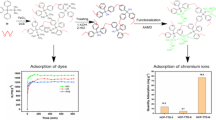
Abstract
As the first generation of macrocyclic hosts, crown ethers have excellent host-guest recognition characteristics and are widely used in molecular recognition, catalysis, drug delivery and other fields. Porous organic polymers (POPs) with adjustable porosity and stable network structure have become advanced materials for molecular storage, heterogeneous catalysis, water purification and energy storage. In recent years, crown ether-based porous organic polymers have attracted extensive attention due to their excellent performance, which combine supramolecular hosts with traditional polymer, and have created a new field for supramolecular chemistry and material science. In this review, we discusses the research status of crown ether-based porous organic polymers in structure and pollutant adsorption, including selective adsorption of metal ions in water and removal of organic pollutants, and prospected the promising applications and future development direction of crown ether-based porous organic polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nowadays, with the rapid development of economy and society, the threat of pollutants in the water to human production, life and the environment is becoming more and more serious, and the global water environment is showing a deteriorating trend. As the main pollution sources of water environment, heavy metal ions, organic dye molecules, pesticides and other organic micro-pollutants are enriched in large quantities in water, which are difficult to degrade and beyond the normal purification capacity of water. This is a huge challenge for environmental protection and sustainable development [1,2,3,4]. At present, for these pollutants, although chemical precipitation [5], membrane separation [6], ion exchange [7], solvent extraction [8], biological method [9], electrochemical method [10] and adsorption separation [11, 12] have been developed for wastewater treatment, the development of efficient, rapid and low-cost treatment technologies is still the focus of research on wastewater treatment. Among many methods, the adsorption method has the advantages of strong anti-interference ability and low environmental pollution, and the adsorption material has the advantages of low synthesis cost, variety and recyclability, which is used in water purification. However, the adsorption capacity, selectivity and chemical stability in water environment of adsorption materials still restrict the wide application of adsorption method. Therefore, the design and synthesis of adsorption materials with high adsorption capacity, high selectivity and good chemical stability is a research hotspot in recent years. Among many adsorption materials, porous organic polymers [13,14,15,16,17,18,19] show unique advantages in improving adsorption capacity and selectivity: on the one hand, porous structure provides more adsorption sites for pollutants, on the other hand, organic polymer materials are easy to modify, which can further improve the adsorption capacity and selectivity of adsorption materials.
As a class of macrocyclic molecules connected by multiple heteroatoms, crown ethers are a very important class of macrocyclic receptors. The abundant oxygen atoms in crown ethers can selectively recognize some metal ions through coordination interactions and electrostatic interactions and catch some organic molecules depends on the hydrophobic cavity of the crown ether and the hydrogen bond interaction between the guest and the oxygen atoms in the crown ether. This excellent recognition selectivity makes it widely concerned in the field of adsorption and separation. Introducing crown ether structure into porous organic polymers can not only improve the adsorption capacity of polymers materials for pollutants, but also improve the adsorption selectivity. This method of introducing macrocyclic host into the polymer can obtain high performance polymers materials [20, 21].
In this paper, we discuss the adsorption of different types of pollutants by crown ether-based porous organic polymers, summarize the relationship between structural design and adsorption performance, and propose some challenges and future development directions of crown ether-based polymers in the field of pollutant treatment.
Adsorption of metal ions by crown ether-based porous organic polymers
The selective adsorption of metal ions by crown ether porous organic polymers is fully dependent on the ability of the crown ether cavity to different metal ions. Especially in water, the difference of binding ability of crown ether to different metal ions indicates that ion recognition is closely related to the environment of aqueous solution. Yudan Zhu [22] et al. explained the underlying mechanism of selective recognition of crown ether monomers in water through experiments and simulation calculations. As shown in Fig. 1a, in water, this recognition difference is directly reflected in the distance between different metal ions and the crown ether cavity, the degree of aggregation of metal ions in a certain area near the crown ether and the length of the existence time, and the energy barrier of metal ions passing through the crown ether. Studies have shown that the adsorption interaction of 18-crown-6 ether to K+ is stronger than other alkali metal ions and divalent metal ions. The selective recognition ability of monomer crown ether provides a reference for the design and synthesis of porous organic polymers with ultra-high selective adsorption based on crown ether. Due to the excellent performance of crown ether in selective recognition, many adsorption materials with crown ether as the identification sites have been developed, such as amorphous polymers containing crown ether rings, MOF, COF and other materials [23,24,25]. These works indicate that the development of organic polymer adsorption materials containing crown ether has a good application prospect.
L. I. Trakhtenberg [26] et al.prepared two types of crown ether-containing polymers by chemical binding and immobilization of acrylamide and monomers containing crown ether structure, and studied the adsorption of Cu2+ and Pb2+ in aqueous solution. The results showed that the adsorption efficiency was improved by introducing crown ether into the polymer system through chemical combination. When the content of crown ether increased from 3 to 6%, the adsorption efficiency of these adsorbents for metal ions was increased. In the coexistence system of copper and lead ions, the adsorbent containing 18-crown-6 ether showed high adsorption selectivity for Pb2+. However, when the polymers containing crown ether were prepared by chemical grafting, the grafting rate became one of the factors affecting the adsorption performance of the adsorption material. Due to the existence of benzene ring, dibenzo-18-crown-6 ether showed higher grafting rate on polyacrylamide than 18-crown-6 ether, and its adsorption material has better performance. Comparing the adsorption performance of the adsorption materials prepared by introducing crown ether into the polymer through chemical binding and non-chemical binding, the results showed that the non-chemical binding crown ether polymer has the loss of crown ether molecules during use, resulting in poor adsorption performance and recycling performance. Therefore, when designing and synthesizing crown ether-based adsorption materials, chemical binding should be considered. At the same time, the adsorption performance of crown ether-based organic polymer will be greatly improved by rationally designing polymers with specific binding sites and specific topological structures.
Bao-HangHan [27]et al.used Friedel-Crafts reaction to crosslink 1,4-dimethoxybenzene with dibenzo-18-crown-6 ether (DB18C6) and dibenzo-24-crown-8 ether (DB24C8) respectively, and successfully synthesized crown ether-based hypercrosslinked porous polymers, as shown in Fig. 1b. There is a uniformly distributed crown ether structure in the polymer system, and the rigid cross-linked chain ensures the stability of the polymer microporous structure. The adsorption results of the crown ether-based hypercrosslinked porous polymer for metal ions show that the adsorption performance of the two polymers for Au3+ is much higher than that of K+ ( the adsorption capacities are 1096 mg g− 1 and 1667 mg g− 1, respectively ). This adsorption behavior is attributed to the change of the structure of the crown ether in the polymer, which leads to a higher binding of smaller Au3+.
In the above amorphous polymers, the disordered arrangement of the polymer chains easily leads to the distortion of the crown ether structure, which makes the recognition properties of the crown ether in the polymer have the opposite results to the recognition properties of the monomer crown ether. However, the long-range ordered arrangement of the polymer chains can maintain the stability of the crown ether structure, which is beneficial to predict the adsorption selectivity of the polymer. In 2019, Xin Zhao [28] et al. successfully synthesized crown ether-based crystalline COF materials for the first time, as shown in Fig. 1c, it opened the way for the exploration of crown ether-functionalized COF. The research group used crown ether-functionalized p-triphenyldiamine monomer and trimesic aldehyde to prepare three different crown ether-functionalized COF materials: 12C4-COF, 15C5-COF and 18C6-COF. With the increase of crown ether size in the building blocks, the specific surface area of the three COFs decreased, and all of them showed mesoporous with single pore size. 12C4-COF, 15C5-COF and 18C6-COF showed different binding preferences for Li+, Na+ and K+, respectively. For example, the equilibrium adsorption capacities of 18C6-COF for K+, Na+ and Li+ were 1.2, 0.7 and 0.2 mmol/g, respectively, the adsorption capacity can intuitively reflect that the adsorption selectivity of crown ether polymers is consistent with the identification characteristics of crown ether monomers. The COF material of the side chain crown ether blocks the pores of the polymer, and the specific surface area of the synthesized COF material is not high, resulting in limited adsorption capacity.
In order to improve the specific surface area of crown ether-based COF, the introduction of crown ether into the main chain through reasonable structural design can effectively reduce the pore blockage of crown ether. Honglai Liu [29] et al.integrated crown ether molecules into a covalent organic framework to synthesize two main-chain crown ether-based COF materials ( Py-B18C6-COF, Py-B24C8-COF ), as shown in Fig. 1d. Compared with the side chain type 18C6-COF [28], the Py-B18C6-COF synthesized by this group has an ultra-high BET specific surface area of 1356 m2 g− 1. Py-B18C6-COF and Py-B24C8-COF were used to adsorb Li+, Na+, K+ and Cs+ solutions with the same initial concentration. In a short time, the maximum K+ capture capacity of Py-B18C6-COF was 244.21 mg g− 1, and the maximum Cs+ capture capacity of Py-B24C8-COF was 223.05 mg g− 1. The pore size of the crown ether channel in the main chain crown ether-based COF and the high specific surface area of the polymer make its adsorption performance far superior to the same type of adsorption material.
Adsorption of organic molecules by crown ether-based porous organic polymers
The oxygen atoms in the crown ether structure provide abundant lone electron pairs, which can not only recognize metal ions, but also recognize cationic organic molecules containing ammonium ions or neutral organic molecules through hydrogen bonds, electrostatic interactions, hydrophobic interactions, etc. The introduction of crown ether into the polymer, using the recognition selectivity of crown ether, through reasonable structural design, the synthesized crown ether-based porous organic polymer can be used for the adsorption and separation of organic matter in water.
WanQi Zhang [30] et al.prepared nanofibers with different ratios of polystyrene (PS) and polydibenzo-18-crown-6-ether by electrospinning, as shown in Fig. 2a. Compared with crown ether monomer modified nanofibers, PDB18C6 functionalized nanofibers provide a polymer skeleton, which solves the solubility problem of dissolution of crown ether monomers in most solvents. When the concentration of PS was 8% ~ 15%, the composite nanofibers had the highest removal efficiency of catecholamine under near neutral conditions, which could be used as an adsorbent for the pretreatment of trace catecholamine. The introduction of polycrown ether structure increases the surface roughness and porosity of the fiber to a certain extent. However, too much polycrown ether content cannot form continuous fibers, which hinders the further improvement of adsorption performance. However, the bottom-up construction strategy can effectively ensure the uniform distribution and high content of the crown ether structure in the polymer, and the use of non-planar building blocks can significantly increase the specific surface area of the porous material. Cheng-Xiong Yang [31] et al. constructed microporous organic networks using the reaction of crown ether bromide and arylene with different structures, as shown in Fig. 2b. Polymers with different topologies were synthesized by using building blocks with different structures. TEPM-MON constructed with non-planar arylene blocks has a larger specific surface area and pore structure, which is attributed to the rigid twisted structure that inhibits the close packing of polymer chains. The microporous organic network showed good removal ability for chlorophenol organic pollutants, and the adsorption capacity of TEPM-MON for 2,4, 6-trichlorophenol was up to 294.6 mg g− 1. Hydrophobic, π-π and hydrogen bonding interactions and size effect during the adsorption process together improved the adsorption capacity and selectivity of chlorophenol organic pollutants.
In the design and synthesis of porous organic polymers, in order to obtain more porous and structurally stable polymers, monomers with rigid non-planar structures are usually introduced into the polymer. Honglai Liu [32] et al. selected trienes as the building unit, which not only ensured the high porosity of the polymer, but also introduced crown ether into the polymer skeleton, which significantly enhanced the affinity for organic dye molecules. The structure is shown in Fig. 2c. POP-T CE-15 has a high BET specific surface area (up to 848.27 m2 g− 1) and a hierarchical pores. The polymer showed good adsorption performance for the target adsorbent cationic dyes methylene blue, Rhodamine B and anionic dyes methyl orange. This is due to the selective advantage of crown ether structure, the adsorption of cationic dyes can reach equilibrium quickly and the adsorption capacity is maximum in a short time, while it takes a longer time to reach equilibrium for anionic dyes. In the polymer system, the electrostatic interaction between the crown ether and dye molecules and the screening of organic dye molecules by the pore size of the polymer jointly determined the selective adsorption behavior of the crown ether polyporous organic polymer.
COF materials have become a research hotspot due to their excellent porous properties and structural stability. Although crown ether-based COF materials are difficult to synthesize, some COF materials have been continuously developed with the deepening of research, and have shown good application prospects in the adsorption and separation of organic matter. Zilin Chen [33] et al. successfully synthesized covalent organic nanospheres CON ADBC-Tp containing crown ether, which showed good adsorption and separation performance for imidazole compounds. Under acidic conditions, imidazole compounds with different substituents exhibit different structural sizes and protonation properties, and these differences are utilized to realize the separation of different imidazole compounds by crown ether-based materials. On the other hand, according to the difference in the hydrophobicity of the adsorbate, the separation of the crown ether-based polymer CON ADBC-Tp from the mixture can also be achieved. The separation characteristics were tested with several adsorbates with different hydrophobicity. The results showed that the retention time was positively correlated with hydrophobicity, and the adsorption and separation performance of the material was synergistically enhanced through hydrophobic interaction, electrostatic interaction, and size effect. Yong Cui [34] et al. synthesized chiral crown ether-based COFs with fluorescence properties, which can be used for enantioselective recognition of chiral molecules and used as a fluorescence sensor for the detection of chiral amino alcohols. The structure is shown in Fig. 2d. The adsorption test of chiral molecule phenylglycol by CCOFs showed that it showed obvious fluorescence enhancement to D-phenylglycol, while the fluorescence enhancement of L-phenylglycol to COF was not obvious. From the perspective of binding energy, the binding of L-type guests to chiral crown ethers requires higher energy, indicating that chiral crown ethers in fluorescent COF have preferential binding and sensing ability to D-enantiomers. The chirality of crown ethers in crown ether-based COF materials is used to achieve selective adsorption of organic matter. The fluorescence characteristics of COF are also used to detect organic pollutants, which broadens the application of crown ethers in the removal of organic pollutants.
Sumary and outlook
In summary, crown ether-based porous organic polymers, as a new type of polymer containing macrocyclic host, have shown great application prospects in wastewater treatment due to their advantages of adjustable function, high specific selectivity, high specific surface area and good stability. Based on the research status in recent years, this paper systematically discusses the structural design and selective adsorption of crown ether-based porous organic polymers. The results show that crown ether-based porous organic polymers show high selective adsorption performance for metal ions, which depends on the stable existence of crown ether structure in polymer system. In the polymer system, crown ether not only has the effect of pore-forming, but also can interact with metal ions, organic dye molecules and other pollutants as an adsorption site, which has higher selectivity than traditional amino, carboxyl, hydroxyl and other functional groups.
From the perspective of future research direction, the adsorption method will be combined with fluorescence detection and other means to detect pollutants in the water environment, which also requires the design and synthesis of materials must be developed in the direction of multi-function. Although crown ether based porous organic polymers have made some achievements in wastewater treatment, there are still some challenges. The first aspect is the structural design. The selective adsorption of metal ions by crown ether based polyporous organic polymers depends on the structure of crown ether. How to regulate the conformational change of flexible crown ether rings in polymers more accurately is a problem that needs further explored. Secondly, the selective adsorption of metal ions by crown ether based porous organic polymers is more demanding than the adsorption of organic pollutants, while the difference in the selective adsorption of organic pollutants by amorphous and crystalline polymers has not been explored. Thirdly, the adsorption tests on actual water samples of the crown ether-based porous organic polymer are less, which is also the key data for optimizing its performance in the future. We believe that with more in-depth research, these problems will eventually be resolved.
References
Mututuvari, T.M., Tran, C.D.: Synergistic adsorption of heavy metal ions and organic pollutants by supramolecular polysaccharide composite materials from cellulose, chitosan and crown ether. J. Hazard. Mater. (2013)
Sounthararajah, D.P., Loganathan, P., Kandasamy, J., Vigneswaran, S.: Adsorptive removal of heavy metals from water using sodium titanate nanofibres loaded onto GAC in fixed-bed columns. J. Hazard. Mater. (2015)
Wang, P., Du, M., Zhu, H., Bao, S., Yang, T., Zou, M.: Structure regulation of silica nanotubes and their adsorption behaviors for heavy metal ions: pH effect, kinetics, isotherms and mechanism. J. Hazard. Mater. (2015)
Naef, A.A.Q., Ramy, H.M., Dahiru, U.L.: Removal of heavy metal ions from wastewater: A comprehensive and critical review. npj Clean. Water (2021)
Das, S., Heasman, P., Ben, T., Qiu, S.: Porous Organic materials: Strategic Design and structure–function correlation. Chem. Rev. (2016)
Jiang, J.-X., Su, F., Trewin, A., Wood, C.D., Campbell, N.L., Niu, H., Dickinson, C., Ganin, A.Y., Rosseinsky, M.J., Khimyak, Y.Z., Cooper, A.I.: Conjugated Microporous Poly(aryleneethynylene) Networks. Angewandte Chemie International Edition (2008)
Xu, C., Zhu, Y., Yao, C., Xie, W., Xu, G., Zhang, S., Zhao, Y., Xu, Y.: Facile synthesis of tetraphenylethene-based conjugated microporous polymers as adsorbents for CO2 and organic vapor uptake†. New J. Chem. (2019)
Hei, Z.-H., Huang, M.-H., Luo, Y., Wang, Y.: A well-defined nitro-functionalized aromatic framework (NO2-PAF-1) with high CO2 adsorption: Synthesis via the copper-mediated Ullmann homo-coupling polymerization of a nitro-containing monomer†. Polym. Chem. (2015)
Bavykina, A.V., Olivos-Suarez, A.I., Osadchii, D., Valecha, R., Franz, R., Makkee, M., Kapteijn, F., Gascon, J.: Facile Method for the Preparation of Covalent Triazine Framework coated Monoliths as Catalyst Support: Applications in C1 Catalysis. ACS Applied Materials & Interfaces (2017)
Babel, S., Kurniawan, T.A.: Low-cost adsorbents for heavy metals uptake from contaminated water: A review. J. Hazard. Mater. (2003)
Comarmond, M.J., Payne, T.E., Harrison, J.J., Thiruvoth, S., Wong, H.K., Aughterson, R.D., Lumpkin, G.R., Müller, K., Foerstendorf, H.: Uranium Sorption on Various Forms of Titanium dioxide–influence of Surface area, Surface Charge, and Impurities. Environmental Science & Technology (2011)
Yusan, S., Akyil Erenturk, S.: Adsorption equilibrium and kinetics of U(VI) on beta type of akaganeite. Desalination (2010)
Uribe-Romo, F.J., Hunt, J.R., Furukawa, H., Klöck, C., O’Keeffe, M., Yaghi, O.M.: A crystalline imine-linked 3-D porous covalent Organic Framework. J. Am. Chem. Soc. (2009)
Zhu, L., Zhang, Y.-B.: Crystallization of Covalent Organic Frameworks for Gas Storage Applications. Molecules (2017)
Xu, M., Wang, T., Gao, P., Zhao, L., Zhou, L., Hua, D.: Highly fluorescent conjugated microporous polymers for concurrent adsorption and detection of uranium†. J. Mater. Chem. A (2019)
Shuanyan, K., Zhiguang, Z., Lei, W., Shan, X., Guolong, H., Xiaohua, M., Nanwen, L.: Synthesis and gas separation properties of polyimide membranes derived from oxygencyclic pseudo-Tröger’s base. J. Membr. Sci. (2021)
Yin, Z.-J., Xu, S.-Q., Zhan, T.-G., Qi, Q.-Y., Wu, Z.-Q., Zhao, X.: Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. (2017)
An, S., Xu, Q., Ni, Z., Hu, J., Peng, C., Zhai, L., Guo, Y., Liu, H.: Construction of Covalent Organic frameworks with Crown Ether struts. Angew. Chem. Int. Ed. (2021)
Chen, L., Zhu, X., Shen, J., Zhang, W.: Selective solid-phase extraction of catecholamines from plasma using nanofibers doped with crown ether and their quantitation by HPLC with electrochemical detection. Anal. Bioanal. Chem. (2016)
Yin, Z., Cui, C., Chen, H., Duoni, Yu, X., Qian, W.: The Application of Carbon Nanotube/Graphene-Based Nanomaterials in Wastewater Treatment. Small (2019)
Umeh, C., Asegbeloyin, J.N., Akpomie, K.G., Oyeka, E.E., Ochonogor, A.E.: Adsorption Properties of Tropical Soils from Awka North Anambra Nigeria for Lead and Cadmium Ions from Aqueous Media. Chemistry Africa (2019)
Pan, X., Wang, Q., Ma, Z., Qin, Y., Lu, X., Jin, W., Zhu, Y.: Assisting role of water molecules in ionic recognition by 18-crown-6 ether in aqueous solutions. Journal of Molecular Liquids 371. (2023)
Giri, A., Sahoo, A., Dutta, T.K., Patra, A.: Cavitand and Molecular Cage-based porous Organic polymers. ACS Omega. 5(44), 28413–28424 (2020)
Mei, D., Liu, L., Yan, B.: Adsorption of uranium (VI) by metal-organic frameworks and covalent-organic frameworks from water. Coord. Chem. Rev. 475. (2023)
Yu, J., Qi, D., Li, J.: Design, synthesis and applications of responsive macrocycles. Commun. Chem. 3 (1). (2020)
Gromov, V.F., Gerasimov, G.N., Ikim, M.I., Spiridonova, E.Y., Trakhtenberg, L.I.: Sorption of Metal ions from Aqueous solutions by Crown Ethers. Russian J. Phys. Chem. B. 14(3), 492–497 (2020)
Kong, H.-Y., Wang, T.-X., Tao, Y., Ding, X., Han, B.-H.: Crown ether-based hypercrosslinked porous polymers for gold adsorption. Separation and Purification Technology 290. (2022)
Shen, J.C., Jiang, W.L., Guo, W.D., Qi, Q.Y., Ma, D.L., Lou, X., Shen, M., Hu, B., Yang, H.B., Zhao, X.: A rings-in-pores net: Crown ether-based covalent organic frameworks for phase-transfer catalysis. Chem. Commun. (Camb). 56(4), 595–598 (2020)
An, S., Xu, Q., Ni, Z., Hu, J., Peng, C., Zhai, L., Guo, Y., Liu, H.: Construction of Covalent Organic frameworks with Crown Ether struts. Angew Chem. Int. Ed. Engl. 60(18), 9959–9963 (2021)
Chen, L., Zhu, X., Huang, D., Xu, Z., Shen, J., Zhang, W.: Polystyrene/poly(dibenzo-18-crown-6) composite nanofibers for the selective adsorption of plasma catecholamines. RSC Adv. 7(22), 13263–13271 (2017)
Wang, Y.X., Bi, Y.P., Cui, Y.Y., Yang, C.X.: Synthesis of crown ether-based microporous organic networks: A new type of efficient adsorbents for chlorophenols. J. Hazard. Mater. 443, 130268 (2023). (Pt B)
Xu, T., He, Y., Qin, Y., Zhao, C., Peng, C., Hu, J., Liu, H.: Facile preparation of porous organic copolymer based on triptycene and crown ether for efficient organic dye adsorption. RSC Adv. 8(9), 4963–4968 (2018)
Hu, C., Li, Z., Hu, Z., Li, Q., Fu, Y., Chen, Z.: Synthesis of multifunctional crown ether covalent organic nanospheres as stationary phase for capillary electrochromatography. J. Chromatogr. A. 1677, 463323 (2022)
Yuan, C., Fu, S., Yang, K., Hou, B., Liu, Y., Jiang, J., Cui, Y.: Crystalline C-C and C horizontal lineC bond-linked Chiral Covalent Organic frameworks. J. Am. Chem. Soc. 143(1), 369–381 (2021)
Author information
Authors and Affiliations
Contributions
Wang jianchun wrote the main manuscript text and other co-authors revised the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest regarding this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, JC., Guo, JT., Gou, RT. et al. Crown ether-based porous organic polymers for the removal of environmental pollutants in water. J Incl Phenom Macrocycl Chem 104, 1–6 (2024). https://doi.org/10.1007/s10847-023-01216-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-023-01216-y