Abstract
β-Cyclodextrin plays a crucial role in augmenting the activity of metal complexes by ameliorating their solubility, stability, and reactivity, therefore the thio-functionalized β-cyclodextrin based amino ligand mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin (4) was synthesized and analyzed by elemental analyses, AAS, UV–visible, FTIR, and 1H NMR spectroscopy. The newly synthesized Fe III) complex was soluble in water. The Fe(III) complex's spectral analysis using FTIR confirmed that the ligand aided the metal ion coordinate by way of the sulfur atom of the β-cyclodextrin moiety and the nitrogen atoms of the two ligand molecules, with two H2O occupying the fifth and sixth coordination sites. Additionally, the mass spectrometry verifies that the intended Fe(III) complex has synthesized. Density functional theory (DFT) was used to calculate different electronic parameters of the optimized structure of Fe(III) complex to reveal its stability. Antimicrobial metal complexes that are suitable for therapeutic application are known to be more stable and bioavailable when β-cyclodextrin is introduced, therefore studies have been done to inquire into the possible comparative in vitro antibacterial activity of the Fe(III) complex and free ligand against two gram positive (Bacillus subtilis, and Staphylococcus aureus) and two gram negative bacteria (Escherichia coli, Klebsiella pneumoniae) strains. Molecular docking was used to further corroborate these capabilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, supramolecular chemistry has drawn more attention from several chemistry disciplines. After presenting their host–guest chemistry conclusions for the first time, Cram, Lehn, and Pedersen were granted the Nobel Prize in 1987 [1,2,3,4,5]. Cyclodextrins are cyclic oligosaccharides consisting a combination of six (α-CD), seven (β-CD), eight (η-CD), or more glucose subunits connected by α-1,4 glycosidic linkages [6, 7]. A non-toxic derivative having a hydrophilic surface and a hydrophobic internal cavity, β-cyclodextrin is the most affordable, most helpful, and easily accessible when compared to other derivatives. The fact that β-CD, functionalized derivatives and its metal complex, can be yielded and applied to a variety of biological applications is the focus of extensive study currently underway in this field [8, 9]. A similar combination of hydrophobicity and molecular rearrangement capabilities is also shown when β-CD is substituted with a ligand at the primary face. On the other hand, other transformations in aqueous media have also been carried out using metal complexes of β-CD. The derivation of β-CD underwent a significant change with the introduction of mono tosylation, which made it possible to couple or derive β-CD at the site of the main hydroxyl group [5, 10].
Transition metal complexes with polydentate ligands have emerged as a major stay of chemistry [11]. In polydentate ligands, donor hetero atoms such as nitrogen, phosphine, oxygen, and sulfur tend to be the most frequent coordinating atoms [12, 13]. Due to the significant roles that nitrogen and sulfur ligands can play in a wide range of metallic biomolecules, chemists worldwide have recently focused their attention on the study of metal complexes containing these elements. Under excessive pressure, sulfur- and nitrogen-containing ligands and their transition metal complexes can be added to lubricants, functioning as additional corrosion inhibitors [14,15,16]. The metal complexes of certain chelating ligands, such as S and N, exhibit biological activities like antifungal [17], antibacterial [18], and anti-humentery [19] characteristics. Unfortunately, the potential applications of these complexes for biological activity can be restricted by their limited solubility in water. Enhancing the solubility of the aforementioned complexes can be achieved by tagging the β-cyclodextrin moiety [20,21,22] in the ligand structure and thereafter in the complex structure.
Overall, metal complexes based on β-cyclodextrin present encouraging prospects for the synthesis of potent antibacterial medicines that exhibit improved solubility, stability, targeting specificity, and synergistic activity. Their distinct qualities render those appealing options for battling bacterial infections and tackling issues related to antibiotic resistance. Therefore, this work focuses on the synthesis, physico-chemical characterization, and antibacterial activity of an intriguing Fe(III) complex and ligand that is soluble in water with components S and N. Computational simulation is an effective tool to understand the stability of the complex through different electronic property calculations [23]. In this study, different electronic properties e.g., highest occupied molecular orbital (HOMO)-lowest unoccupied molecular orbital (LUMO) gap, electrostatic potential (ESP) maps, Mulliken charge distribution, reduced density gradient (RDG) were calculated with computational simulation using density functional theory (DFT) to establish the stable geometry of the complex. Additionally, the antibacterial activity of both ligand and Fe(III) complex has been studied which was further supported with the help of molecular docking study. According to both experimental and theoretical studies, the ligand and complex may be an effective antibacterial agent, and small modifications to the ligand system could enhance the activity.
Materials, reagents and methods
The chemicals β-cyclodextrin, p-Toluenesulfonyl chloride (TsCl), o-chloroaniline, FeCl3⋅6H2O, sodium hydroxide (NaOH), ammonium chloride (NH4Cl), thiourea, hydrochloric acid (HCl), trichloroethylene (C2HCl3), sodium carbonate (Na2CO3) and ethanol were purchased from Sigma Aldrich in Germany. Double-distilled water was utilized in each experiment. Solvents were purified prior to their use following standard literature procedures.
Instruments
The Euro VECTOR EA 3000 analyzer was used to conduct elemental microanalyses of C, H, and N. The Perkin-Elmer Spectrum FTIR spectrometer (RX-1) was used to record IR spectra in the 4000–400 cm−1 range at room temperature using KBr pellets. The JascoV-530 double beam spectrophotometer was used to record UV–visible spectra using a quartz cell with a path length of 1 cm that was equipped with a thermostated bath (maintained at 25 ± 0.1 °C) using DMSO and water as solvent references. Using D2O and DMSO-d6 as solvents at room temperature, 1H NMR spectra were acquired on a Bruker Advance-II 400 MHz spectrometer, and chemical shifts (δ) were stated in ppm with respect to TMS. The Waters ZQ-40000 equipment was utilized to measure the ESI–MS of both the complex and ligand. AAS (Varian, SpectraAA 50B) was used to determine the metal content using a standard metal solution from Sigma-Aldrich, Germany. A magnetic susceptibility balance (Magway MSB Mk1) made by Sherwood Scientific Ltd. was used to test the magnetic susceptibility at room temperature. A Systronics conductivity meter (TDS-308) was used to evaluate the synthesized complex's specific conductance in room-temperature water. The generated ligand and complex's antibacterial activity (in vitro) against four distinct bacterial species—gram positive (Staphylococcus aureus, Bacillus subtilis) and gram negative (Escherichia coli, Klebsiella pneumoniae)—was examined using the disc diffusion method.
Synthesis of mono-6-deoxy-6-(p-tosylsulfonyl)-β-cyclodextrin (β-CDOTs) (1)
p-Toluenesulfonyl chloride (2.5 g, 1.5 equiv.) was added drop wise to a round bottom flask containing β-cyclodextrin (β-CD) (10.0 g, 8.8 mmol) dissolved in pyridine (500 mL) and stirred overnight at room temperature. After 24 h, the solution gave three components as observed by TLC (SiO2; butanol:ethanol:water = 5:4:3 (vol.)) and the reaction was ceased by adding 10 mL water. Then the reaction mixture was concentrated to one-fifth in volume and acetone was added dropwise until the precipitation was over. The precipitate was filtered, washed with acetone and dried under vacuum. The precipitate was dissolved in DMF and purified on a reversed-phase column (silica gel 60 silanized, 200 g in dry weight, 4.1 × 30 cm2) using 5–40% DMF (aq) as eluent. The fraction of 5–10% contained β-CD while TsO-β-CD was in 10–20% and 20–40% contained (TsO)2-β-CD. Then the fraction of 10–20% was concentrated and poured into acetone to obtain white pure product. The product was filtered, dried and stored in a vacuum desiccator. Color: white; yield (45%); IR cm−1, KBr: 3391 (OH), 2926 (C–H), 1646 (C = C), 1366 (SO2 asym), 1153 (SO2 sym) cm−1; 1H-NMR (400 MHz, DMSO-d6, 25 °C), δ (ppm): 7.49 (m, 2H Ph), 7.12 (m, 2H Ph), 5.94–5.72 (m, 14H, OH2 and OH3 CD), 4.87–4.76 (m, 7H, H1 CD), 4.58–4.43 (m, 6H, OH6 CD), 4.27 (m, 2H, H6/CD), 4.02 (m, 1H, H5/CD), 3.92–3.54 (m, 25H, H3, H5 and H6 CD), 3.47–3.14 (m, H2, H4 overlap with water), 2.28 (s, 3H, Ph-CH3) [13]; 13C-NMR (DMSO-d6), d (ppm): 146.52, 143.15, 127.91, 124.26 (aromatic), 101.84 (C1), 81.06 (C4), 73.03,72.06, 71.68 (C3,C2,C5), 62.88 (C6/), 60.16 (C6), 23.18 (C9) [14]; Anal. Calcd for C49H76O37S: C, 45.65; H, 5.94; O, 45.92. Found: C, 45.05; H, 5.29; O, 45.53.
Synthesis of mono-6-deoxy-6-mercapto-β-cyclodextrin (2)
After dissolving 2 g of powdered β-CDOTs (1) and 2 g of thiourea in 100 mL of 80% aqueous methanol, the mixture was refluxed for two days at 60 °C. The solution was refluxed and then evaporated in a vacuum. The residue was combined with 30 mL of methanol and stirred for 1 h. The residue was filtered and dissolved to ̴ 34 mL of 10% NaOH and was kept at 50–60 °C for 5 h. By adding 2.5 mL of trichloroethylene (CHCl3) and 10% HCl, the pH of the solution was kept at 2. It was then stirred all night at room temperature. The precipitate that was produced was filtered and repeatedly cleaned with water. After that the trichloroethylene (CHCl3) was eliminated in a vacuum, compound 2 was produced by repeatedly recrystallizing it from water.
Color: White; Yield (56%); IR, KBr cm−1: 2562 (-SH), 1650, 1365–1200 cm−1; 1H NMR (400 MHz, DMSO-d6, 25 °C): 5.95–5.86 (m, 14H, OH2 and OH3 CD), 4.92–4.85(m, 7H, H1 CD), 4.59–4.51 (m, 6H, OH6 CD), 4.35 (m, 2H, H6/CD), 3.98 (m, 1H, H5/CD), 3.71–3.61 (m, 25H, H3, H5 and H6 CD), 3.43–3.34 (m, H2, H4 overlap with water), 2.14 (s,1H,SH), ppm [21, 24].Anal. Calcd for C42H70O34S: C, 43.82; H, 6.12; O, 47.25; S, 2.78. Found: C, 43.28; H, 5.87; O, 46.86; S, 2.27. m/z (ESI): calculated 1151.02 found 1152.24 [M + H]+.
Synthesis of mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin (3)
After dissolving 344.7 mg of mono-6-deoxy-6-mercapto-β-cyclodextrin (2) and 38.25 mg of o-chloroaniline in 70 mL of aqueous Na2CO3 (pH 10) with 20 mL ethanol, the mixture was stirred under nitrogen for two days at room temperature. The pH of the solution was adjusted to 3 by adding 1N HCl, and was vacuum-concentrated to about 40 mL. After adding 5 mL of trichloroethylene, the liquid was stirred for an entire day. The precipitate formed was collected with trichloroethylene using separating funnel. After the trichloroethylene was evaporated in vacuo, the solid product that remained was obtained and purified by repeated recrystallization.
Color: White; Yield (78%); IR cm−1: 1612, 1262, 753 cm−1; 1H NMR (400 MHz, D2O, 25 °C): δ = 7.53 (m,1H, Ph), 7.28 (m, 1H, Ph), 7.12 (m, 1H, Ph), 6.91 (m, 1H, Ph), 5.93–5.89 (m, 14H, OH2 and OH3 CD), 4.71–4.64 (m, 7H, H1 CD), 4.45–4.38 (m, 6H, OH6 CD), 4.25 (s, 2H, NH2), 4.11 (m, 2H, H6/CD), 3.89 (m, 1H, H5/CD), 3.78–3.64 (m, 25H, H3, H5 and H6 CD), 3.54–3.41 (m, H2, H4).;UV: 206 nm, 312 nm; Anal. Calcd for C48H75NO34S: C, 46.41; H, 6.08; N, 1.12; O, 43.79; S, 2.58. Found: C, 46.03; H, 5.19; N, 0.97; O, 43.24. m/z (ESI): calculated 1242.13, found 1242.63 [M]+.
Synthesis of Fe(III)-complex of mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin (4)
An aqueous solution containing 0.1 mmol (27.03 mg) FeCl3⋅6H2O was stirred at room temperature and then was added drop wise to a stirred aqueous solution containing 2 mmol (248 mg) of ligand (3). Afterwards, the mixture was refluxed at 60 °C for 10 to 12 h. The solution was then concentrated to a volume of 15 mL. Then, by adding 100 mL of acetone, white precipitate appeared. After being vacuum-filtered through a Buchner funnel and twice being washed with acetone, the precipitate was dried in a desiccator to yield a purple color product.
Color: Purple; Yield (80%); IR cm−1, KBr: 840, 735, 525, 466; UV: 232 nm, 370 nm, 524 nm; Anal. Calcd for C84H142N2O70S2Cl3 Fe: C, 39.94; H, 5.66; N, 1.10; O, 44.31; S, 2.53, Fe, 2.21. Found: C, 39.42; H, 5.11; N, 0.94; O, 44.07; S, 2.06; Fe, 1.93. m/z (ESI): calculated 2525.80, found 2525.54 [M]+.
Computational study
Gaussian 09 software was used for the DFT calculation of the optimized Fe(III) complex structure with B3LYP hybrid functional [25]. 6–31 g (d,p) basis set was used for non-metals (C H O N S) while an effective core potential LANL2DZ basis set was used for Iron metal in aqueous medium [26, 27]. Frequency calculation of optimized geometry (no imaginary frequencies) revealed that the geometry of the complex lies on the minima to the potential energy surface [27].To reduce the computational cost we have considered one unit of thio-functionalized β-cyclodextrin attached with Fe(III) for computational simulation [28, 29]. Different electrochemical parameters like highest HOMO –LUMO energy gap, Mulliken charge distribution, and ESP maps, RDG of the Fe complex were also analyzed with same level of theory.
Antibacterial activity
Two pathogenic gram positive bacteria Staphylococcus aureus, Bacillus subtilis and two pathogenic gram negative bacteria Escherichia coli, Klebsiella pneumonia were used for assessing the antibacterial activities using well diffusion method. The Mumbai, India-based Hi-media laboratory Pvt Ltd was the supplier of the nutrient agar (NA) medium. Boiling NA (2.8 g) in 100 mL of distilled water while suspended in it resulted in its complete dissolution. It was then autoclaved for 15 min at 15 lbs of pressure (121 degrees Celsius) and transferred onto a sterile Petri plate to set. A sterility check was carried when the agar solidified [30]. Pure cultures of each bacteria were prepared in nutrient broth and kept at 37 °C for the duration of the night. After incubation, each of the required plates was inoculated using a non-toxic, sterile cotton swab dipped into the microbial growth. Extra inoculate was removed by firmly pressing and spinning the swab against the tube wall above the liquid's level. To inoculate the media, the swab was equally streaked across the whole surface of the plate in three distinct directions. The antibacterial activity assay was carried out using the agar well diffusion method. Sterile nutritional agar (NA) was applied to sterile Petri dishes containing the test organisms after they had been inoculated. Using a sterile cork borer, every Petri plate (9 mm) was carefully prepared. Each well (microorganism-inoculated plate) received approximately 200 μl of a synthetic chemical (10 mg/mL) using a sterile pipette. The combination was then incubated for 24 h at 37 °C. After the incubation period, all tested plates were inspected and the inhibitory zone widths were measured.
Molecular docking
The selected protein PDB ID (https://www.rcsb.org) was utilized to carry out the molecular docking of the complex using the Auto Dock Vina tool (version 1.1.2). The grid dimensions for all the proteins were kept 1 Å spacing were all the proteins dimensions to being stiff for the receptor structure. The dimensions of X, Y, and Z for 5ZH8 (Staphylococcus aureus) was 62, 58, and 60 for 2GCX (Klebsiella pneumoniae) was 40, 44, and 40, for 2BH0 (Bacillus subtilis) was 56, 54, and 40 and for 1XFF (Escherichia coli) was 40, 46, and 48, respectively.
Results and discussion
Mono-6-deoxy-6-(p-tosylsulfonyl)-β-cyclodextrin (β-CDOTs) (1) was converted into mono-6-deoxy-6-mercapto-β-cyclodextrin (2). After synthesis, o-chloroaniline reacts with the mercapto modified β-cyclodextrin (2) to generate mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin (3). Later, it is permitted to react with FeCl3⋅6H2O in aqueous medium to give amino functionalized mercapto modified β-cyclodextrin based Ferric complex(4) (Scheme.1).The structure of Fe(III) complexes was verified by a variety of analytical spectroscopic techniques, such as elemental analysis, FTIR, 1H NMR, UV–visible spectra, ESI–MS, and molar conductance.
FTIR spectra
The FTIR spectra of compounds 1 and 2 were shown in (Fig. S1 and Fig. S2). The usual absorption peaks at 3391 cm−1 is due to the presence of OH of the β-cyclodextrin moiety. The other peaks such as 1646, 1366, and 1153 cm−1 in the IR spectra of 6-OTs-β-CD (1) is due to presence of C=C, SO2 Asymmetric and SO2 Symmetric respectively [31]. Owing to the thio group (–SH) substitution for the tosyl group, these peaks are absent from the compound 2 IR spectra. On the other hand, the characteristic absorption peak of 2562 cm−1 is visible in the spectra of 2. The absorption peak at 2562 cm−1, which was found to match with SH stretching vibrational bands [24], suggesting the detection of a thiolated β-cyclodextrin. The absorption peak at 1200–1055 cm−1, appears along this because of the C–O vibration in polysaccharide and antisymmetric glycosidic (C–O–C) bond [32]. The polysaccharide C–C stretching is responsible for the band at 1650 cm−1. In the FTIR spectra of compound 3 (Fig S3), three new, distinct absorption peaks appeared at 1612 (N–H bending), 1262 (C–N stretching), and 753 (N–H wagging) cm−1. The absorption peaks at 2562 cm−1 in compound 2 disappeared. The results showed that the thiol group had been replaced with the aniline group. The coordinated wagging and rocking vibration of the water molecule was identified as the cause of the bands that appeared at 840 and 735 cm−1 in the infrared spectra of 4 (Fig. S4) [21]. In complex 4, v(M–S) vibrations were identified in the bands at 466 cm−1 and v(M–N) vibrations were identified in the band at 525 cm−1 [33].
NMR spectra
For the mono-tosylated β-cyclodextrin (1), mono-6-deoxy-6-mercapto-β-cyclodextrin (2), and mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin (3), the 1H NMR spectra were recorded in D2O and DMSO-d6. The metal compound is paramagnetic, so it’s 1H NMR spectra were not able to be obtained. The 1H NMR of the mono-tosylated β-cyclodextrin (1) is shown in figure S5. The 1H NMR spectra of Compound 2 (figure S6) shows that the –SH group peak is situated at δ = 2.14 ppm [24]. It is proposed that the signals of the H6 protons with a thiol group (H6'a. H6'b) that occur at 0.74 ppm lower field than those of the H6 protons of other rings of β-cyclodextrin suggesting the formation of mono-6-deoxy-6-mercapto-β-cyclodextrin (2).
Compound (3) 1H NMR spectrum (figure S7) reveals that the aromatic protons' signals showed as multiplets in the range of δ ≈6.91–7.53 ppm, and the –NH2 group appeared as a singlet at 4.25 ppm [21]. This observation supports the theory that compound 3 substituted the thiol proton with an aromatic amine. In compound (3) 1H NMR spectra, the peak of the –SH proton that was present in compound (2) disappeared.
UV–visible spectra and magnetic moment studies
The UV–visible spectra of the mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin(3) and Fe(III)-complex of mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin (4) were recorded in water, (figure S8 and S9). Two bands are visible at 312 and 206 nm in compound 3, which are caused by the n → π* and π → π* transitions. Fe(III) complex (4) exhibits a band shift from 206 to 232 nm (bathochromic), indicating a coordinated behavior of the metal ion (Fe3+) with ligand. The coordination of Fe and the ligand's N-atom was shown by the complexes' disappearance of their peaks at 312 nm relative to the ligands. The MLCT/LMCT transition is responsible for the complex (4) peaks that appear at 370 nm. In contrast, transitions at 714–625 nm have been discovered to characterize the low-spin (S = 1/2) form, while transitions at 555–500 nm have been shown to represent the high spin (S = 5/2) form in distinct spin equilibrium systems [34]. The Fe(III) complexes' spectra show a single band at 524 nm, which is consistent with the 6A1g → 4T1g transition characteristic of the octahedral structure [34]. As a result, the Fe-complex has octahedral, high spin geometry. A computation of the magnetic moment of the synthesized Fe(III) complex showed that it was paramagnetic. The magnetic moment value (µeff) at room temperature is 5.88 B.M. According to these values, the Fe(III) complex has a high spin octahedral structure.
ESI–MS
The ESI–MS spectra of compounds 2, 3, and 4 were recorded and are shown in figure S10, S11 and S12 respectively. Compounds 2 and 3 includes m/z peaks at 1152.24 and 1242.63 corresponding to [M + H]+and [M]+, respectively. In Fe(III) complex (4), an additional peak was found at 2525.54 [M]+ which corresponds to the molecular weight of the complexes. Consequently, these results were in good agreement with the comparable structures that had been earlier demonstrated by the elemental and other spectrum examinations.
Molar conductance
After dissolving the synthesized Fe(III) complex in water, the molar conductance of 10−3 mol dm−3 complex solutions was measured at room temperature. A 1:3 electrolytic behavior was suggested by the conductance value of 405 Ω−1 cm2 mol−1 of the Fe(III) complex (4) [35].
Electronic properties study using DFT
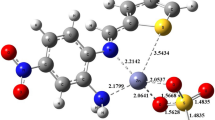
Optimized structure of the complex [considering one unit of thio-functionalized β-cyclodextrin attached with Fe(III)] is represented by the Fig. 1a. Metal Iron is well capped by the Sulphur, Oxygen and Nitrogen atoms. A strong binding reflects by the bond distances of 1.83 Å (N-Fe) and 2.27 Å (S-Fe). Two Oxygen atoms are also apart from Fe with an average distance of 2.1 Å. Mulliken charge analysis data (Fig. 1b) also supports the compact structure of the complex. Positive Fe (green) is well attached with the negative N, O and S (reddish).
ESP study (Fig. 2a) reflects a moderate negative surface present in the thio end of the complex. As per RDG analysis, van der Waals and strong interaction majorly dominate the complex as green and blue color covers the maximum part of Fig. 2b. Repulsive steric interaction (red color) is very less as per RDG analysis. Hence, RDG analysis reflects a stable complex was synthesized. HOMO–LUMO energy gap (Fig. 2c) is quite small in the complex as reflected from the DFT study [36].
Antibacterial activity
The antibacterial activity of the ligand and complex was assessed using two gram positive (Staphylococcus aureus, Bacillus subtilis) and gram negative (Escherichia coli, Klebsiella pneumonia) bacteria. Given the existence of biologically active donor sites (N and S), it was highly expected that the ligand and complex would have good activity against bacteria, as shown in Fig. 3 and Table 1. Comparing two gram-positive bacteria with complex and ligand activity, Staphylococcus aureus and Bacillus subtilis had a stronger response than the other two gram-negative bacteria. The ligand and complex have demonstrated inhibitory zones of 11 and 12 mm against Staphylococcus aureus and 6 and 9 mm against Bacillus subtilis. The inhibitory zones obtained for Escherichia coli have been calculated to be 6 and 8 mm, while Klebsiella pneumonia showed similar patterns of diminishing ligand and complex activity sequences at 7 and 8 mm. The efficiency of common medications against various bacterial strains was investigated in a previous paper. The results suggested that small adjustments to the ligand and complex might enhance the antibacterial activity [37,38,39].
Molecular docking
The complex was examined for interactions with different bacteria using the Auto Dock Vina tool (Fig. 4; Table 2). The molecular docking data further validate our previous claims. The receptor proteins of Staphylococcus aureus (5ZH8), Bacillus subtilis (2BH0), Klebsiella pneumoniae (2GCX), and Escherichia coli (1XFF), were used for the molecular docking of the complex. According to reports, the binding affinities were − 6.5, − 5.0, − 4.7, and − 4.2 in that order. Overall, the data point to the complex's strong interactions with a variety of bacterial protein receptors, supporting its claimed antibacterial efficacy.
Conclusions
To summarize, a water soluble octahedral Fe(III) complex was synthesized using mono-6-deoxy-(o-aminobenzylthio)-β-cyclodextrin, an amino ligand derived from thio modified β-cyclodextrin. FTIR spectra of the Fe(III) complexes confirmed that the ligands coordinate the metal ion via the sulfur and nitrogen atoms of the β-cyclodextrin moiety and amino groups respectively of the two ligand molecules and two H2O occupied the fifth and sixth coordination. The ESI–MS study suggested that it was a 1:2 metal–ligand complex. Electronic properties study using DFT also reflected a stable complex was synthesized. The synthesized compounds show potentiality to inhibit the bacterial efflux systems and enhancing the antibiotic activity which was further supported with the help of molecular docking.
Availability of data and materials
All data generated or analyzed during this study are included in the manuscript.
References
Cram, D.J., Cram, J.M.: Host-guest chemistry. Science (80-) 183, 803–809 (1974)
Dietrich, B., Lehn, J.M., Sauvage, J.P.: Les Cryptates. Tetrahedron Lett. 10, 2889–2892 (1969)
Dietrich, B., Lehn, J.M., Sauvage, J.P., Blanzat, J.: Cryptates-X. Syntheses et proprietes physiques de systemes diaza-polyoxa-macrobicycliques. Tetrahedron 29, 1629–1645 (1973)
Pedersen, C.J.: Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 7017–7036 (1967)
Dindulkar, S.D., Jeong, D., Kim, H., Jung, S.: Functionalized β-cyclodextrin as supramolecular ligand and their Pd(OAc)2 complex: highly efficient and reusable catalyst for Mizoroki-Heck cross-coupling reactions in aqueous medium. Carbohydr. Res. 430, 85–94 (2016)
Dass, C.R., Jessup, W.: Apolipoprotein A-I, cyclodextrins and liposomes as potential drugs for the reversal of atherosclerosis. A review. J. Pharm. Pharmacol. 52, 731–761 (2010)
Del Valle, E.M.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Tang, W., Zou, C., Da, C., Cao, Y., Peng, H.: A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. (2020). https://doi.org/10.1016/j.carbpol.2020.116321
Tahir, M.N., Nielsen, T.T., Larsen, K.L.: β-cyclodextrin functionalized on glass micro-particles: a green catalyst for selective oxidation of toluene to benzaldehyde. Appl. Surf. Sci. 389, 1108–1112 (2016)
Tripodo, G., Wischke, C., Neffe, A.T., Lendlein, A.: Efficient synthesis of pure monotosylated beta-cyclodextrin and its dimers. Carbohydr. Res. 381, 59–63 (2013)
Kettle, S.F.A.: Physical inorganic chemistry. Phys. Inorg. Chem. (1996). https://doi.org/10.1007/978-3-662-25191-1
Cook, T.R., Stang, P.J.: Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 115, 7001–7045 (2015)
Lu, H., Zhang, X.P.: Catalytic C–H functionalization by metalloporphyrins: recent developments and future directions. Chem. Soc. Rev. 40, 1899–1909 (2011)
Singh, M.M., Rastogi, R.B., Upadhyay, B.N., Yadav, M.: Thiosemicarbazide, phenyl isothiocyanate and their condensation product as corrosion inhibitors of copper in aqueous chloride solutions. Mater. Chem. Phys. 80, 283–293 (2003)
Rastogi, R.B., Singh, M.M., Yadav, M., Singh, K.: Substituted thiobiurets and their molybdenum and tungsten complexes as corrosion inhibitors for mild steel in 1.0 N sulphuric acid. Indian J. Eng. Mater. Sci. 10, 155–160 (2003)
Rastogi, R.B., Yadav, M., Bhattacharya, A.: Application of molybdenum complexes of 1-aryl-2,5-dithiohydrazodicarbonamides as extreme pressure lubricant additives. Wear 252, 686–692 (2002)
Singh, H., Yadav, L.D.S., Mishra, S.B.S.: Studies on some antifungal transition metal chelates of N-(5-phenyl-1, 3, 4-thiadiazol-2-yl) dithiocarbamic acid. J. Inorg. Nucl. Chem. 43, 1701–1704 (1981)
Melha, K.S.A.: In-vitro antibacterial, antifungal activity of some transition metal complexes of thiosemicarbazone Schiff base (HL) derived from N4-(7′-chloroquinolin-4′-ylamino) thiosemicarbazide. J. Enzyme Inhib. Med. Chem. 23, 493–503 (2008)
Clegg, W., Lockhart, J.C., Musa, F.H.: Preparation and complexation of polydentate and macrocyclic ligands incorporating benzimidazole. X-ray crystal structure of 6,7,9,10,12,13,15,16-octahydro-23H,25H-bis(benzimidazo[1,2-j:2′,1′- o])[1,4,7,13,10,16]tetraoxadiazacyclo-octadecine. J. Chem. Soc. Dalt. Trans. 47–53 (1986)
Das, A., Mishra, D.K., Sinha, D.B.: A Pd(II) complex of a β-cyclodextrin-based polydentate ligand: an efficient catalyst for the Suzuki reaction in aqueous media. J. Coord. Chem. 70, 3035–3047 (2017)
Das, A., Dutta, S., Sinha, B.: Effects of β-cyclodextrin-based Schiff-base Zn(II) complexes: synthesis, physicochemical characterization and their role in alleviating oxidative stress related disorder. J. Coord. Chem. 71, 3731–3747 (2018)
Fujita, K., Ueda, T., Imoto, T., Tabushi, I., Toh, N., Koga, T.: Guest-induced conformational change of β-cyclodextrin capped with an environmentally sensitive chromophore. Bioorg. Chem. 11, 72–84 (1982)
Pradhan, S., Gurung, P., Chettri, A., Singha, U.K., Chhetri, P., Dutta, T., Sinha, B.: Synthesis of novel [{(2-amino-5-nitro-n-[(e)-thiophen-2-yl-methylidene]aniline-κ3N1:N4:S)(sulphato-κ2O1:O3)}zinc(II)] complex with physico-chemical and biological perspective exploration: a combined experimental and computational studies. J. Fluoresc. (2024). https://doi.org/10.1007/s10895-024-03612-0
Wang, J., Kong, L., Guo, Z., Xu, J., Liu, J.: Synthesis of novel decorated one-dimensional gold nanoparticle and its application in ultrasensitive detection of insecticide. J. Mater. Chem. 20, 5271–5279 (2010)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993)
Ghosh, K., Mridha, N.K., Khan, A.A., Baildya, N., Dutta, T., Biswas, K., Ghosh, N.N.: CO2 activation on transition metal decorated graphene quantum dots: an insight from first principles. Phys. E Low-Dimens. Syst. Nanostruct. 135, 114993 (2022)
Baildya, N., Mazumdar, S., Mridha, N.K., Chattopadhyay, A.P., Khan, A.A., Dutta, T., Mandal, M., Chowdhury, S.K., Reza, R., Ghosh, N.N.: Comparative study of the efficiency of silicon carbide, boron nitride and carbon nanotube to deliver cancerous drug, azacitidine: a DFT study. Comput. Biol. Med. (2023). https://doi.org/10.1016/j.compbiomed.2023.106593
Dutta, T., Ghosh, N.N., Das, M., Adhikary, R., Mandal, V., Chattopadhyay, A.P.: Green synthesis of antibacterial and antifungal silver nanoparticles using Citrus limetta peel extract: experimental and theoretical studies. J. Environ. Chem. Eng. (2020). https://doi.org/10.1016/j.jece.2020.104019
Dutta, T., Chattopadhyay, A.P., Ghosh, N.N., Khatua, S., Acharya, K., Kundu, S., Mitra, D., Das, M.: Biogenic silver nanoparticle synthesis and stabilization for apoptotic activity; insights from experimental and theoretical studies. Chem. Pap. 74, 4089–4101 (2020)
Atlas, R.M., Snyder, J.W.: Handbook of media for clinical microbiology. Handb. Media Clin. Microbiol. 1–524 (2006)
Raoov, M., Mohamad, S., Abas, M.R.: Synthesis and characterization of β-cyclodextrin functionalized ionic liquid polymer as a macroporous material for the removal of phenols and As(V). Int. J. Mol. Sci. 15, 100–119 (2014)
Dernaika, H., Chong, S.V., Artur, C.G., Tallon, J.L.: Spectroscopic identification of neurotoxin tetramethylenedisulfotetramine (TETS) captured by supramolecular receptor β -cyclodextrin immobilized on nanostructured gold surfaces. J. Nanomater. (2014). https://doi.org/10.1155/2014/207258
Pansuriya, P., Patel, M.N.: Iron(III) complexes: preparation, characterization, antibacterial activity and DNA-binding. J. Enzyme Inhib. Med. Chem. 23, 230–239 (2008)
Shabani, F., Saghatforoush, L.A., Ghammamy, S.: Synthesis, characterization and anti-tumour activity of iron(III) Schiff base complexes with unsymmetric tetradentate ligands. Bull. Chem. Soc. Ethiop. 24, 193–199 (2010)
Ali, I., Wani, W.A., Saleem, K.: Empirical formulae to molecular structures of metal complexes by molar conductance. Synth. React. Inorg. Met. Nano-Met. Chem. 43, 1162–1170 (2013)
Sheng, Y., Suhartono, T., Hazmatulhaq, F., Kamil, M.P., Assfour, B., Al Zoubi, W., Ko, Y.G.: Conjugation of defect-controlled nucleation and self-assembly growth achieving uniform amelioration of organic micro-flowers: surface properties and DFT analysis. Chem. Eng. J. (2024). https://doi.org/10.1016/j.cej.2023.148230
Al Zoubi, W., Kim, M.J., Kim, Y.G., Ko, Y.G.: Dual-functional crosslinked polymer-inorganic materials for robust electrochemical performance and antibacterial activity. Chem. Eng. J. (2020). https://doi.org/10.1016/j.cej.2019.123654
Hassan, A.U., Sumrra, S.H.: Exploring the bioactive sites of new sulfonamide metal chelates for multi-drug resistance: an experimental versus theoretical design. J. Inorg. Organomet. Polym. Mater. 32, 513–535 (2022)
Behrami, A.: Antibacterial activity of coumarine derivatives synthesized from 4-chloro-chromen-2-one. The comparison with standard drug. Orient. J. Chem. 30, 1747–1752 (2014)
Acknowledgements
The authors would like to acknowledge Departmental Special Assistance Scheme under the University Grants Commission, New Delhi (SAP-DRS-III, No. 540/12/DRS/2013) & University of North Bengal, Govt. of West Bengal for financial and instrumental support.
Funding
This declaration is “not applicable”.
Author information
Authors and Affiliations
Contributions
Pritika Gurung: Design and Synthesis, Analytical and Spectroscopic Data Analysis, Writing—original draft. Ananya Das: Analytical and Spectroscopic Data Analysis. Sudarshan Pradhan: Molecular docking study. Anmol Chettri: Analytical and Spectroscopic Data Analysis. Tanmoy Dutta: DFT Study. Biswajit Sinha: Design and Synthesis, Analytical and Spectroscopic Data Analysis, Writing—final draft, Editing and Communication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
This is an observational study. The University of North Bengal Ethics Committee has confirmed that no ethical approval is required.
Consent to participate
This declaration is “not applicable”.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gurung, P., Das, A., Pradhan, S. et al. A new amino functionalized thio-modified β-cyclodextrin based ligand and its Fe(III) complex with physico‑chemical and antibacterial activity: an integrated experimental and computational investigation. J Incl Phenom Macrocycl Chem 104, 461–471 (2024). https://doi.org/10.1007/s10847-024-01247-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01247-z