from the patient. This patented procedure comprises a cold-sprayed metallic coating either within the opening or on the metallic fastener. The cold-sprayed metallic coating comprises a biocompatible metallic material having a third composition that is different than the first and second compositions.
Ceramic-based biocoatings
Specifically, bioactive ceramic coatings highlight their direct bond to living tissues when implanted. Looking for fixation, bioactive fixation forms a bond with higher strength than mechanical fixation. Nevertheless, TiO2 coatings are currently investigated by CGS despite its good mechanical properties and biocompatibility. Kilemann et al. [123] studied the formation of TiO2 particles onto metallic substrates. TiO2 particles interact as solid spheres with the substrate bonding in a ring-like zone. Particles break into small remnants and remain in the bonding zones. Only if substrate material is brought to the surface and is available to bind other particles, a second layer or parts of it are likely to be attached to the coating on impact. Salim et al. [124] proposed a novel synthesis of TiO2 powders for CS in which makes it possible the deposition of those particles by CS and the growing up of a layer without the addition of binder, but onto Cu not in biocompatible material. Nevertheless, investigations are currently running out.
HA biocoatings
Previously, the advantages of CS over conventional thermal spray processes have been mainly associated to high-temperature-related features. HA coatings have been found to promote fast and enhanced fixation strength, but the long-term stability of the fixation has been reported to still be a challenge in TS techniques; for this main reason, CS is proposed as an alternative to produce HA coatings with high density and controlled crystallinity. In front of other low-temperature processes such as sol–gel, biomimetic deposition, solution deposition, electrochemical deposition, and atomic layer deposition, HA cold spray technique highlights for its simple and economic process of producing coatings at low temperatures being able to control coatings’ microstructure.
Despite the common sense that HA particles bombardment is like blasting the metal surface of the implant, some approaches have been applied in this direction [125–129] and even more successful by dealing with a shot-penning route [130]. Cold Gas Spray of ceramics has been actually compared to other low-temperature powder-based dry manufacturing processes, i.e., aerosol deposition (AD), sometimes known as vacuum cold spray (VCS) and nanoparticle deposition system (NPDS), which appeared in the 1990s and 2000s, respectively. AD is based on the acceleration of submicrometer particles, but low-vacuum conditions are necessary to control the supersonic flow. In NPDS, the source of bonding is attributed to the dissipation of the kinetic energy of the particles. The use of submicrometer feedstock particles seems to be also important, and some plasticity features have been revealed [131–133]. Dense HA coatings have been deposited on titanium by this method [134, 135].
Different numerical and simulation studies have been developed to come upon optimal conditions for cold spraying of spraying HA. Zhang et al. [136] studied the factors influencing HA particle acceleration using a computational fluid dynamics program FLUENT. The simulation results showed that the HA particle is accelerated by the combination of throat diameter and exit diameter whose expansion ratios lie within the optimal range of 1.5–4. HA particle velocity increases with the increasing of gas pressure notably from 0.2MPA (150 mm/s) to 0.6 MPa (360 mm/s) and with the decrease of HA particle size until a minimum of 5 μm, where it decelerates steeply, being 5–20 μm particle size suitable for spray with CS. The taguchi method was used by Singh [137] to optimize HA conditions in CS; they calculated the percentage contribution of all factors on exit particle velocity of HA powder, being as follows in descending order: gas type > particle diameter > gas inlet pressure > particle temperature > gas inlet temperature. Moreover, they observed that the combination of those parameters can alter the result [138]; the increase of gas pressure and particle temperature was found to increase the particle velocity, while the increase of HA particle diameter was found to decrease the particle velocity and its influence was found to be more than respective influences of gas pressure, gas temperature, and particle temperature. Therefore, HA particle velocity is inversely proportional to particle size, despite the increase of gas pressure and gas temperature.
Recent investigations concern biodegradable implants and biocompatible coatings on implant materials, for example, magnesium-based alloys. Despite its excellent properties, magnesium-based alloys have not seen tangible applications in biomedical field industry. To date, they have been studied within the development of cardiovascular stents, bone fixation material, and porous scaffolds for bone repair. Nevertheless, the main limitation to the medical application is their rapidly and localized corrosion behavior. In order to control the degradation rates, it is useful to coat with HA. APS studies have not been developed for its high temperatures that could melt magnesium substrate and decompose HA in other calcium phosphate phases, and the crystallinity of HA may also be lowered due to rapid solidifications. CS has offered solution to both problems [139].
On the other hand, pure HA coatings have been produced on PEEK substrates by CS, therefore providing bioactivity to a material that avoids the stress shielding phenomenon normally occurring between a metallic material and the bone and the weak mechanical properties of ceramic substrates [140]. Coating polymeric biomaterials with calcium phosphate is also one of the most effective methods to enhance biocompatibility. However, calcium phosphate ceramic coatings necessitate a heat treatment at a high temperature in order to induce crystallization of the coating layer, or necessitate a cost-consuming vacuum deposition method for low-temperature crystallization in order to control/obtain other calcium phosphate phases. In the case of polymeric biomaterials, a heat treatment at a high temperature brings about deformation of polymers, and such deformation eventually deteriorates the performance of polymers, preventing the polymers from being used as biomaterials. Furthermore, a vacuum deposition method at a low temperature may also damage the surfaces of polymers, causing deformation, and requires high production cost to increase productivity, which is not preferable. CS overcomes the limitations of various conventional coating methods and enables coating of the surfaces of polymeric biomaterials while maintaining the intrinsic properties of both the powder and the polymer, with low production cost and high productivity. This patent includes as bioactive coatings HA, bioglass compounds such as bioglasses containing CaO, SiO2, and P2O5 as main ingredients, and crystallized bioglasses, and mixtures thereof [140]. Lee et al. [141] also evaluated the bioactivity of HA coatings on PEEK substrates by CS; these proved to be homogeneous and strongly adhered without any deformation of the substrate material.
HA-composite biocoatings
Due to the intrinsic brittle nature of ceramics, a direct deposition of a uniform layer with proper adhesion is still a challenge via CS, especially onto the typical metallic prosthesis, i.e., titanium and SS, on account of the inelastic deformation that ends in failure fragmentation. This has already been observed by the few studies reported in the previous section. For a better understanding of this behavior, lots of studies are being carried out on the investigation of failure mechanisms of ceramics at dynamic impacts [142, 143]. Significant efforts are thus addressed in the direction of using metal–ceramic and polymer–ceramic composite powders. Some works deal with HA–Ti mixtures [144, 145]. The results showed that, compared to pure Ti coating, cold-sprayed HA/Ti composite coating exhibits higher corrosion current and lower corrosion resistance. However, a post-spray heat treatment can improve the corrosion property of HA/Ti composite coating remarkably. In addition, the mechanical properties such as microhardness and ultimate shear strength of cold-sprayed 20wt% HAP/Ti composite coating also improved up to three times by a post-spray heat treatment process. Further, the recrystallization also favored the interfacial bonding and hence improved the mechanical properties [146]. Choudhuri et al. [147] also demonstrated that HA–Ti mixture powders can be cold sprayed achieving a better bond strength (24.45 MPa) than APS (~10–15 MPa); two different titanium powders were used in those mixtures: a vacuum atomized commercial pure Ti (Cp-Ti) and a sponge titanium powder both from a particle size ~45 μm.
Cp-Ti showed difficulties to build up the coating by encapsulating HA particles, whereas the use of sponge Ti powder was more effective. The maximum incorporation of HA was of 20 %; above that percentage, it was found that HA particles got crushed into fragments due to high impacts.
As reported before, aluminum powders have been used as a porogen, in combination with titanium, to achieve porous titanium coatings with higher interconnected macroporosity and larger specific surface area; in order to make these coatings bioactive, HA was added to facilitate bone cell attachment and ingrowth, leading to outstanding in vitro HA mineralization, although long-term studies are required [117]. Such authors used two types of HA, a crystalline and an amorphous calcium phosphate nanocrystalline HA (NC-HA), where it could be observed that NC-HA reaches a maximum Ca2+ mineralization efficiency promoting an early bone fixation.
Other attempts in the case of cold-sprayed HA-composite coatings include HA–graphene nanohseet (GN) [148], with the aim to avoid the concerns related to its long-term performance, i.e., the intrinsic brittleness and low fracture toughness of HA, and doping HA with silver [149], with the advantages that silver involves. The addition of graphene has been proved to be very suitable for load-bearing applications, exhibiting a very reasonable biocompatibility as well; it was even embedded in HA matrix and plastic deformation of certain nano HA particles was revealed. The GN-containing HA coatings markedly enhanced attachment and proliferation of the osteoblast cells, which is most likely attributed to fast adsorption of key serum proteins like fibronectin with elongated stretching conformation on GN. Table 6 shows different cases of CS conditions for biomedical applications.
Clinical performance
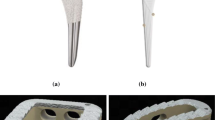
This is a very novel topic and since many few researchers have optimized their coating systems, not many in vitro and in vivo results exist within the literature. In vitro performance can be evaluated by the evaluation of morphological changes of coatings after immersion in SBF. Qiu et al. reported the formation of clusters of fine precipitates for their HA–Ti porous coatings, with similar calcium mineralization efficiencies when using either crystalline HA or amorphous nanocrystalline HA [117]. In addition, these authors used the human osteosarcoma-derived SaOS-2 line with the aim to evaluate the cytotoxicity; cell viabilities after 48 h proved that neither of the coatings was cytotoxic. On the other hand, Gardon et al. [150] studied the differentiation and proliferation of cultured trabecular bone of Ti coatings onto PEEK obtaining a better biological response from Ti than PEEK from 3 days of culture, although optimal properties were shown with nanostructured titanium dioxide. Lee et al. [136] performed similar studies with cultured Human bone marrow mesenchymal stem cells hBMSCs (Human Bone Marrow Stromal Osteoprogenitor Cells) on HA–CS-coated PEEK samples. The HA coating facilitated the differentiation and proliferation of cultured hBMSCs and promoted bone fusion with the surrounding iliac bone without the presence of any fibrous layer. Figure 9 illustrates some in vivo results showing an association of the cylinders with the bone tissue improved as the recovery period in HA-coated PEEK disk group was increased. In contrast, the association of the cylinders with the bone tissue decreased for the animals implanted with the bare PEEK cylinder. Noorakma et al. [139] deposited an HA layer onto magnesium alloy substrate and demonstrated that in vitro behavior and superior cells adherence with numerous cellular micro extensions on porous Ta samples compared to Ti samples clearly suggest that Ta surfaces are biocompatible and cause no inhibition to bone cells (hFOB) adhesion and growth. Presence of relatively high extra cellular matrix (ECM) mineralization on porous Ta samples also indicates that osteoblast cells have started differentiating and ECM remodeling [151]. In vivo, this porous tantalum biomaterial has desirable characteristics for bone ingrowth; further studies are warranted to ascertain its potential for clinical reconstructive orthopedics [152].
In vivo evaluation of bare PEEK and HA-PEEK at 4 weeks (a) and 8 weeks (b) [141]
The addition of graphene to HA coatings significantly enhanced the attachment and proliferation of human osteoblast cells, which is most likely attributed to fast adsorption of key serum proteins like fibronectin with elongated stretching conformation on graphene [143].
Antibacterial/antimicrobial coatings
Although the use of titanium and its alloys in biomedicine is still important, the infection around the implants remains as a concern. Infection not only makes the patients suffer serious damage, but bacterial infection after implant placement can cause significant complications thereby increasing medical cost. The paradigm of bacterial attachment and proliferation on surfaces was first recognized in the 1930s. It was established that bacteria prefer to colonize a solid substrate than living in a planktonic state. The creation of antibacterial surfaces seeks to repel or resist the initial attachments of bacteria by either exhibiting an antibiofouling effect or by inactivating any cells coming into contact with the surface. Antibacterial surfaces can be divided in two groups: (i) antibiofouling surfaces that may resist or prevent cellular attachment due to the presence of an unfavorable surface topography or surface chemistry and (ii) bactericidal surfaces that disrupt the cell on contact, causing cell death. The CS process has also emerged as a promising process to functionalize surfaces in such way.
The use of inorganic antimicrobial agents has attracted interest for its improved safety and stability versus organic antimicrobial agents. There has been a great development during recent years in antibacterial coatings, but they are not still clinically much used; however, more developments and investigations are being explored to achieve both excellent tissue integration ability and good antibacterial properties [153]. Silver (Ag) has already been highlighted as an antibacterial material. The combination of bioactivity (HA) and antibacterial properties (Ag) has been previously reported, and the results indicated that the antibacterial activity increased with increasing HA–Ag nanopowder concentrations [144]. Alternatively, ceramic powder of zinc oxide (ZnO), calcium oxide (CaO), and magnesium oxide (MgO) has found antibacterial activity. Combinations of ZnO/Ti powders with different ratios have been performed to produce composite-coated implants [154]; the results show that the viability of cells on ZnO20/Ti80 was higher than that on ZnO50/Ti50 and ZnO80/Ti20 samples, thus proving that the cell viability decreased with increasing ZnO concentration in the coating composition. On the other hand, the bactericidal effect of TiO2 coatings has also been extensively studied; specifically, CS anatase coatings were investigated by Kliemann et al. [123]. A kill rate of 99.99 % was obtained after 5 min of exposition of the bacteria Pseudomonas Aeruginosa to UV light with a peak intensity of 360 nm. Certain stagnation of the decay of the bacteria was found, which could be attributed to non-coated areas present due to the impossibility of covering all the surface of the substrate by means of anchoring TiO2 particles. Other coatings that are committed to antibacterial properties thank to ZnO are made of Novaron VZ 600 (a commercial available inorganic antimicrobial powder made from glass, with the functional material being ZnO) onto Ti [155]. Those studies were developed to analyze the differences among surfaces using different processing pressures and analysis of the antimicrobial with CS due to the low heat powder resistance. Results have shown that S.Areus cells on samples decreased after 24-h culture, even on non-coated plates. Two possibilities were reported: (i) Roughness can contribute to antimicrobial ability and (ii) medium concentration may have been too low for this bacterium.
Moreover, antibacterial coatings not only focus on orthopedic and implant applications, but also in touch surfaces where there is certain risk of infection. Metals like copper (Cu) have been employed for this purpose. In the case of copper, its antibacterial activity not only comes from itself but also the utilized technique. The specific mechanism by which copper affects cellular structures is not yet proven, but the active agent of cell destruction is generally considered to be the copper ion [156]. Since CS involves high strain rates which lead to extreme work hardening and high dislocation density within the deposit, it causes an increase of ion diffusion through the grain dislocations leading to microbial destruction [156]. Champagne et al. [156] produced copper surfaces onto aluminum using three thermal spray methods: plasma spray, wire spray, and CS, in order to analyze the microbiologic differences and decrease the risk of infection of bacterial contamination on touch surfaces such as hospital table. CS produced the minimum percentage of MRSA (Methicillin-Resistant Staphylococcus Aureus) due to the high number of dislocations within the coating.
Other attempts were performed with aluminum powder as ductil metal for blend antibacterial powders such as chitosan–Cu [157] and ZnO [158]. The use of aluminum is cause for a number of cosmetics used, repair, corrosion, and protection applications, also for its low density that could be accelerated to very high velocities in CS and the available commercial variety of composition of Al powders. Table 7 summarizes the CS spraying conditions used for the antibacterial coatings referenced within this section.
Summary
All the above coating systems try to satisfy the main requirements for a biocoating, either in biological (biocompatibility and bioactivity), mechanical, or antibacterial terms. Terms like “structural design” and “deposition techniques” are involved in the development of the fabrication process to obtain cost-efficient products making it commercially reproducible and attainable to all types of markets. From this point of view, it is worth taking into consideration the valuable advantages that CS has to offer non-microstructural changes from feedstock powder, high deposition efficiency, low temperature rates, and overcoating the wide range of materials that could be applied. Day-by-day constant work and research demonstrate CS as a new technique to produce coatings.
However, still a big step has to be taken in order to translate the experimental studies to the real market. More studies in vitro and in vivo from CS technique are required, and the addition of antibacterial components must be performed as a necessity upturn in human health.
References
Gaona M (2007) Recubrimientos biocompatibles obtenidos por Proyección Térmica y estudio in vitro de la función osteoblástica. PhD thesis. Universitat de Barcelona
Hench LL (1998) Biomaterials: a forecast for the future. Biomaterials 19:1419–1423
Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (2013) Introduction—biomaterials science: an evolving, multidisciplinary endeavor. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE (eds) biomaterials science, 3rd edn. Academic Press, London
Ramakrishna S, Mayer J, Wintermantel E, Leong KW (2001) Biomedical applications of polymer-composite materials: a review. Compos Sci Technol 61:1189–1224
William DF (1987) Consensus and definitions in biomaterials. In: de Potter C, de Lange K, de Groot K, Lee AJC (eds) Advances in biomaterials. Elsevier, Amsterdam, pp 11–16
Cao W, Hench LL (1996) Bioactive materials. Ceram Inter 22:493–507
Jones J, Clare A (2012) Bio-glasses: an introduction. Wiley, Chichester
Kalita S (2008) Nanostructured biomaterials. In: Seal S (ed) Functional nanostructures. Springer, New York
Bronziono JD (2000) The biomedical engineering handbook, vol 1, 2nd edn. CRC Press LLC, Boca Raton
Salinas AJ, Vallet-Regi M (2013) Bioactive ceramics: from bone grafts to tissue engineering. RSC Adv 3:11116–11131
Katti KS (2004) Biomaterials in total joint replacement. Colloid Surf B 39:133–142
Bauer S, Schmuki P, von der Mark K, Park J (2013) Engineering biocompatible implant surfaces: Part I: materials and surfaces. Prog Mater Sci 58:261–326
Khan SP, Auner GG, Newaz GM (2005) Influence of nanoscale surface roughness on neural cell attachment on silicon. Nanomed Nanotechnol Biol Med 1:125–129
Yun H-S, Park J-W, Kim S-H, Kim Y-J, Jang J-H (2011) Effect of the pore structure of bioactive glass balls on biocompatibility in vitro and in vivo. Acta Biomater 7:2651–2660
Singhatanadgit W (2009) Biological responses to new advanced surface modifications of endosseous medical implants. Bone Tissue Regen Insights 2:1–11
Lin L, Wang H, Ni M, Rui Y, Cheng T-Y, Cheng C-K (2014) Enhanced osteointegration of medical titanium implant with surface modifications in micro/nanoscale structures. J Orthop Transl 2:35–42
Yang YZ, Tian JM, Tian JT, Chen ZQ, Deng XJ, Zhang DH (2000) Preparation of graded porous titanium coatings on titanium implant materials by plasma spraying. J Biomed Mater Res 52:333–337
Endres S, Wilke M, Knöll P, Frank H, Kratz M, Wilke A (2008) Correlation of in vitro and in vivo results of vacuum plasma sprayed titanium implants with different surface topography. J Mater Sci Mater Med 19:1117–1125
Borsari V, Giavaresi G, Fini M, Torricelli P, Tschon M, Chiesa R (2005) Comparative in vitro study on a ultra-high roughness and dense titanium coating. Biomaterials 26:4948–4955
Chen Y, Zheng X, Ji H, Ding C (2007) Effect of Ti–OH formation on bioactivity of vacuum plasma sprayed titanium coating after chemical treatment. Surf Coat Technol 202:494–498
Jaeggi C, Mooser R, Frauchiger V, Wyss P (2009) 3D characterization of open porous vacuum plasma sprayed titanium coatings by means of high resolution micro computer tomography. Mater Lett 63:2643–2645
Kinos T, Chen SL, Siitonen P, Kettunen P (1996) Densification of plasma-sprayed titanium and tantalum coatings. JTST 5:439–444
Stanisic J, Kosikowsky D, Mohanty PS (2005) High temperature erosion behavior of thermal sprayed tantalum. In: Sudarshan TS, Stiglich JJ (eds). Proceedings of the 19th International Conference on Surface Modification Technologies, pp 28–33. ASM International, Materials Park
Balla VK, Bodhak S, Bose S, Bandyopadhyay A (2010) Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater 6:3349–3359
Matson DW, Merz MD, McClanahan ED (1992) High rate sputter deposition of wear resistant tantalum coatings. J Va Sci Technol A 10:1791–1796
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Knetsch MLW, Koole LH (2011) New strategies in the development of antimicrobial coatings: the example of increasing usage of silver and silver nanoparticles. Polymer 3:340–366
Melaiye A, Youngs WJ (2005) Silver and its application as an antimicrobial agent. Expert Opin Ther Patents 15:125–130
Schierholz JM, Lucas LJ, Rump A, Pulverer G (1998) Efficacy of silver-coated medical devices. J Hosp Infect 40:257–262
Gosheger G, Hardes J, Ahrens H, Streitburger A, Buerger H, Erren M et al (2004) Silver-coated megaendoprostheses in a rabbit model—an analysis of the infection rate and toxicological side effects. Biomater 25:5547–5556
Tobin EJ, Bambauer R (2003) Silver coating of dialysis catheters to reduce bacterial colonization and infection. Ther Apher Dial 7:504–509
Gray JE, Norton PR, Alnouno R, Marolda CL, Valvano MA, Griffiths K (2003) Biological efficacy of electroless-deposited silver on plasma activated polyurethane. Biomaterials 24:2759–2765
Dowling DP, Donnelly K, McConnell ML, Eloy R, Arnaud MN (2001) Deposition of anti-bacterial silver coatings on polymeric substrates. Thin Solid Films 398–399:602–606
Bosetti M, Massè A, Tobin E, Cannas M (2002) Silver coated materials for external fixation devices: in vitro biocompatibility and genotoxicity. Biomaterials 23:887–892
Noda I, Miyaji F, Ando Y, Miyamoto H, Shimazaki T, Yonekura Y et al (2009) Development of novel thermal sprayed antibacterial coating and evaluation of release properties of silver ions. J Biomed Mater Res Part B 89B:456–465
Ando Y, Miyamoto H, Noda I, Sakurai N, Akiyama T, Yonekura Y et al (2010) Calcium phosphate coating containing silver shows high antibacterial activity and low cytotoxicity and inhibits bacterial adhesion. Mater Sci Eng C 30:175–180
Miola M, Ferraris S, Di Nunzio S, Robotti PF, Bianchi G, Fucale G et al (2009) Surface silver-doping of biocompatible glasses to induce antibacterial properties. Part II: plasma sprayed glass-coatings. J Mater Sci Mater Med 20:741–749
Li B, Liu X, Meng F, Chang J, Ding C (2009) Preparation and antibacterial properties of plasma sprayed nano-titania/silver coatings. Mater Chem Phys 118:99–104
Li B, Liu X, Cao C, Meng F, Dong Y, Cui T et al (2008) Preparation and antibacterial effect of plasma sprayed wollastonite coatings loading silver. Appl Surf Sci 255:452–454
Zheng X, Chen Y, Xie Y, Ji H, Huang L, Ding C (2009) Antibacterial property and biocompatibility of plasma sprayed hydroxyapatite/silver composite coatings. J Therm Spray Technol 18:463
Fernández J, Gaona M, Guilemany JM (2004) Tribological study of plasma hydroxyapatite coatings. Key Eng Mater 254–256:383–386
Heimann RB, Vu TA (1997) Low-pressure plasma-sprayed (LPPS) bioceramic coatings with improved adhesion strength and resorption resistance. J Therm Spray Technol 6:145–149
Sun L, Berndt CC, Gross KA, Kucuk A (2001) Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res 58:570–592
Tsui YC, Doyle C, Clyne TW (1998) Plasma sprayed hydroxyapatite coatings on titanium substrates. Part 1: mechanical properties and residual stress levels. Biomater 19:2015–2029
Fernández J, Gaona M, Guilemany JM (2007) Effect of heat treatments on HVOF hydroxyapatite coatings. J Therm Spray Tech 16:220–228
Lima RS, Khor KA, Li H, Cheang P, Marple BR (2005) HVOF spraying of nanostructured hydroxyapatite for biomedical applications. Mater Sci Eng A 396:181–187
Khor K, Li H, Cheang P (2003) Processing–microstructure–property relations in HVOF sprayed calcium phosphate based bioceramic coatings. Biomater 24:2233–2243
Cho JS, Kang YC (2008) Nano-sized hydroxyapatite powders prepared by flame spray pyrolysis. J Alloys Compd 464:282–287
Melero H (2014) Recubrimientos biocompatibles de Hidroxiapatita-Titania obtenidos mediante Proyección Térmica de Alta Velocidad (HVOF). PhD thesis. Universitat de Barcelona
Pajares López M, Hernández Cortés P, Peregrinaf Palomares M, Hernández Hernández MA (1998) Vástagos cementados y no cementados en artroplastias totales de cadera por coxopatías mecánicas. Rev Esp Cir Osteoartic 33:59–65
Chern Lin JH, Liu ML, Ju CP (1994) Structure and properties of hydroxyapatite-bioactive glass composites plasma sprayed on Ti6Al4V. J Mater Sci Mater Med 5:279–283
Wheeler DL, Montfort MJ, McLoughlin SW (2001) Differential healing response of bone adjacent to porous implants coated with hydroxyapatite and 45S5 bioactive glass. J Biomed Mater Res 55:603–612
Floroian L, Popescu A, Servan N, Mihailescu Ion N. Polymer-bioglass composite coatings: a promising alternative for advanced biomedical implants. http://cdn.intechopen.com/pdfs-wm/16716.pdf. Accessed 11 April 2015
Cai F, Miyata C, Huang X, Yang Q (2014) Microstructure, bioactivity and wear resistance of sintered composite Co-Cr-Mo/Bioglass((R)) for medical implant applications. Int J Surf Sci Eng 8:264–281
Pourhashem S, Afshar A (2014) Double layer bioglass-silica coatings on 316L stainless steel by sol-gel method. Ceram Int 40:993–1000
Ananth KP, Suganya S, Mangalaraj D, Ferreira JMF, Balamurugan A (2013) Electrophoretic bilayer deposition of zirconia and reinforced bioglass system on Ti6Al4V for implant applications: an in vitro investigation. Mater Sci Eng C 33:4160–4166
Wang DG, Chen CZ, Jin QP, Li HC, Pan YK (2014) HA/bioglass composite films deposited by pulsed laser with different substrate temperature. Appl Phys A 114:897–902
Sun L, Berndt CC, Khor KA, Cheang HN, Karlis A (2002) Gross, Surface characteristics and dissolution behavior of plasma-sprayed hydroxyapatite coating. J Biomed Mater Res 62:228–236
Formin AA, Steinhauer AB, Lyasnikov VN, Wenig SB, Zakharevich AM (2012) Nanocrystalline structure of the surface layer of plasma-sprayed hydroxyapatite coatings obtained upon preliminary induction heat treatment of metal base. Tech Phys Lett 38:481–483
Kurzweg H, Heimann RB, Troczynski T, Wayman ML (1998) Development of plasma-sprayed bioceramic coatings with bond coats based on titania and zirconia. Biomaterials 19:1507–1511
F1609-08 (2014) Standard Specification for calcium phosphate Coatings for Implantable Materials
ISO Standard 13779-1:2008 Implants for surgery—Hydroxyapatite—Part 1: Ceramic hydroxyapatite
ISO Standard 13779-1:2008 Implants for surgery—Hydroxyapatite—Part 2: Coatings of hydroxyapatite
ISO Standard 13779-1:2008 Implants for surgery—Hydroxyapatite—Part 3: Chemical analysis and chartacterization of crystallinity and phase purity
ASTM F 2068-00 Standard Specification for Femoral Prostheses—Metallic Implants
Mohseni E, Zalnezhad E, Bushroa AR (2014) Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: a review paper. Int J Adhes Adhes 48:238–257
Kuo MC, Yen SK (2002) The process of electrochemical deposited hydroxyapatite coatings on biomedical titanium at room temperature. Mater Sci Eng C 20:153–160
Yoshimura M, Byrappa K (2008) Hydrothermal processing of materials: past, present and future. J Mater Sci 43:2085–2103. doi:10.1007/s10853-007-1853-x
Darr JA, Guo ZX, Raman V, Bououdina M, Rehman IU (2004) Metal organic chemical vapour deposition (MOCVD) of bone mineral like carbonated hydroxyapatite coatings. Chem Commun 2004:696–697
Li H, Khor KA, Cheang P (2002) Titanium dioxide reinforced hydroxyapatite coatings deposited by high velocity oxy-fuel (HVOF) spray. Biomaterials 23:85–91
Melero H, Fargas G, Garcia-Giralt N, Fernández J, Guilemany JM (2014) Mechanical performance of bioceramic coatings obtained by high-velocity oxy-fuel spray for biomedical purposes. Surf Coat Technol 242:92–99
Melero H, Torrell M, Fernández J, Gomes JR, Guilemany JM (2013) Tribological characterization of biocompatible HAp-TiO2 coatings obtained by high velocity oxy-fuel spray. Wear 305:8–13
Bhadang KA, Gross KA (2004) Influence of fluorapatite on the properties of thermally sprayed hydroxyapatite coatings. Biomaterials 25:4935–4945
Gu YW, Khor KA, Pan D, Cheang P (2004) Activity of plasma sprayed yttria stabilized zirconia reinforced hydroxyapatite/Ti–6Al–4V composite coatings in simulated body fluid. Biomaterials 25:3177–3185
Fathi MH, Azam F (2007) Novel hydroxyapatite/tantalum surface coating for metallic dental implant. Mater Lett 61:1238–1241
Roy M, Fielding GA, Beyenal H, Bandyopadhyay A, Bose S (2012) Mechanical, in vitro antimicrobial, and biological properties of plasma-sprayed silver-doped hydroxyapatite coating. ACS Appl Mater Interfaces 4:1341–1349
Yonekura Y, Miyamoto H, Shimazaki T, Ando Y, Noda I, Mawatari M et al (2011) Osteoconductivity of thermal-sprayed silver-containing hydroxyapatite coating in the rat tibia. J Bone Jt Surg 93B:644–649
Shimazaki T, Miyamoto H, Ando Y, Noda I, Yonekura Y, Kawano S et al (2010) In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. J of Biomed Mater Res Part B 92B:386–389
Balani K, Anderson R, Laha T, Andara M, Tercero J, Crumpler E et al (2007) Plasma-sprayed carbon nanotube reinforced hydroxyapatite coatings and their interaction with human osteoblasts in vitro. Biomaterials 28:618–624
Green SM, Schlegel J (2001) A polyaryletherketone biomaterial for use in medical implant applications. Polym for the Med Ind Proc, Brussels, 14–15 May 2001, pp 1–7
Li H, Zou X, Woo C, Ding M, Lind M, Bünger C (2007) Experimental lumbar spine fusion with novel tantalum-coated carbon fiber implant. J Biomed Mater Res Part B 81B:194–200
Ma R, Tang T (2014) Current strategies to improve the bioactivity of PEEK. Int J Mol Sci 15:5426–5445
Roeder RK, Conrad TL (2012) Bioactive polyaryletherketone composites. In: Kurtz SM (ed) PEEK biomaterials handbook. William Andrew Publishing, Oxford, pp 163–179
Riner M, Roth A, Brandsberg F, Wintermantel E, Mayer J. Development of a human hip endoprosthesis stem made by injection molding of carbon fiber reinforced PEEK. http://www.iccm-central.org/Proceedings/ICCM13proceedings/SITE/PAPERS/paper-1409.pdf
Brydone AS, Meek D, Maclaine S (2010) Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering Proceedings of the Institution of Mechanical Engineers, Part H. J Eng Med 224:1329–1343
Furlong R, Osborn J (1991) Fixation of hip prostheses by hydroxyapatite ceramic coatings. J Bone Jt Surg 73B:741–745
Jaffe W, Scott D (1999) Total hip arthroplasty with hydroxyapatite-coated prostheses. In: Imura S, Wada M, Omori H (eds) Joint arthroplasty. Springer, Tokyo, pp 159–187
Pramanik S, Agarwak AK, Rai KN (2005) Chronology of total hip joint replacement and materials development. Trends Biomater Artif Organs 19:15–26
Cross MJ, Parish EN (2005) A hydroxyapatite-coated total knee replacement. J Bone Jt Surg Br 87B:1073–1076
Manley MTC, D’Aantonio WN, Edin JA, Geesink JA, Rudolph GT (1998) Fixation of acetabular cups without cement in total hip arthroplasty. A comparison of three different implant surfaces at a minimum duration of follow-up of five years*. J Bone Jt Surg 80:1175–1185
Nilsson KG, Kärrholm J, Carlsson L, Dalén T (1999) Hydroxyapatite coating versus cemented fixation of the tibial component in total knee arthroplasty: prospective randomized comparison of hydroxyapatite-coated and cemented tibial components with 5-year follow-up using radiostereometry. J Arthroplast 14:9–20
Zhang S (2011) Biological and biomedical coatings handbook, vol 2. Taylor & Francis, Boca Raton
Davis JR (2004) Introduction to thermal spray processing. In: Davis JR (ed) Handbook of thermal spray technology. ASM International, Materials Park, pp 3–13
Davis JR (2004) Thermal spray processes. In: Davis JR (ed) Handbook of thermal spray technology. ASM International, Materials Park, pp 54–76
Fernández J, Guilemany JM, Gaona M (2005) La proyección térmica en la obtención de recubrimientos biocompatibles: ventajas de la proyección térmica por alta velocidad (HVOF) sobre la proyección térmica por plasma atmosférico (APS). Biomechanics 13:16–39
Kang AS, Singh G, Chawla V (2013) Some problems associated with thermal sprayed ha coatings: a review. Int J Surf Eng Mater Surf Eng Mater Technol 3:10–20
Villa M, Dosta S, Fernández J, Guilemany JM (2012) La proyección fría (CGS): Una alternativa a las tecnologías convencionales de deposición. Rev Metal 48:175–191
Champagne Victor K (2007) The cold spray materials deposition process: fundamentals and applications. Woodhead, Cambridge
Ghelichi R, Guagliano M (2009) Coating by the cold spray process: a state o the art. Fratt Integr Strutt 8:30–44
Singh HR, Sidhu TS, Kalsi SBS (2012) Cold spray technology: future of coating deposition processes. Frat Integr Strutt 22:69–84
Morgan R, Fox P, Pattison J, Sutcliffe C, O’Neill W (2004) Analysis of cold gas dynamically sprayed aluminium deposits. Mater Lett 58:1317–1320
Suhonen T, Varis T, Dosta S, Torrell M, Guilemany JM (2013) Residual stress development in cold sprayed Al, Cu and Ti coatings. Acta Mater 61:6329–6337
Champagne VK, Helfritch DJ (2014) Mainstreaming cold spray—push for applications. Surf Eng 30:396–403
Moridi A, Hassani-Gangaraj SM, Guagliano M, Dao M (2014) Cold spray coating: review of material systems and future perspectives. Surf Eng 30:369–395
Richer P et al (2005) Effect of particle geometry and substrate preparation in cold spray. ITSC 2005 “Thermal spray connects: explore its surfacing potential!” Basel, Switzerland, pp.193–199
Makinen H, Langeborn J, Vuoristo P (2007) Adhesion of cold sprayed coatings: effect of powder, substrate and heat treatment. In: Marple BR, Hyland MM, Lau Y, Lia C, Lima RS, Montavon G (eds) Thermal spray global solutions. ASM International, Materials Park, pp 31–36
Sakaki K, Tajima K, Li H, Shinkai S, Shimitzu Y (2004) Influence of substrate conditions and traverse speed on cold sprayed coatings. In: International Thermal Spray Conference 2004: Advances in Technology and Application, Osaka (Japan), pp 358–362. ASM International, Material Park
Wu J, Yang J, Fang H, Yoon S, Lee C (2006) The bond strength of Al–Si coating on mild steel by kinetic spraying deposition. Appl Surf Sci 252:7809–7814
Marrocco T, McCartney DG, Shipway PH, Sturgeon AJ (2006) Production of titanium deposits by cold-gas dynamic spray: numerical modeling and experimental characterization. J Therm Spray Tech 15:263–272
Price TS, Shipway PH, McCartney DG (2006) Effect of cold spray deposition of a titanium coating on fatigue behavior of a titanium alloy. J Therm Spray Tech 15:507–512. doi:10.1361/105996306X147108
Eason PD (2012) A structure property processing comparison of cold rolled pm copper and cold gas dynamically sprayed copper. J Powder Metall Min 1:101
Miguel JM, Vizcaíno S, Dosta S, Cinca N, Lorenzana C, Guilemany JM (2014) Recubrimientos de materiales compuestos metal-cerámico obtenidos por nuevas tecnologías de proyección térmica: Proyección fría (CGS) y su resistencia al desgaste. Rev Metal 47:390–401
Li W-Y, Zhang C, Guo X, Xu J, Li C-J, Liao H et al (2007) Ti and Ti-6Al-4V coatings by cold spraying and microstructure modification by heat treatment. Adv Eng Mater 9:418–423
Wong W, Rezaeian A, Yue S, Irissou E, Legoux J-G (2009) Effect of gas temperature, gas pressure, and particle characteristics on cold sprayed pure titanium coatings, thermal spray 2009. In: Marple BR, Hyland MM, Lau Y-C, Li C-J, Lima RS, Montavon G (eds) Proceedings of the International Thermal Spray Conference. ASM International, Materials Park, pp 231–236
Li C-J, Li W-Y (2003) Deposition characteristics of titanium coating in cold spraying. Surf Coat Technol 167:278–283. doi:10.1016/S0257-8972(02)00919-2
Sun J, Han Y, Cui K (2008) Innovative fabrication of porous titanium coating on titanium by cold spraying and vacuum sintering. Mater Lett 62:3623–3625
Qiu D, Zhang M, Grøndahl L (2013) A novel composite porous coating approach for bioactive titanium-based orthopedic implants. J Biomed Mater Res Part A 101A:862–872
Gardon M, Latorre A, Torrell M, Dosta S, Fernández J, Guilemany JM (2013) Cold gas spray titanium coatings onto a biocompatible polymer. Mater Lett 106:97–99
Guilemany JM, Dosta S, Cinca N, Fernández J, Garcia I. Feasibility of cold gas spraying to produce metal coatings onto activaated polymeric substrates. Thermal Spray Centre (CPT). Intellectual properties protection (iPP). Ref.1240B p 10
Al-Mangour B, Mongrain R, Irissou E, Yue S (2013) Improving the strength and corrosion resistance of 316L stainless steel for biomedical applications using cold spray. Surf Coat Technol 216:297–307
Steenkiste T, Gorkiewicz DW (2004) Analysis of tantalum coatings produced by the kinetic spray process. J Therm Spray Technol 13:265–273
Lozier A, Popoola OO, Mason JJ, Forstein M (2009) Bone fracture fixation system. US Patent 0198286 A1, August 6, 2009
Kliemann J-O, Gutzmann H, Gaertner F, Huebner H, Borchers C, Klassen T (2011) Formation of cold-sprayed ceramic titanium dioxide layers on metal surfaces. J Therm Spray Technol 20:292–298
Salim NT, Yamada M, Nakano H, Fukumoto M (2011) The synthesis of titanium dioxide (TiO2) powder for cold spray process. In: 3rd International Congress on Ceramics (ICC): Novel Chemical Processing Sol-Gel and Solution-Based Processing 18:032019. doi:10.1088/1757-899X/18/3/032019
Ishikawa K, Miyamoto Y, Nagayama M, Asaoka K (1997) Blast coating method: new method of coating titanium surface with hydroxyapatite at room temperature. J Biomed Mater Res 38:129–134
O’Hare P, Meenan BJ, Burke GA, Byrne G, Dowling D, Hunt JA (2010) Biological responses to hydroxyapatite surfaces deposited via a co-incident microblasting technique. Biomaterials 31:515–522
Gbureck U, Masten A, Probst J, Thull R (2003) Tribochemical structuring and coating of implant metal surfaces with titanium oxide and hydroxyapatite layers. Mater Sci Eng C 23:461–465
O’Neill L, O’Sullivan C, O’Hare P, Sexton L, Keady F, O’Donoghue J (2009) Deposition of substituted apatites onto titanium surfaces using a novel blasting process. Surf Coat Technol 204:484–488
O’Sullivan C, O’Hare P, O’Leary ND, Crean AM, Ryan K, Dobson ADW et al (2010) Deposition of substituted apatites with anticolonizing properties onto titanium surfaces using a novel blasting process. J Biomed Mater Res Part B 95B:141–149
Byrne GD, O’Neill L, Twomey B, Dowling DP (2013) Comparison between shot peening and abrasive blasting processes as deposition methods for hydroxyapatite coatings onto a titanium alloy. Surf Coat Technol 216:224–231
Chun D-M, Ahn S-H (2011) Deposition mechanism of dry sprayed ceramic particles at room temperature using a nano-particle deposition system. Acta Mater 59:2693–2703
Chun D-M, Choi J-O, Lee CS, Ahn S-H (2012) Effect of stand-off distance for cold gas spraying of fine ceramic particles (<5 μm) under low vacuum and room temperature using nano-particle deposition system (NPDS). Surf Coat Technol 206:2125–2132
Akedo J (2006) Aerosol deposition of ceramic thick films at room temperature: densification mechanism of ceramic layers. J Am Ceram Soc 89:1834–1839
Hahn B-D, Park D-S, Choi J-J, Ryu J, Yoon W-H, Kim K-H et al (2009) Dense nanostructured hydroxyapatite coating on titanium by aerosol deposition. J Am Ceram Soc 92:683–687. doi:10.1111/j.1551-2916.2008.02876.x
Park D-S, Kim I-S, Kim H, Chou AHK, Hahn B-D, Li L-H et al (2010) Improved biocompatibility of hydroxyapatite thin film prepared by aerosol deposition. J Biomed Mater Res B 94B:353–358. doi:10.1002/jbm.b.31658
Zhang L, Zhang WT (2011) Numerical investigation on particle velocity in cold spraying of hydroxyapatite coating. Adv Mater Res 18:717–722
Singh RP (2011) Numerical evaluation, optimization and mathematical validation of cold spraying of hydroxyapatite using taguchi approach. Inter J Eng Sci Technol 3:7006–7015
Singh RP, Batra B (2013) Effect of cold spraying parameters and their interaction an hydroxyapatite deposition. J Appl Fluid Mech 6:555–561
Noorakma AW, Zuhailawati H, Aishvarya V, Dhindaw BK (2013) Hydroxyapatite-coated magnesium-based biodegradable alloy: cold spray deposition and simulated body fluid studies. J Mater Eng Perform 22:2997–3004
Noh JH, Kim DW, An JS, Chang HR, Kim DH, Hong KS, Chin DK (2012) Method for modifying the surface area of a bioinert material. US Patent 0009341 A1, January 12, 2012
Lee JH, Jang HL, Lee KM, Baek H-R, Jin K, Hong KS et al (2013) In vitro and in vivo evaluation of the bioactivity of hydroxyapatite-coated polyetheretherketone biocomposites created by cold spray technology. Acta Biomater 9:6177–6187
Chen MW, McCauley JW, Dandekar DP, Bourne NK (2006) Dynamic plasticity and failure of high-purity alumina under shock loading. Nat Mater 5:614–618
Mukhopadhyay A, Joshi K, Dey A, Chakraborty R, Rav A, Biswas S et al (2010) Shock deformation of coarse grain alumina above Hugoniot elastic limit. J Mater Sci 45:3635–3651. doi:10.1007/s10853-010-4409-4
Zhou X, Mohanty P (2012) Electrochemical behavior of cold sprayed hydroxyapatite/titanium composite in Hanks’ solution. Electrochim Acta 65:134–140
Shukla V, Elliott G, Kear B, McCandlish L (2001) Hyperkinetic deposition of nanopowders by supersonic rectangular jet impingement. Script Mater 44:2179–2182
Zhou X (2012) Hydroxyapatite/titanium composite coating for biomedical applications. PhD thesis. University of Michigan
Choudhuri A, Mohanty PS, Karthikeyan J (2009) Bio-ceramic composite coatings by cold spray technology. In: Proceedings of the International Thermal Spray Conference, pp 391–96
Liu Y, Dang Z, Wang Y, Huang J, Li H (2014) Hydroxyapatite/graphene-nanosheet composite coatings deposited by vacuum cold spraying for biomedical applications: inherited nanostructures and enhanced properties. Carbon 67:250–259
Sanpo N, Tan M, Cheang P, Khor KA (2009) Antibacterial property of cold-sprayed HA-Ag/PEEK coating. J Therm Spray Tech 18:10–15
Gardon M, Melero H, Garcia-Giralt N, Dosta S, Cano IG, Guilemany JM (2014) Enhancing the bioactivity of polymeric implants by means of cold gas spray coatings, J Biomed Mater Res Part B
Balla VK, Bodhak S, Bose S, Bandyopadhyay A (2010) Porous tantalum structures for bone implants: fabrication, mechanical and in vitro biological properties. Acta Biomater 6:3349–3359
Bobyn JD, Stackpool GJ, Hacing SA, Tanzer M, Krygier JJ (1999) Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J Bone Jt Surg Br 81:907–914
Zhao L, Chu PK, Zhang Y, Wu Z (2009) Antibacterial coatings on titanium implants. J Biomedl Mater Res Part B 91B:470–480
Sanpo N, Hailan C, Loke K, Keng KP, Cheang P, Berndt CC, et al. (2010) Biocompatibility and antibacterial property of cold sprayed ZnO/titanium composite coating. In: Mendez-Vilas A (ed). Science and Technology against Microbial Pathogens. Research, Development and Evaluation. In: Proceedings of the International Conference on Antimicrobial Research, World Scientific pp 140-44
Tamai K, Kawate K, Kawahara I, Takakura Y, Sakaki K (2009) Inorganic antimicrobial coating for titanium alloy and its effect on bacteria. J Orthop Sci 14:204–209
Champagne V, Helfritch D (2013) A demonstration of the antimicrobial effectiveness of various copper surfaces. J Biol Eng 7:1–7
Sanpo N, Ang S, Cheang P, Khor KA (2009) Antibacterial property of cold sprayed chitosan–Cu/Al coating. J Therm Spray Tech 18:600–608
Sanpo N, Saraswati T, Tan Meng Lu, Cheang P (2008) Anti-bacterial property of cold sprayed ZnO–Al coating. In: Proceedings of the 2008 international conference on biomedical engineering and informatics (BMEI 2008), vol 1, pp 488–491
Acknowledgements
The authors wish to thank the Generalitat de Catalunya for the Project 2014 SGR 1558.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vilardell, A.M., Cinca, N., Concustell, A. et al. Cold spray as an emerging technology for biocompatible and antibacterial coatings: state of art. J Mater Sci 50, 4441–4462 (2015). https://doi.org/10.1007/s10853-015-9013-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9013-1