Abstract
Flavors have been widely used in many products. They are volatile compounds and do not often last long in air especially at high temperature. The techniques for slow flavor release and long-lasting scents, for the stability of sensory perception, are much desired. The inclusion complex method encompasses the idea of molecular recognition and interactions through noncovalent bonding. It can provide protection and prevent the loss of volatile aroma materials, and improve shelf-life and enhance the stability of the entrapped ingredients of flavors. In this article, the production of control-release flavor by inclusion complex method was reviewed in considerable detail. The host materials, the method of production flavor inclusion complex, and the methods of characterization of flavor inclusion complex were depicted. The complexation thermodynamics and calculations were also discussed. Cyclodextrins and their derivatives as the host materials were discussed in this paper. Methods of production flavor inclusion complex include solution method (water or water as the main component of the solution, cosolublizer), suspension method, gas/liquid interface method, and solid phase method (co-grinding method, heating in a closed container method, shaking method at room temperature). The methods of characterization of solid inclusion complex mainly include x-ray diffraction technique, Infrared and Raman spectra, solid nuclear magnetic resonance spectroscopy, thermal analysis, mass spectrometry analysis, scanning and transmission electron microscopy. Structures, energies, and some properties of molecules can be studied by computational chemistry. The application of computational chemistry to the study of flavor compound inclusion complexes were discussed. The review indicates that inclusion complex method is an efficient way for encapsulation of flavor compounds. By this method slow flavor release and long-lasting scents can be obtained.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flavors and perfumes have been known to mankind since time immemorial [1, 2]. Materials for flavors have attracted attention since the dawn of human civilization [3]. In general, a flavor, blended by flavorist, is a mixture of two to dozens of aroma materials [2, 4]. The material for composing a flavor is a substance that can give aroma smell or taste [5]. Flavors play important roles in the daily life [6, 7]. Without imparting a characteristic taste or aroma of the flavors’ themselves, they are usually added in foods, beverage, medicine and cigarettes to supplement, enhance, or modify the original taste and aroma of these products [4, 5, 8]. The materials for blending flavors are derived from natural and synthetic sources. The natural ingredients of flavor include essential oil, concrete, resinoid, balsam, resin, absolute, tincture, while the synthetic ingredients of flavor include aldehydes, alcohols, acids, ketone, esters, ethers, and compounds containing sulfur or nitrogen [9,10,11]. Flavors are composed of volatile aroma compounds; therefore, they do not often last long in air especially at high temperature. The flavor performance is usually measured by its lastingness, together with the pleasantness [12]. Furthermore, the sensory perception of some flavors can also be changed easily as a result of volatilization, heating, oxidation or chemical interactions [13]. The use of techniques for slow flavor release, long-lasting scents, and for the stability of sensory perception, is much desired.
There are a number of methods to solve these problems. Among these methods, the inclusion complex method is a typical one [14]. By this method, long-lasting flavors can be developed [15]. The inclusion complex method encompasses the idea of molecular recognition and interactions through noncovalent bonding. Hydrogen bonds, steric fit, dispersive forces, dipole–dipole forces, van der Waals forces, charge-transfer interactions, electrostatic interactions and hydrophobic interactions are several types of non-covalent interactions [16, 17]. An inclusion compound is a complex of host and guest molecules. In the host–guest chemistry, the chemical compound, called host molecule, forms a cavity. The second compound located in the cavity is called guest molecule. Of course, the definition of inclusion compounds is very broad, extending to channels formed between molecules in which guest molecules can fit [18]. Menthol is a highly volatile compound with a peppermint-like odor and cooling sensation and has been widely used in flavor and pharmaceuticals. An example of an inclusion complex consisting of a menthol bound with hydroxypropyl-β-cyclodextrin is shown in Fig. 1. The inclusion complex method can provide protection and prevent the loss of volatile core materials. It can improve shelf-life and enhance the stability of the entrapped ingredients.
The host materials
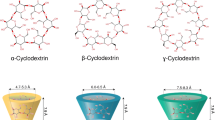
In host–guest chemistry, cyclodextrins, cyclotriveratrylenes, foldamers, pillararenes, calixarenes, cucurbiturils, porphyrins, zeolites, metallacrowns, crown ethers and carcerands are common host molecules. In flavor encapsulation, cyclodextrins are the most widely used host materials. Cyclodextrins, produced from starch by means of enzymatic conversion, are a family of compounds made up of sugar molecules bound together in a ring [19]. Typical cyclodextrins contain a number of glucose monomers ranging from six to eight units in a ring and are called α (alpha)-cyclodextrin (6-membered sugar ring molecule), β (beta)-cyclodextrin (7-membered sugar ring molecule), γ (gamma)-cyclodextrin (8-membered sugar ring molecule) respectively [20,21,22]. Because cyclodextrins are hydrophobic inside and hydrophilic outside (see Fig. 2), they can form complexes with hydrophobic compounds.
The hydrophobic inner cavities of cyclodextrins can encapsulate flavor molecules with suitable sizes to form flavor inclusion complexes [23]. Except for the native cyclodextrins, α-, β- and γ-cyclodextrin, some cyclodextrin derivatives are also used in production of inclusion complex. Hydroxypropyl-β-cyclodextrin (HP-β-CD), with improved water solubility, is a hydroxyalkylated cyclodextrin. The HP-β-CD complex has allowed a homogenous delivery system [20]. HP-β-CD is one of the most widely used cyclodextrin derivatives in current scientific researches and industry applications. It mainly used in food, pharmaceutical and cosmetics industries. Carvacrol, a known antioxidant molecule, has a characteristic pungent warm odor and is commonly used as a flavor and fragrance agent. By forming inclusion complexes with HP-β-CD and HP-γ-CD, the thermal stability, water solubility and shelf-life of carvacrol can be enhanced [24].
Cyclodextrin sulfobutyl ether sodium (SBE-β-CD, SBE-γ-CD) exhibit greater water solubility and a more desirable safety profile. SBE-β-CD and SBE-γ-CD could successfully form inclusion complexes with flavor compounds such as camphene, β-caryophyllene, p-cymene, eucalyptol, estragole, limonene, myrcene, α-pinene, β-pinene and γ-terpinene. SBE-β-CD showed the best binding ability compared with HP-β-CD and SBE-γ-CD and could delay the release of these flavor compounds and allow the generation of controlled release system [25]. It is a potential carrier and solubilizing agent for flavor components. Methylated-β-cyclodextrin (Me-β-CD), a methylated cyclodextrin, has a larger surface tension in common cyclodextrin derivatives. Me-β-CD with an asymmetric molecular structure has higher water solubility. The main reason for the solubility enhancement of methyl derivative is that chemical manipulation transforms the crystalline β-CD into amorphous derivative, while the methyl derivative provides a deeper cavity and better bioaffinity. Turmeric oleoresin has a spicy, fresh odor reminiscent of sweet orange and ginger and a slightly pungent, bitter flavor. The turmeric oleoresin can be successfully solubilized in aqueous solution by forming inclusion complex with Me-β-CD [26]. Benzophenone, which often used as a flavoring agent, has a delicate, persistent, rose-like odor. It also is an oil soluble photoinitiator. A water-soluble complex of this flavoring agent can be synthesized using Me-β-CD as wall material [27]. Fixolide is a synthetic, polycyclic musk fragrance widely used in the manufacturing of personal care and household products. It is semivolatile and is degraded under light exposure and high temperature. Its water solubility at 25 °C is only 1.25 mg/l. These unsatisfied properties can be improved by formation inclusion complex with CDs including Me-β-CD [28].
Hesperetin has a faint oily and fatty odor with aroma of vanilla. It is a common flavor component and has some biological effects such as blood lipid- and cholesterol-lowering effects, anti-inflammatory and anticancer activities. Solubility and bioactivity of hesperetin can be increased by complexation with Me-β-CD and 2,6-di-O-methylβ-cyclodextrin (DM-β-CD) [29].
Monochlorotriazinyl-β-cyclodextrin (MCT-β-CD), with monochlorotriazinyl groups as reactive anchor able to form covalent bonds to nucleophilic groups such as –OH in cellulose, is a commercially available reactive form of β-CD. Each MCT-β-CD molecule contains on average two to three monochlorotriazinyl groups. Using MCT-β-CD as wall material, d-limonene can be encapsulated in cavity of MCT-β-CD and the maximum molar inclusion ratio is 0.85. d-limonene-MCT-β-CD inclusion complex has a pleasant, lemon-like odor free from camphoraceous and turpentine-like notes [30]. Hinokitiol has a woody odor and shows antibacterial activity. Hinokitiol molecule enters into the cavity of MCT-β-CD and forms inclusion complex. The maximum molar inclusion ratio of hinokitiol in the immobilized MCT-β-CD is around 0.8. MCT-β-CD can be covalently bonded to the paper. This implies that the paper prepared with hinokitiol-MCT-β-CD is very useful as antibacterial and aroma material [31]. Essential oils of Eucalyptus, Peppermint, Lavender, Jasmine, Clove and Cedarwood are applied directly on cotton as well as with anchoring hosts as MCT-βCD to assess their stability of retention on the fabric surface. MCT-β-CD showed enhanced fragrance stability with added advantage of exhibiting no major change in tensile strength, stiffness and air permeability of cotton. The application of MCT-β-CD had outstood with highly durable essential oil retention lasting for five or more washes and also for longer span [32].
Carboxymethyl-β-cyclodextrin (CM-β-CD) is a carboxymethylated cyclodextrin. In solution that pH < 4, its solubility is low. But in pH > 4 solution that carboxyl group dissociates, CM-β-CD can dissolve in water in any concentration [33, 34].
Organic amine groups in a neutral or acid condition can be positively charged which has a very good composite ability to guest molecules. Amino-β-cyclodextrin in neutral or acidic pH conditions can be protonated. Borneol has a piney, camphor-like odor and burning taste somewhat reminiscent of mint. The nonbonded interaction and the binding constant between amino-β-cyclodextrin and borneol were obtained by Takenaka et al. and the values are 30 kcal/mol and 1149 ± 287 respectively [35].
Oligo (lactic acid) -β-cyclodextrin (OLA-β-CD) is a new cyclodextrin derivative. Oligo-(lactic acid) group can be degraded in the human body. As a modified cyclodextrin with the general characteristics of common cyclodextrin derivatives, it also has unique properties as follows: excellent biocompatibility; excellent controlled release of specific guest molecular; adjustable water-solubility; capability to form duel interactions with specific drugs. OLA-β-CD can be used to improve the solubility of water insoluble compounds, enhance compounds stability and bioavailability, and achieve controlled release system or delayed release system of compounds. Sulphur is lively group by oxidation or replacement to create higher levels of supramolecular structure. Mercaptoacetic group is extremely easy adherent to gold surface, thus it has novel applications. These cyclodextrin derivatives with sulphur mainly contain: (6-mercapto-6-deoxy)-β-cyclodextrin, (6-Mercapto-6-deoxy)-α-Cyclodextrin. Iodine groups are lively reactive groups, easily replaced by other groups. Iodine generation alpha, beta, gamma—cyclodextrins are cyclodextrins derivatives synthesis important intermediates. Octakis-(6-Iodo-6-Deoxy)- β-Cyclodextrin is a widely used cyclodextrin derivative containing iodine group. Organic amine groups in a neutral or acid condition can be positively charged which has a very good composite ability to guest molecules. These cyclodextrin derivatives can also be used as hosts for encapsulation of flavor compounds.
Method of production inclusion complex
Preparation inclusion complex in solution
In the water or water as the main component of the solution
Mixing CDs and guests in solution is the most common way to prepare the inclusion complex. Water is absolutely necessary when using this method. Of course, a mixed solvent of water can also be used. But only a limited solvent, such as methanol, ethanol, acetone, ether, and acetonitrile, can be used [36]. Eucalyptus essential oil has a characteristic aromatic, somewhat camphoraceous odor and a pungent, spicy, cooling taste, and has been widely applied to food, toiletry, perfumery as well as pharmaceutical because of its various functionalities, such as antibacterial, antioxidant, insecticide, damp clearing, and detoxification effects. In order to improve the utilization rate and prevent the volatilization of the volatile component, preparation of the inclusion complex of eucalyptus essential oil with β-CD in solution was investigated by Ren et al. They found that encapsulation enhanced the stability and prolonged the acting time of eucalyptus essential oil [37]. In HP-β-CD aqueous solution, lavender flavor as a core material can be encapsulated by HP-β-CD. By this method lavender HP-β-CD complex can be produced and the lavender flavor loading capacity is about 5.7%. The product is a transparent lavender flavor nanocapsule aqueous solution with average diameter 15 nm [38].
In most cases, the solubility of CDs decreases rapidly after formation of inclusion complex. This provides favorable conditions for the separation of the products. The resulting inclusion complex can be recovered from solution by simple filtration. When solvents are the mixtures of water and organic solvent, ternary inclusion complex containing solvent molecules may be separated sometimes. This must be noted in the experimental design and analysis of the products. The effect of solvent interactions on flavor inclusion complex was investigated with ethyl butyrate, ethyl heptanoate, l-menthol, methyl anthranilate, neral and geranial being selected as flavor compounds. The smallest and most polar solvent molecule represented by propylene glycol had the least effect on flavor/CD inclusion complex [39].
Usually, essential oils, flavors and fragrances are insoluble in water. These insoluble liquids may be blended or dissolved in a little solvent and added dropwise to the stirred aqueous solution of CDs. Stirring is continued at room temperature. The inclusion complex precipitate is separated by vacuum filtration. After washing with water and the corresponding readily soluble volatile organic solvent to remove the guest attached on the surface of CDs, the relatively pure inclusion complex is obtained. The product is naturally dried or dried under low temperature in vacuum to constant weight [40]. The advantages of the direct mixing process are simple. Furthermore, solvent can’t be mixed in the inclusion complex. However, because it is heterogeneous reaction, a long time is needed. When the inclusion complex is prepared using a supersaturated solution of CDs, the CDs themselves tend to precipitate crystals during long time agitation. Therefore, the share of the guest reduces in the inclusion complex [36].
In the encapsulation process, heating can not only speed up the formation of inclusion complex, but also maintain some physical and chemical properties of the inclusion complex. Perpetration at higher temperatures can adopt a small reaction volume and a concentrated solution which is beneficial for large amounts of preparation [36]. In order to speed up the formation of inclusions, in some cases, ultrasound technology was used to increase the intensity of agitation. In the production process of citral MCT-β-CD inclusion complex, the mixture of citral and water was agitated using a JY92-2D Ultrasonic Cell Disruptor. The citral load capacity was about 9% [41].
Preparation of inclusion complex in the presence of cosolublizer
In the actual handing of some guests and modified CDs, solubilizers are usually adopted, such as ethanol, isopropanol, ammonium hydroxide and so on. For example, guests and HP-β-CD are dissolved in as little as 75–90% ethanol solution. The filtrate through membrane is evaporated at room temperature or dried under vacuum. The residue is dissolved in distilled water, filtered and lyophilized to give a powdery product [36]. Cinnamon oil has an odor of cinnamon and a spicy burning taste. In the process of preparation of cinnamon oil complex with β-CD, a predetermined quantity of cinnamon oil dissolved in ethanol (10% w/v) was slowly added to the warm β-CD solution (ethanol/water, 1/2). The maximum inclusion efficiency of β-CD was achieved at the ratio of 15:85, in which the complex powder contained 117.2 mg of oil/g of β-CD [42]. Mexican oregano essential oil has a particular herb odor. It contains the aromatic and bioactive compounds carvacrol and thymol in different concentrations according to their chemotype. In the complexation of mexican oregano essential oil with β-CD and γ-CD, ethanol was used as cosolublizer to dissolve mexican oregano essential oil by Barbieri et al. [43]. Evidence of inclusion complexation was demonstrated by contrasting analytical techniques. According to the quantity and stability of carvacrol and thymol, they found that the best complex combinations were formed between γ-CD-Thymol and β-CD-Carvacrol chemotypes. In the complexation of lemon oil with β-CD, ethanol was used as cosolublizer to dissolve lemon oil by Barbieri et al. [44]. They found that the maximum inclusion capacity of β-CD and a maximum powder recovery were achieved at the ratio of 12:88, in which the β-CD complex contained 9.68% (w/w) lemon oil. Using the same method as described by Barbieri et al., an inclusion complex of lemon oil and β-CD was also produced by Padukka et al. [45]. They also investigated extraction of lemon oil from the inclusion complex. In the complexation of benzaldehyde, citral, l-menthol and vanillin, with β-CD, ethanol was used as cosolublizer. These four aromatic compounds retention was assessed by instrumental analysis. Complexation of benzaldehyde, citral, l-menthol and vanillin in β-CD markedly improved heat stability in some cases [46].
Preparation of inclusion complex in the suspension
If a guest does not have a suitable solvent, it can be added directly to CDs suspension. In general, the ratio of CDs to water is 50% in the suspension. After the mixture is stirred for several hours, the inclusion complex can be formed [36]. For comparison of flavor retention in alpha, beta, and gamma cyclodextrins, encapsulation of flavor compounds (2,3-butanedion, 2,3-petanedion, dimethyl sulfide, dimethyl disulfide, ethyl acetate, ethyl butyrate, ethyl valerate, ethyl hexanoate, 2-hexenal, 2-heptenal, 2-octenal, 2-nonenal, 2-decenal, 2-methylpyrazine, 2,5-dimethylpyrazine, 2-methyl-3-ethylpyrazine, 3,5(6)-dimethyl-2-ethylpyrazine isomers, benzaldehyde, limonene, methyl salicylate, 4-hydroxy-2,5-dimethyl-3(2H)-furanone, eugenol, isoeugenol, vanillin, ethylvanillin) in three types of cyclodextrins was adopted this method [47]. Lippia sidoides essential oil has an aromatic herbal odor. The volatility of the essential oil is one of its main characteristics. However, when it is exposed to oxygen, light and heat, it can be easily oxidized, decomposed or become resinous. In order to improve its physicochemical stability, inclusion complexes of lippia sidoides essential oil and β-CD were obtained by addition of lippia sidoides essential oil to β-CD slurry containing 50% of solid and its solid powdered form was prepared subsequently using spray drying [48]. Using suspension method, Fenyvesi et al. investigated encapsulation of borneol, citral, linalool, menthol and thymol in β-CD. They found that thermal stability and on storage stability of these aroma components were improved by formation inclusion complexes [49].
Gas/liquid interface method
Another recommended method is the gas/liquid interface method. The container containing the appropriate concentration of the aqueous solution of CDs is placed in the atmosphere of a volatile guest. The CDs in the surface of the liquid phase combine with the guest in the gas phase and the inclusion complex is produced. Crystallization is achieved when the saturated concentration is reached. The solid inclusion complex is separated from the precursor in the solution by filtration. A purer CDs inclusion complex is obtained. Cis-cyclooctene, methyl benzoate and CD ternary inclusion complex was produced through this method [36].
Preparation of inclusion complex by solid phase method
Co-grinding method
The solid CDs is mixed with a liquid or a solid guest molecule can form inclusion complex in the general humidity (relative humidity: 60–75%) under the condition of strong grinding. With this solid phase technique, the moisture content of the initial CDs plays an important role. Solid phase method is more favorable for the preparation of those water-soluble guest compounds [36].
The essential oil of Mentha × villosa Hudson is often used for cooking because of its flavouring properties. It also has different pharmacological activities such as anti-parasitic and central nervous system depressant. The Mentha × villosa Hudson oil inclusion complex can be prepared by this method with a 1:9 mass/mass oil: β-CD ratio [50]. Bhandari et al. encapsulated lemon oil in β-CD and the inclusion complex prolong the aroma of lemon oil [51]. Solid phase grinding method is also successfully used for the preparation of some essential oil inclusion complexes. However, long time for grinding will convert the inclusion complex from microcrystal to amorphous powders.
Heating in a closed container method
In the presence of trace amounts of water, CDs and guests are placed in a closed container and heated. The guest-CD inclusion complex can be produced by this method. Some aromatic acids inclusion complexes, such as benzoic acid and salicylic acid, have been successfully prepared. Generally, the heating temperature range is 43–142 °C. A certain amount of water is necessary in this process. When the initial CDs are completely dry, the inclusion complex cannot be produced [14, 36, 52].
Shaking method at room temperature
CDs crystal powder and guest are sealed in a glass bottle and shook for a certain time. The gust molecules diffuse into the cavities of CDs and the inclusion complex can be formed [36, 40].
Methods of characterization of solid inclusion complex
X-ray diffraction technique (XRD)
X-ray powder diffraction technique is a useful method for determining the formation and purity of solid inclusion complex. When the guest is gas or liquid, it usually does not produce any diffraction peaks itself. However, the resulting inclusion complex may be crystal powder; characteristic peaks will appear in the diffraction pattern. Therefore, it is easy to identify whether solid inclusion complex is produced or not. If the guest itself is a crystal, after the formation of the inclusion complex, its own characteristic peaks disappear. The crystal diffraction peaks of the CDs also change. The diffraction peaks of the inclusion complex appear. If guest and CDs are physically mixed, two kind of characteristic diffraction peaks appear simultaneously. The x-ray diffraction results can be expressed in several ways. The first method is to attach powder diffraction photographs. The second method is to attach a diffraction spectrum, indicating the diffraction angle 2θ in the range of 5–60°. The third method is to make a linear graph on the 2θ scale. The second method is often used in papers. For the sake of precision, the diffraction angle 2θ, the surface spacing d and the intensity I are described in an attached table. The diffraction pattern of CDs is related to the water content in the crystal. For example, the diffraction patterns of β-CDs containing 13 and 6% water are different. The polycrystalline with the same chemical composition also shows different diffraction patterns. X-ray technology requires the corresponding standard data or powder data, there is no standard data in the analysis of structures of inclusion complexes. At present, some relevant information can be obtained only from the comparison. The advantage is that the method is simple, and the analysis result is intuitive. Therefore, it is still a better way to judge the formation of inclusion complex and product purity. Menthyl acetate is a common flavor material having a fresh odor and cool mouthfeel. The XRD of the inclusion complex between menthyl acetate and β-CD was reported by Zhu et al. [53] as shown in Fig. 3. From the changes of XRD, it can be used to infer whether menthyl acetate was encapsulated in β-CD or not. This method has also been used for characterization of inclusion complexes such as benzophenone [54] (with a delicate, persistent, rose-like odor), palmarosa and star anise essential oils [55].
Infrared and Raman spectra
The infrared spectra of CDs are similar to those of starch. Mcnamara, Russell [56, 57] and Egyed [58] have studied the peaks of infrared and Raman spectra of CDs and their attribution in detail. The FTIR absorption frequencies of β-CD and their assignments are shown it Table 1.
The characteristic absorption frequency of CDs covers the entire range of 400–3800 cm−1. For organic small molecules, their amount in the inclusion complex is no more than 25% (w/w). Their characteristic peaks are easily covered by the absorption peaks of CDs and are difficult to recognize. If the guest molecule contains a group such as –COOH, –COOR or C=O, there is tensile vibration absorption in the vicinity of 1700 cm−1. Changes in peak shape, peak position and intensity can provide evidence of whether the guest molecule enters the cavity or not and the nature of the interaction force. 2,5-Dimethylpyrazine has a characteristic odor of earthy, potato-like odor. The FTIR changes of host, guest and inclusion complex can be observed by an example of 2,5-dimethylpyrazine-HP-β-CD inclusion complex as shown in Fig. 4 [59]. Most 2,5-dimethylpyrazine bands disappear in the complex spectrum indicating that 2,5-dimethylpyrazine is deeply encapsulated into the host cavity.
FTIR of HP-β-CD (A), 2,5-dimethylpyrazine-HP-β-CD inclusion complex, and 2,5-dimethylpyrazine [59]
Raman spectroscopy is a spectroscopy technique used in the study of molecules. When monochromatic light is incident on molecules, the incident light brings about an electronic transition in the chemical species from its ground state to a virtual energy state, which will emit a photon on returning to the ground state. It results in Raman signal. An example of Raman spectra of a lavender oil and its inclusion complex with HP-β-CD is shown in Fig. 5 [60]. Some bands of the guest disappear or shift in the Raman curve of its inclusion complex.
Raman spectra of a lavender oil (a), lavender oil-HP-β-CD inclusion complex (b), and HP-β-CD (c) [60]
The difference in energy between the absorbed and the emitted photon is unique for each chemical species depending on its electronic environment. The Raman spectra can be used as a supplement to provide a stronger band when the absorption corresponding to a certain vibration on the IR spectrum is very weak or does not occur. When used to study the hydration and dehydration of CDs, Raman spectra have a significant effect. The technique serves as an important tool for study of various binding events [61]. To compare the Raman spectrum of menthol with the Raman spectrum of its inclusion complex compound with HP-β-CD, some marker bands (2859, 1363, 1309, 1240, 1175, 1105 cm−1) of menthol disappeared in the Raman spectrum of the inclusion compound and other marker bands (2963, 2929, 765, 292 cm−1) were shifted. The Raman spectra indicated that menthol was stabilized by in interaction with HP-β-CD via hydrogen bonds and formed the inclusion compound [62].
Solid nuclear magnetic resonance spectroscopy
NMR is a powerful tool to study conformation and structure, and provides valuable data concerning the spatial accommodation of small molecules in inclusion complexes. If inclusion occurs, the alteration in the physical and chemical environment affects the protons of guests and CDs, which may result in their chemical shifts [63]. The 13C NMR spectrum using cross-polarization magic-angle spinning technology is another useful method for analyzing the structure of CD inclusion complex. The splitting state of the host (CD) peaks from the ample NMR spectrum confirms whether the sample is inclusion complex or not. In addition, the solid NMR spectrum may provide information about the chemical composition of the inclusion complex, the asymmetric unit of the crystal, and the water content in the cavity before and after encapsulation and its location. Thymol has a characteristic herbaceous, warm and aromatic odor with a sweet, medicinal, spicy flavor. The chemical shifts (ppm) of β-CD, thymol and thymol-β-CD inclusion complex in CDCl3 are presented in Table 2 [64]. It can be inferred that thymol was encapsulated in β-CD from the chemical shifts. Ethyl propionate has an odor reminiscent of rum and pineapple. Ethyl propionate-β-CD inclusion complex was prepared and characterized by 13C NMR. The chemical shifts (ppm) of β-CD, ethyl propionate-β-CD inclusion complex were discussed by Cao et al. [65]. The valeric acid-α-CD inclusion complex was also characterized by 13C NMR. The chemical shifts confirm the formation of valeric acid-α-CD inclusion complex [66].
Unlike the NMR spectrum in the solution, there is a strong internuclear dipolar coupling between the proton and the carbon nucleus. Due to the average conformation in the solution, only six lines can be observed in the spectrum of CD. Each line corresponds to an unequal carbon core. However, in the solid state, the conformation is locked due to the spatial barrier and the piling effect. Chemically equivalent carbon atoms on the crystal may be unequal. Therefore, each of the chemical equivalent nuclei appears multiple lines in 13C NMR spectrum. In the simplest case, the solid 13C NMR spectrum corresponds to a finger-print identification. It can characterize the structure of the smallest repeating unit of the crystal, and it also possible to analyze the structural properties from the spectrum division [36].
The inclusion of the flavor compounds into the β- CDs cavities can also be better demonstrated by 1H NMR spectroscopy. Because guests enter into the hydrophobic cavities of β- CDs, which may result in an effect on the density of the electron cloud in the cavities that create shielding or deshieding effects on H-5 and H-3 in the β- CDs cavities, significant changes in the proton chemical shifts of H-3 and H-5 can be observed when the inclusion complexes are formed [54, 67]. Therefore, 1H NMR can provide valuable information about the spatial position of guest molecules in the cyclodextrin cavity, as well as the formation and dissociation encapsulation conditions in the solvent [68]. The formation of the inclusion complex between 2-phenylethanol and β-CD was investigated by Yang et al. [69]. In their studies, H-3 and H-5 protons experienced significant upfield shifts (0.065 and 0.132 ppm respectively). This indicated that 2-phenylethanol should penetrate into the β-CD cavity from the narrow side. The nuclear Overhauser effect (NOE) is the transfer of nuclear spin polarization from one population of nuclear spins to another via cross-relaxation. It is a through-space phenomenon that occurs between neighbouring protons (4–5 Å). 2D ROESY NMR could help to confirm the spatial proximity between host and guest molecules. A NOE cross correlation could be observed in ROESY spectroscopy if two protons are closed located in space. A 2D-ROESY of the thymol-β-CD inclusion complex in D2O is shown in Fig. 6. The appreciable correlation of H-1, H-4 and H-3 protons of thymol with the H-3′, H-5′ and H-6′ protons of β-CD suggests that thymol is included in the β-cyclodextrin cavity [70].
2D-ROESY of the thymol-β-CD inclusion complex in D2O [70]
Thermal analysis
Thermal analysis is one of the earlier methods for determination of CDs inclusion complexes. It is a powerful tool to study the complex formation between guest molecules and CDs and to evaluate the inclusion compound stoichiometry [71]. TG and DTG methods are used to determine the quality change (or mass loss) and rate of change of the samples respectively with the increase of temperature as shown in Fig. 7 [72]. Guest vaporized quickly and almost completely before 110 °C. However, some guest still released from inclusion complex in the temperature range of 215–292 °C. This indicates that guest was encapsulated in the host and thermal stability of guest was improved. Changes in mass loss can provide a supporting evidence for the formation of inclusion complexes [73]. Solvents and volatile oils can be released quantitatively at 100 °C under normal pressure or vacuum, while the encapsulated guest molecules remain in the cavity of the CDs. Thus, TG and DTG can be used to distinguish whether the sample is a complex or a mixture, and to identify the percentage of guest molecules in complex or free state [36]. Differential scanning calorimetry (DSC) is another thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. DSC is used to determine the exothermic and endothermic rates of the sample with the increase of temperature. It is possible to determine the composition and melting and decomposition temperature of the sample with the increase of temperature [36]. DSC analysis can also be used to confirm inclusion complex formation by comparing their thermal stability [74].
Thermogravimetric kinetics, which can be explored for insight into the combination of guest and host and the reaction mechanisms of thermal decomposition, can be studied using TG and DTG data. Activation energy, defined as the minimum energy required starting a chemical reaction, of the decomposition process of inclusion complex can be used to estimate the combination of guest and host. Activation energy can be thought as the energy barrier separating guest from guest–host inclusion complex. The value of activation energy can reflect the combination of guest and host. Small activation energy means that guest easily releases from its inclusion complex and the inclusion complex is relatively instable, while high activation energy means that guest is bound to host firmly and the inclusion complex is relatively stable [75]. Kissinger, Coats–Redfern, Freeman-Carroll, and Kissinger–Akahira–Sunose (KAS) methods can be adopted to calculate activation energy. Lavender flavor release activation energy from its HP-β-CD inclusion complex was investigated by Freeman-Carroll method. Because lavender flavor is a mixture, two main peaks can be observed from its inclusion complex DTG curve, and the activation energy values are 24.6 and 86.4 kJ/mol respectively [38]. Menthyl acetate release activation energy from its β-CD inclusion complex was obtained by KAS method and the value is 258.7 kJ/mol [53]. Menthol release activation energy from its HP-β-CD inclusion complex was investigated by Coats–Redfern method and the value is 145.3 kJ/mol [64]. Mentha-8-thiol-3-one release activation energy from its β-CD inclusion complex was studied by Coats–Redfern method and the value is 290.8 kJ/mol [75]. Sweet orange flavor release activation energy from its β-CD inclusion complex was investigated by Freeman-Carroll method. Because sweet orange flavor is blended with many aroma ingredients, three main peaks can be observed from its inclusion complex DTG curve, and the activation energy values are 80.0, 98.3 and 104.5 kJ/mol respectively [76].
Mass spectrometry analysis
Electrospray ionization mass spectrometry (ESI–MS) is routinely used for the detection of cyclodextrin noncovalent complexes. In the early days, fast atom bombardment mass spectrometry (FABMS) and ion spray mass spectrometry (ISMS) were used to study structures of α-, β- and γ-CD. The solid sample is solvent-free and is directly coated on the thioglycerol thin layer attached on FAB target. Each dosage is 2.5–5 ng. Later, with the development of electrospray ionization mass spectrometry, it has been widely used in the field of cyclodextrin superamolecule because of its fast analysis speed, high sensitivity and high accuracy, and its ability to withstand impurity [36]. ESI–MS has shown itself to be a very useful technique in describing the noncovalent interaction between β–CD and various organic molecules. Linalool has a typical pleasant floral odor. Inclusion complex of linalool with β-CD was characterized using ESI–MS. Ions at m/z 1136 and 129.0 corresponding to protonated species of β-CD and protonated species of linalool: β-CD inclusion complex respectively can be observed. It reveals that the inclusion complex was formed in a ratio 1:1 [77]. Methyl jasmonate has a powerful, floral–herbaceous, sweet, persistent odor. To study the interactions of CD with methyl jasmonate, the MS methodology was applied by Oliva et al. From the MS data, they found that methyl jasmonate inclusion complexes with β-CD and methylated CDs were more abundant and more stable than those involving α-CD and γ-CD [78]. Combined thermoanalytical technique (MS-TG) can be used to confirm the inclusion complex formation directly. It is efficient to characterize the inclusion complex formation and to indicate the improvement of the thermal stability of the included guests [48].
Scanning electron microscopy and transmission electron microscopy
Scanning electron microscopy (SEM) is a useful tool for observing the microscopic morphology and structure of solids, especially crystalline materials [36]. Images of a sample are produced by scanning the surface with a focused beam of electrons. The electrons interact with atoms in the sample, producing various signals that contain information about the surface topography and composition of the sample. SEM is used to study the morphology of solid or crystal CD inclusion complex. Ethyl butyrate has a fruity odor with pineapple undertone and sweet, analogous taste. The inclusion complexes of ethyl butyrate with β-CD and γ-CD were prepared by Zhang et al. [79]. The morphologies of β-CD, γ-CD, ethyl butyrate-β-CD and ethyl butyrate-γ-CD by SEM were characterized by SEM and the images are shown in Fig. 8. The morphological differences between the CDs and corresponding complexes indicated that new crystalline phases might have formed after complexation. Based the information, the form of the inclusion complex can be understood. Complex formation of Lippia graveolens essential oil with β-CD and γ-CD was determined by SME. The inclusion complexes showed the typical monoclinic crystalline structures [80].
SEM images of β-CD (a), γ-CD (b), ethyl butyrate-β-CD (c), and ethyl butyrate-γ-CD (d) [79]
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. Transmission electron microscopes are capable of imaging at a significantly higher resolution than light microscopes. This enables the instrument to capture fine detail. The difference between TEM and SEM is that the surface topology can be obtained from SEM while more inner structure is given by TEM [81]. Bonini et al. [82] detected the structural features of cyclodextrin self-aggregates using TEM. They found the occurrence of polymorphism depending on the β-CD concentration: polydisperse nearly spherical objects with diameters of about 100 nm are present at lower concentrations, whereas micrometer planar aggregates are predominant at higher concentrations. TEM images of apple flavor β-CD inclusion complexes under different magnifications are presented in Fig. 9. As shown in Fig. 9, apple flavor β-CD inclusion complexes have many geometric shapes. Regular geometric shape such as d parallelogram aggregate can also be found from Fig. 9. Under high magnification, layered wormlike structure can be found. It suggests that apple flavor β-CD inclusion complexes are expressed through a bicontinuous ‘‘worm-type’’ pore system as reported in Ref. [83]. Using TEM, Hill et al. [84] characterized β-CD inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts). They found that all particles showed a spherical shape and smooth surfaces with a broad size distribution and a strong tendency to form clusters. The characterization of the inclusion complex between 2,5-dimethylpyrazine and HP-β-CD was performed using TEM by Tian et al. [59]. TEM studies revealed that the complex forms aggregate of nanometric size and the inclusion complexes were irregular nanoparticles.
Energy-dispersive X-ray spectroscopy (EDS) is an analytical technique used for the elemental analysis or chemical characterization of a sample. TEM-EDS can be used to further clarify the component and distribution of guest–host inclusion complex. By this method, an element map of mentha-8-thiol-3-one-β-CD inclusion complex obtained by Zhu et al. is presented as Fig. 10 [75]. The distributions of S, C and O can be observed obviously.
Element map of mentha-8-thiol-3-one-β-CD inclusion complex [67]
Complexation thermodynamics
Flavor compound encapsulation on a molecular basis can be performed by cyclodextrins. The inclusion of flavor compounds into the interior changes the properties of these flavor molecules, which may be used for a broad variety of applications. The affinity of flavor compounds for the cavities of various cyclodextrins depends on the stereochemistry and on the interaction forces of the molecules involved. Thermodynamics applies to a wide variety of topics in science and engineering, especially physical chemistry, chemical engineering and mechanical engineering. Calculations of the thermodynamic parameters are highly important for the inclusion reaction [85].
During the process of formation of inclusion complex, there are unbound state (in which guest and host are separate from each other) and bound state (in which guest and host are bounded together) [36]. Equilibrium between the unbound state and the bound state is as shown in Eq. (1) [86, 87]
where H is the host molecule, G is the guest molecule, and HG is the host–guest inclusion complex molecule.
Due to the interaction between guest and host molecules, there is a lower overall Gibbs free energy (ΔG0). The energy and thermodynamic properties of theses interactions found throughout the guest and host molecules are tried to measure to gain further insight into combinatorial outcome of these many, small, non-covalent forces that are used to generate an overall effect on the inclusion complex structure.
The association constant, Ka, is defined as Eq. (2). [36, 86]
where [HG]eq is the concentration of the host–guest complex in equilibrium, [H]eq is the concentration of the host in equilibrium, [G]eq is the concentration of the guest in equilibrium.
It is equal to the concentration of the host–guest complex divided by the product of the concentrations of individual host and guest molecules when the system is in equilibrium. A large Ka value indicates a strong complexation between guest and host molecules to form the host–guest complex.
The Gibbs free energy of the reaction can be obtained by knowing the equilibrium constant (Ka,), because ΔG is a function of Ka as shown in Eq. (3) [88].
where R is gas constant, T is absolute temperature.
To get thermodynamic parameters enthalpy (ΔH) and entropy (ΔS), Van’t Hoff Eq. (4) can be used.
Using the above equations, Ka, ΔG, ΔH and ΔS can be calculated. Some thermodynamic data reported for 1:1 complexation reaction with natural and modified cyclodextrins are presented in Tables 3 [86, 89, 90].
Calculation of flavor compounds inclusion complex with CDs
Computational chemistry, which can help rationalize experimental observation, is a multidisciplinary area of science transcending traditional barriers separating mathematics, physics, chemistry, and biology. Computational chemistry can provide information not amenable to experimentation, and even make predictions concerning the outcome of future experiments. Therefore, it is a valuable adjunct and is becoming more widely accepted by experimental scientists. Computational studies can lead to a more profound understanding of the structure, dynamics and chemical behavior of flavor compounds and cyclodextrins [91].
Geraniol has a characteristic rose-like odor. α-Terpineol has a characteristic lilac odor with a sweet taste reminiscent of peach on dilution. Magdalena et al. [92] investigated β-CD inclusion complexes with volatile molecules geraniol and α-terpineol enantiomers in solid state and in solution. Using an Opix computer program package, they calculated the intermolecular interactions and crystal energies and obtained the packing structure of β-CD/geraniol as shown in Fig. 11.
Packing diagram for β-CD/geraniol structure [92]
Estragole has an odor reminiscent of anise with a corresponding sweet taste, and is widely used in food as a flavoring agent and perfumery and medical industries. Yang et al. [67] simulated the inclusion processes between β-CD and estragole using the semiempirical PM3 and ONIOM [B3LYP/6-31 g(d):PM3] methods. They obtained the structures with energy minimum from ONIOM calculations. The binding energies from ONIOM (B3LYP/6-31G(d):PM3) calculations for the estragole/β-CD head-up and head-down orientation were − 73.49 and − 78.64 kJ/mol, respectively. It revelated that the complex in the head-down orientation is more stable than that in the head-up orientation.
Cinnamaldehyde, as a common flavor material, has a strong cinnamon odor and excellent antifungal activity. Cinnamyl acetate has a characteristic balsamic-floral odor and burning, sweet taste reminiscent of pineapple, and can be used as a flavoring agent in beverages and foods. Chai et al. [87] applied a density functional theory simulation for the inclusion process of cinnamaldehyde and cinnamyl acetate with β-CD. The host–guest complex compounds were constructed by moving the guest through the cavity of β-CD from the wider rim, or from the smaller rim. The optimized geometries of complexes were further calculated at a single point by the two layered hybrid ONIOM2 (B3LYP/6-31G: PM3) method and all calculations were performed with GAUSSIAN 2009. They obtained the stable structures of the inclusion complexes and binding energies. In the molecular conformation of the cinnamyl acetate complex, the aromatic ring of cinnamyl acetate is totally inserted in β-CD cavity, whereas the ester group of cinnamyl acetate is located at its wider edge. In the case of the cinnamaldehyde complex, the aldehyde group lies on the wide rim of the cavity and the aromatic moiety is also totally engulfed within the β-CD macrocycle. Because the binding energy of the cinnamyl acetate complex obtained by calculation is higher than that of the cinnamaldehyde complex, the inclusion complex of cinnamyl acetate is more stable than cinnamaldehyde.
Molecular mechanics (MM), which use an empirical force field, is a nonquantum mechanical method of computing structures, energies, and some properties of molecules. MM is much faster than quantum mechanics and can be used to reliably predict structures of small- to medium-sized molecules quickly or to evaluate very large molecular such as cyclodextrins. The information regarding the inclusion process, energy and structure of inclusion complexes, the formation of inclusion complex at the molecular level can be examined using MM calculations. Geometries of HP-β-CD, l-menthol, l-menthol-HP-β-CD inclusion complex, geranial, neral, geranial-MCT-β-CD and neral-MCT-β-CD were optimized using MM2 calculations. The minimum binding energy and the most stable structure of these inclusion complexes were obtained using MM2 calculations [72, 83]. These results are important for a deep understanding of the interaction between guest and host, and the reaction mechanism.
Conclusions
Flavors play important roles in the daily life and have widely used in many products. Because flavors are composed of volatile aroma compounds, they do not often last long in air especially at high temperature. Furthermore, the sensory perception of a flavor can also be changed easily as a result of volatilization, heating, oxidation or chemical interactions. The flavor performance is usually measured by its lastingness, stability together with the pleasantness. The use of techniques for slow flavor release, long-lasting scents, and for the stability of sensory perception, is much desired. Encapsulation is a method of protecting ingredients that are sensitive oxidation, temperature, moisture, and so on. CDs have been used extensively in encapsulation research. Flavor compounds can be encapsulated in the cavities of CDs by formation of inclusion complexes with CDs. The inclusion complexes formed by flavors and CDs can achieve relatively high temperature stability and prolonged shelf-life. Furthermore, the solubility of some flavor compounds which are insoluble in water can be improved by formation of inclusion complex. The formation of inclusion complex flavor with CDs is an effective method to improve stability and lastingness of flavors.
References
Wells, F.V., Billot, M.: Perfumery Technology: Art, Science, Industry. Ellis Horwood Ltd, New York (1981)
Zhang, C., Wang, Q.: Perfumery. China Light Industry Press, Beijing (1989). (in Chinese)
Yu, G., Wu, G.: Perfumery Technology. China Light Industry Press, Beijing (2006). (in Chinese)
Zhou, Y., Xiao, Z.: The Preparation Technology of Flavors. China Textile & Apparel Press, Beijing (2012). (in Chinese)
Sun, B., Chen, H.: The Technology of Food Flavoring. Chemical Industry Press, Beijing (2016). (in Chinese)
Surburg, H., Panten, J.: Common Fragrance and Flavor Materials. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (2016)
Zhu, G., Xiao, Z.: Creation and imitation of a milk flavor. Food Funct. 8, 1080–1084 (2017)
Zhu, G., Xiao, Z.: Study on creation of an indocalamus leaf flavor. Food Sci. Technol. 35, 647–651 (2015)
Zhu, G., Xiao, Z., Zhou, R., Lei, D.: Preparation and simulation of a taro flavor. Chin. J. Chem. Eng. 23, 1733–1735 (2015)
Burdock, G.A.: Fenaroli’s Handbook of Flavor Ingredients. CRC Press, Boca Raton (2010)
Zhu, G., Xiao, Z., Zhou, R., Zhu, Y., Niu, Y.: Study on development of a fresh peach flavor. Adv. Mater. Res. 781–784, 1570–1573 (2013)
Teixeira, M.A., Rodriguez, O., Gomes, P., Mata, V., Rodrigues, A.E.: Perfume Engineering. Elsevier, Amsterdam (2013)
Zhu, G., Xiao, Z., Zhou, R., Yi, F.: Fragrance and flavor microencapsulation technology. Adv. Mater. Res. 535–537, 440–445 (2012)
Marques, H.M.C.: A review on cyclodextrin encapsulation of essontial oils and volatiles. Flavour Fragr. J. 25, 313–326 (2010)
Ishiguro, T., Sakata, Y., Arima, H., Iohara, D., Anraku, M., Uekama, K., Hirayama, F.: Release control of fragrances by complexation with β-cyclodextrin and its derivatives. J. Incl. Phenom. Macrocycl. Chem. 92, 147–155 (2018)
Saenger, W., Jacob, J., Gessler, K., Steiner, T., Hoffmann, D., Sanbe, H., Koizumi, K., Smith, S.T., Takaha, T.: Structure of the common cyclodextrins and their larger analogues-beyond the doughnut. Chem. Rev. 98, 1787–1802 (1998)
Takahashi, K.: Organic reactions mediated by cyclodextrins. Chem. Rev. 98, 2013–2033 (1998)
Szejtli, J.: Cyclodextrin Technology. Springer, Netherlands (1988)
Zhu, G., Xiao, Z., Zhou, R., Niu, Y.: Pyrolysis characteristics and kinetics of β-cyclodextrin and its two derivatives. Polish J. Chem. Technol. 17, 1–4 (2015)
Yildiz, Z.I., Celebioglu, A., Kilic, M.E., Durgun, E., Uyar, T.: Menthol/cyclodextrin inclusion complex nanofibers: enhanced water solubility and high-temperature stability of menthol. J. Food Eng. 224, 27–36 (2018)
Ciobanu, A., Landy, D., Fourmentin, S.: Complexation efficiency of cyclodextrins for volatile flavor compounds. Food Res. Int. 53, 110–114 (2013)
Cai, R., Yuan, Y., Cui, L., Wang, Z., Yue, T.: Cyclodextrin-assisted extraction of phenolic compounds: current research and future prospects. Trends Food Sci. Tech. 79, 19–27 (2018)
Rakmai, J., Cheirsilp, B., Cid, A., Torrado-Agrasar, A., Mejuto, J.C., Simal-Gandara, J.: Encapsulation of essential oils by cyclodextrins: characterization and evaluation. IntechOpen (2018). https://doi.org/10.5772/intechopen.73589
Yildiz, Z.I., Celebioglu, A., Kilic, M.E., Durgun, E., Uyar, T.: Fast-dissolving carvacrol/cyclodextrin inclusion complex electrospun fibers with enhanced thermal stability, water solubility, and antioxidant activity. J. Mater. Sci. 53, 15837–15849 (2018)
Kfoury, M., Pipkin, J.D., Antle, V., Fourmentin, S.: Captisol®: an efficient carrier and solubilizing agent for essential oils and their components. Flavour Fragr. J. 32, 340–346 (2017)
Hadi, B.J., Sanagi, M.M., Ibrahim, W.A.W., Jamil, S., AbdullahiMu’azu, M., Aboul-Enein, H.Y.: Ultrasonic-assisted extraction of curcumin complexed with methyl-β-cyclodextrin. Food Anal. Methods 8, 1373–1381 (2015)
Temel, G., Parali, T., Tulu, M., Arsu, N.: Photopolymerization of acrylamide with benzophenone/methylated-β-cyclodextrin inclusion complex in the presence of jeffamine based dendrimers as coinitiators in aqueous media. J. Photoch. Photobio. A 213, 46–51 (2010)
Chittiteeranon, P., Soontaros, S., Pongsawasdi, P.: Preparation and characterization of inclusion complexes containing fixolide, a synthetic musk fragrance and cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 57, 69–73 (2007)
Sangpheak, W., Kicuntod, J., Schuster, R., Rungrotmongkol, T., Wolschann, P., Kungwan, N., Viernstein, H., Mueller, M., Pongsawasdi, P.: Physical properties and biological activities of hesperetin and naringenin in complex with methylated β-cyclodextrin. Beilstein J. Org. Chem. 11, 2763–2773 (2015)
Rehmann, L., Yoshii, H., Furuta, T.: Characteristics of modified β-cyclodextrin bound to cellulose powder. Starch 55, 313–318 (2003)
Furuta, T., Kusuya, Y., Neoh, T.-L., Rehmann, L., Beak, S.-H., Yoshii, H.: Inclusion and release of hinokitiol into/from MCT-β-CD fixed on Japanese Washi paper. J. Incl. Phenom. Macrocycl. Chem. 56, 107–111 (2006)
Khanna, S., Sharma, S., Chakraborty, J.N.: Performance assessment of fragrance finished cotton with cyclodextrin assisted anchoring hosts. Fash. Text. 2, 19 (2015)
Wang, J., Chen, J.-Z.: Preparation of carboxymethyl-β-cyclodextrin. Food Sci. 30, 98–100 (2009). (in Chinese)
Zhang, Y., Jing, D.: Improved preparation of carboxymethyl-β-cyclodextrin. Spec. Petrochem. 30, 55–58 (2013). (in Chinese)
Takenaka, Y., Nakashima, H., Yoshida, N.: Fluorescent amino-β-cyclodextrin derivative as a receptor for various types of alcohols having cyclic and macrocyclic rings. J. Mol. Struct. 871, 149–155 (2007)
Tong, L.: Cyclodextrin Chemistry: Fundamentals and Application. China Science Publishing & Medial Ltd., Beijing (2001)
Ren, X., Yue, S., Xiang, H., Xie, M.: Inclusion complexes of eucalyptus essential oil with β-cyclodextrin: preparation, characterization and controlled release. J. Porous Mat. 25, 1577–1586 (2018)
Zhu, G., Xiao, Z., Zhou, R., Feng, N.: Production of a transparent lavender flavour nanocapsule aqueous solution and pyrolysis characteristics of flavour nanocapsule. J. Food Sci. Technol. 52, 4607–4612 (2015)
Reineccius, T.A., Reineccius, G.A., Peppard, T.L.: The effect of solvent interactions on alpha-, beta-, and gamma-cyclodextrin/flavor molecular inclusion complexes. J. Agric. Food Chem. 53, 388–392 (2005)
Jin, Z.: Cyclodextrin Chemistry: Preparation and Application. Chemical Industry Press, Beijing (2009). (in Chinese)
Xiao, Z., Feng, N., Zhu, G., Niu, Y.: Preparation and application of citral-monochlorotriazine-β-cyclodextrin inclusion complex nanocapsule. J. Text. I. 107, 64–71 (2016)
Petrović, G.M., Stojanović, G.S., Radulović, N.S.: Encapsulation of cinnamon oil in β-cyclodextrin. J. Med. Plants Res. 4, 1382–1390 (2010)
Barbieri, N., Sanchez-Contreras, A., Canto, A., Cauich-Rodriguez, J.V., Vargas-Coronado, R., Calvo-Irabien, L.M.: Effect of cyclodextrins and Mexican oregano (Lippia graveolens Kunth) chemotypes on the microencapsulation of essential oil. Ind. Crop Prod. 121, 114–123 (2018)
Bhandari, B.R., D’Arcy, B.R., Bich, L.L.T.: Lemon oil to β-cyclodextrin ratio effect on the inclusion efficiency of β-cyclodextrin and the retention of oil volatiles in the complex. J. Agric. Food Chem. 46, 1494–1499 (1998)
Padukka, I., Bhandari, B., D’Arcy, B.: Evaluation of various extraction methods of encapsulated oil from β-cyclodextrin-lemon oil complex powder. J. Food Compos. Anal. 13, 59–70 (2000)
Reineccius, T.A., Reineccius, G.A., Peppard, T.L.: Utilization of β-cyclodextrin for improved flavor retention in thermally processed foods. J. Food Sci. 69, 58–62 (2004)
Reineccius, T.A., Reineccius, G.A., Peppard, T.L.: Encapsulation of flavors using cyclodextrins: comparison of flavor retention in alpha, beta, and gamma types. J. Food Sci. 67, 3271–3279 (2002)
Fernandes, L.P., Oliveira, W.P., Sztatisz, J., Szilágyi, I.M., Novák, C.: Solid state studies on molecular inclusions of lippia sidoides essential oil obtained by spray drying. J. Therm. Anal. Calorim. 95, 855–863 (2009)
Fenyvesi, E., Zemlényi, C., Orgoványi, J., Oláh, E., Szente, L.: Can conversion mixture substitute beta-cyclodextrin in encapsulation of essential oils and their components? J. Incl. Phenom. Macrocycl. Chem. 86, 55–66 (2016)
Martins, A.D.P., Craveiro, A.A., Machado, M.I.L., Raffin, F.N., Moura, T.F., Novák, C., Éhen, Z.: Preparation and characterization of mentha x villosa hudson oil-β-cyclodextrin complex. J. Therm. Anal. Calorim. 88, 363–371 (2007)
Bhandari, B.R., D’Aecy, B.R., Padukka, I.: Encapsulation of lemon oil by paste method using β-cyclodextrin: encapsulation efficiency and profile of oil volatiles. J. Agric. Food Chem. 47, 5194–5197 (1999)
Valle, E.M.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Zhu, G., Xiao, Z., Zhou, R., Zhu, G., Niu, Y.: Kinetics and release characteristics of menthyl acetate from its β-cyclodextrin inclusion complex by thermogravimetric analysis. J. Incl. Phenom. Macrocycl. Chem. 84, 219–224 (2016)
Deng, S., Liu, H., Qi, C., Yang, A., Li, Z.: Study on preparation and inclusion behavior of inclusion complexes between β-cyclodextrin derivatives with benzophenone. J. Incl. Phenom. Macrocycl. Chem. 90, 321–329 (2018)
Neto, A.C.D.R., Rocha, ABDOd, Maraschin, M., Piero, R.M.D., Almenar, E.: Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocoll. 77, 509–523 (2018)
Mcnamara, M., Russell, N.R.: FT-IR and Raman spectra of a series of metallo-β-cyclodextrin complexes. J. Incl. Phenom. Mol. Recognit. Chem. 10, 485–495 (1991)
Russell, N.R., Mcnamara, M.: FT-IR and Raman spectral evidence for metal complex formation with β-cyclodextrin as a first sphere ligand. J. Incl. Phenom. Mol. Recognit. Chem. 7, 455–460 (1989)
Egyed, O.: Spectroscopic studies on β-cyclodextrin. Vib. Spectrosc. 1, 225–227 (1990)
Tian, H., Xu, T., Dou, Y., Li, F., Yu, H., Ma, X.: Optimization and characterization of shrimp flavor nanocapsules containing 2,5-dimethylpyrazine using an inclusion approach. J. Food Process. Pres. 41, 1–8 (2017)
Guan, X.-L., Fu, J.-H., Tang, J.: Preparation process and property analysis of inclusion complex of lavender oil with hydroxypropyl-β-cyclodextrin. Food Sci. Technol. 42, 230–234 (2017). (in Chinese)
Amado, A.M., Ribeiro-Claro, P.J.A.: Selection of substituted benzaldehyde conformers by the cyclodextrin inclusion process: a Raman spectroscopic study. J. Raman Spectrosc. 31, 971–978 (2000)
Chen, Q., Guo, P.: Study on the inclusion compound of menthol with hydroxypropyl-beta-cyclodextrin by Raman spectroscopy. China J. Pham. Anal. 29, 1528–1532 (2009). (in Chinese)
Zhang, M.-M., Liu, Y.-P., Yin, L.: Nuclear magnetic resonance technology to establish the interaction of cyclodextrin system. Guangzhou Chem. Ind. 39, 13–16 (2011)
Locci, E., Lai, S., Piras, A., Marongiu, B., Lai, A.: 13C-CPMAS and 1H-NMR study of the inclusion complexes of β-cyclodextrin with carvacrol, thymol, and eugenol. Prepared in supercritical carbon dioxide. Chem. Biodivers. 1, 1354–1366 (2004)
Cao, S.-N., Zhang, Y., Jin, S.-S., Zhang, S., Yang, L.-P., Zhou, Y.-B.: Preparation and release characterization of inclusion complex of ethyl propionate with β-cyclodextrin. Sci. Technol. Food Ind. 36, 124–127 (2015). (in Chinese)
Cao, S., Zhang, Y., Jin, S., Zhang, S., Yang, L., Zhou, Y.: Preparation and release characterization of valeric acid-α-cyclodextrin inclusion complex. J. Anhui Agr. Univ. 42, 700–705 (2015)
Yang, Z., Huang, L., Yao, X., Ji, H.: Host-guest complexes of estragole with β-cyclodextrin: an experimental and theoretical investigation. Flavour Fragr. J. 32, 102–111 (2017)
Zhang, G., Yuan, C., Sun, Y.: Effect of selective encapsulation of hydroxypropyl-β-cyclodextrin on components and antibacterial properties of star anise essential oil. Molecules 23, 1126 (2018)
Yang, Z., Yao, X., Xiao, Z., Chen, H., Ji, H.: Preparation and release behaviour of the inclusion complexes of phenylethanol with β-cyclodextrin. Flavour Fragr. J. 31, 206–216 (2016)
Dou, S., Ouyang, Q., You, K., Qian, J., Tao, N.: An inclusion complex of thymol into β-cyclodextrin and its antifungal activity against Geotrichum citri-aurantii. Postharvest Biol. Technol. 138, 31–36 (2018)
Barbieri, N., Sanchez-Contreras, A., Canto, A., Cauich-Rodriguez, J.V., Vargas-Coronado, R., Calvo-Irabien, L.M.: Effect of cyclodextrins and Mexican oregano (Lippia graveolens Kunth) chemotypes on the microencapsulation of essential oil. Ind. Crop. Prod. 121, 114–123 (2018)
Zhu, G., Xiao, Z., Zhu, G., Zhou, R., Niu, Y.: Encapsulation of l-menthol in hydroxypropyl-β-cyclodextrin and release characteristics of the inclusion complex. Polish J. Chem. Technol. 18, 110–116 (2016)
Wadhwa, G., Kumar, S., Chhabra, L., Mahant, S., Rao, R.: Essential oil–cyclodextrin complexes: an updated review. J. Incl. Phenom. Macrocycl. Chem. 89, 39–58 (2017)
Ozdemir, N., Pola, C.C., Teixeira, B.N., Hill, L.E., Bayrak, A., Gomes, C.L.: Preparation of black pepper oleoresin inclusion complexes based on beta-cyclodextrin for antioxidant and antimicrobial delivery applications using kneading and freeze drying methods: a comparative study. LWT—Food Sci. Technol. 91, 439–445 (2018)
Zhu, G., Xiao, Z., Zhu, G.: Preparation, characterization and the release kinetics of mentha-8-thiol-3-one-β-cyclodextrin inclusion complex. Polym. Bull. 74, 2263–2275 (2017)
Zhu, G., Xiao, Z., Zhou, R., Zhu, Y.: Study of production and pyrolysis characteristics of sweet orange flavor-β-cyclodextrin inclusion complex. Carbohyd. Polym. 105, 75–80 (2014)
Bonetti, P., Moraes, F.F.S., Zanin, G.M., Bergamasco, R.D.C.: Thermal behavior study and decomposition kinetics of linalool/b-cyclodextrin inclusion complex. Polym. Bull. 73, 279–291 (2016)
Oliva, E., Mathiron, D., Bertaut, E., Landy, D., Cailleu, D., Pilard, S., Clément, C., Courot, E., Bonnet, V., Djedaïni-Pilard, F.: Physico-chemical studies of resveratrol, methyl jasmonate and cyclodextrin interactions: an approach to resveratrol bioproduction optimization. RSC Adv. 8, 1528–1538 (2018)
Zhang, Y., Zhou, Y., Cao, S., Li, S., Jin, S., Zhang, S.: Preparation, release and physicochemical characterisation of ethyl butyrate and hexanal inclusion complexes with β- and γ-cyclodextrin. J. Microencapsul. 32, 711–718 (2015)
Barbieri, N., Sanchez-Contreras, A., Canto, A., Cauich-Rodriguez, J.V., Vargas-Coronado, R., Calvo-Irabien, L.M.: Effect of cyclodextrins and Mexican oregano (Lippia graveolens Kunth) chemotypes on the microencapsulation of essential oil. Ind. Crop. Prod. 121, 114–123 (2018)
He, Y., Hu, P., Shen, X., Gao, H.: Cyclodextrin-based aggregates and characterization by microscopy. Micron 39, 495–516 (2008)
Bonini, M., Rossi, S., Karlsson, G., Almgren, M., Nostro, P.L., Baglioni, P.: Self-assembly of β-cyclodextrin in water. Part 1: cryo-TEM and dynamic and static light scattering. Langmuir 22, 1478–1484 (2006)
Zhu, G., Feng, N., Xiao, Z., Zhou, R., Niu, Y.: Production and pyrolysis characteristics of citral-monochlorotriazinyl-β-cyclodextrin inclusion complex. J. Therm. Anal. Calorim. 120, 1811–1817 (2015)
Hill, L.E., Gomes, C., Taylor, T.M.: Characterization of beta-cyclodextrin inclusion complexes containing essential oils (trans-cinnamaldehyde, eugenol, cinnamon bark, and clove bud extracts) for antimicrobial delivery applications. LWT—Food Sci. Technol. 51, 86–93 (2018)
Charumanee, S., Titwan, A., Sirithunyalug, J., Weiss-Greiler, P., Wolschann, P., Viernstein, H., Okonogi, S.: Thermodynamics of the encapsulation by cyclodextrins. J. Chem. Technol. Biotechnol. 81, 523–529 (2006)
Tian, X.-N., Jiang, Z.-T., Li, R.: Inclusion interactions and molecular microcapsule of Salvia sclarea L. essential oil with β-cyclodextrin derivatives. Eur. Food Res. Technol. 227, 1001–1007 (2008)
Chai, K., Xu, Z., Zheng, L., Zhou, L., Tong, Z., Ji, H.: Facile separation of cinnamyl acetate and cinnamaldehyde based on host–guest complexation with β-cyclodextrin. Flav. Fragr. J. 33, 285–293 (2018)
Jiang, S., Li, J.-N., Jiang, Z.-T.: Inclusion reactions of β-cyclodextrin and its derivatives with cinnamaldehyde in Cinnamomum loureirii essential oil. Eur. Food Res. Technol. 230, 543–550 (2010)
Rekharsky, M.V., Inoue, Y.: Complexation thermodynamics of cyclodextrins. Chem. Rev. 98, 1875–1917 (1998)
Baránková, E., Dohnal, V.: Effect of additives on volatility of aroma compounds from dilute aqueous solutions. Fluid Phase Equilibr. 407, 217–223 (2016)
Lipkowitz, K.B.: Applications of computational chemistry to the study of cyclodextrins. Chem. Rev. 98, 1829–1873 (1998)
Magdalena, C., Kamila, S., Monika, A., Małgorzata, W.-R., Ewa, K., Kinga, S.: Study of β-cyclodextrin inclusion complexes with volatile molecules geraniol and α-terpineol enantiomers in solid state and in solution. Chem. Phys. Lett. 641, 44–50 (2015)
Acknowledgements
This work was financially supported by the National Key R&D Program of China (2016YFA0200300), Shanghai Alliance Program (LM201844), and sponsored by Shanghai Gaofeng & Gaoyuan Project for University Academic Program Development.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhu, G., Zhu, G. & Xiao, Z. A review of the production of slow-release flavor by formation inclusion complex with cyclodextrins and their derivatives. J Incl Phenom Macrocycl Chem 95, 17–33 (2019). https://doi.org/10.1007/s10847-019-00929-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-019-00929-3