Abstract
Three novel chromogenic cone azocalix[4]arenes 5a–c, which have cavity and the azo groups as metal-binding sites and as coloration sites were synthesized. They were studied by the liquid–liquid extraction of selected metal cations (Sr2+, Ag+, Hg+, Hg2+, Co2+, Ni2+, Cu2+, Zn2+, Cr3+, Al3+). Through examination of the extraction, a novel selectivity of these compounds toward Hg2+ cations has been determined. Besides, it has been also found that azocalix[4]arene 5c is highly sensitive to acid–base titration, which can be detected by the naked eye.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calix[4]arenes have been shown to be useful molecular scaffold in the development of chromoionophores, especially for metal ion recognition [1–3]. Azocalix[n]arenes, generated by the insertion of nitrogen atoms into the p- position unit of the calix[n]arene structure, have several isomers based on the positions of the nitrogen atoms and its ring cavity. The first reported calixarene diazo coupling has involved the reaction of p-nitrobenzenediazonium tetrafluoroborate with calix[4]arene [4, 5].
The synthesis of new chemosensors for metal ions plays an important role in the field of supramolecular chemistry due to their fundamental agent in biological, environmental, and chemical processes. Chromogenic ionophores have been intensively investigated as a specific metal ion indicator for the use of 4-(4-nitrophenyl)azo-coupled crowns and azacrowns as chromoionophores. They showed large UV–Vis band shifts when cations were added [6–8].
Over the last few decades, as a major activity in supramolecular chemistry, chemists have synthesized many types of receptors for complexing cations, anions and neutral molecules both in solution and in the solid state [9]. Ungaro et al. [10] have first reported the binding of potassium by calix[4]crown-5 ligands. The use of crown ethers covalently bound to calix[4]arenes has been studied extensively for selective extraction of cations. Their studies have expanded to include calix[4]arene in modified crown structures. Among them, the family of calix[4]azacrowns refers to molecules combining calix[4]arene elements, which may also serve as linking functions and chelating groups [11]. The linkage of the azacrown unit on the calix[4]arene is often conducted by coupling diacylchloride or diethylester calix derivatives with polyamino alkylene diamino compounds to give bridged calix(aza)crowns [12].
Much of our earlier work in this area has concentrated on calix[n]arene with lower rim and upper rim in the form of mono oxime, vic-dioxime, polymeric, Schiff base, azo groups and telomeric structure [13, 14]. Extraction, transport, thermal behavior and stability constant which have been investigated by spectrophotometric studies have provided evidences that many of these lower rim derivatives have very significant ionophoric properties for cations, several with good selectivity within groups of metals [15–17].
In the crystal state, some calix[4]azacrowns have been shown to form tubular architectures, in which the channels are filled by methanol [18].
In the examples given in the literature, the calix core of the azocalix[4]arenes is bridged either by one or two bridge. In this Letter, we report here the synthesis of a novel chromogenic 5,17-bisazocalix[4]arenes using the click chemistry [19] of a calix ester and an aniline derivatives to form a diazo cationic binding site. Three azocalix[4]arenes 5a–c are connected via diazo coupling reaction in cone conformation and compared with the extraction properties of both compounds.
In continuation of our work, our research group has been also interested in the designing selective coloring chemosensor towards specific metal cations. In the end, we found an outstanding result that combinational use of the compounds 5a–c are able to selectively discriminate metal ions.
Experimental
Chemicals
All of the chemical reagents and solvents used were of analytical grade purity and used without further purification. All aqueous solutions were prepared with deionized water purified by human power plus I + UV water purification system.
Instrumentation
Melting points were measured using an Electrothermal IA9100 digital melting point apparatus in capillaries sealed under nitrogen and were uncorrected. 1H NMR spectra were referenced to tetramethylsilane (TMS) at 0.00 ppm as internal standard solution and recorded on a Bruker 400 MHz spectrometer at room temperature (25 ± 1 °C). IR spectra were recorded by a Mattson 1000 FTIR spectrometer as KBr pellets. UV–Vis spectra were recorded by a Shimadzu 1601 UV–Visible spectrophotometer. The elemental analyses were performed in the The Scientific and Technological Research Council of Turkey (TUBITAK) Laboratories.
Synthesis
The synthesis of bisazocalix[4]arene derivatives 5a–c were depicted in Scheme 1. Our synthesis began p-tert-butylcalix[4]arene and their dibenzoyl ester 1 were obtained in literature [20, 21]. First, 11,23-di(p-tert-butyl)calix[4]arene dibenzoyl ester 2 was prepared by debutylation of calix 4arene 1. Secondly, the synthesized calix 4arene 2 was hydrolized by NaOH in THF/Water/EtOH [22].
After then, the synthesis of azocalix[4]arenes 4a–c were coupled with 11,23-di(tert-butyl)calix[4]arene 3 followed by diazo coupling reaction using 4-methoxy, 4-ethyl and 4-nitro aniline in HCl and NaNO2 in DMF and THF gave the desired product 4 [23]. AlCl3-catalyzed debutylation reaction of azocalixarenes 4a–c with toluene/phenol in the Click condition afforded the 5,17-bis(p-substituephenyl)azocalix[4]arenes 5a–c in 68–83 % yield [22]. Azocalixarene 5a was synthesized in 79 % yield from azocalixarene 4a. Compound 5c was purified by column chromatography from calix[4]aren [24].
25,26,27,28-Tetrahydroxy-5,17-(4-methoxyphenylazo)calix[4]arene (5a)
A mixture of compound 4a (1 g, 1.24 mmol), phenol (1.17 g, 12.42 mmol) and AlCl3 (2.15 g, 16.15 mmol) in 100 mL of toluene was stirred at room temperature for 24 h. HCl (50 mL, 0.2 M) was added dropwise into the toluene solution. The toluene phase was separated and washed with water (3 × 50 mL), and the combined organic phases evaporated to dryness. The residue was precipitated with MeOH (100 mL). After removal of solvents, the crude mixture was crystallized with CHCl3/CH3OH (50 mL, 2:3 v/v)) to give 0.68 g of compound 5a (79 %), brown precipitate, m.p. (318 °C (dec.)). Found: C: 72.96 %; H: 5.17 % N: 7.98 %; C42H36N4O6 requires C: 72.82 %; H: 5.24 % N: 8.09 %. IR (KBr) υ: 1,458 cm−1 (–N=N). 1H-NMR (CDCl3, 25 °C) δH: 3.67 (s, 4H, ArCH 2Ar), 3.85 (s, 6H, –OCH 3 ), 4.31 (s, 4H, ArCH 2Ar), 6.79 (t, J = 7.56 Hz, 2H, ArH), 6.95 (d, J = 9.05 Hz, 4H, ArH), 7.18 (d, J = 7.60 Hz, 4H, ArH), 7.65 (s, 4H, ArH), 7.80 (d, J = 9.07 Hz, 4H, ArH), 10.21 (s, 4H, ArOH).
25,26,27,28-Tetrahydroxy-5,17-(4-ethylphenylazo)calix[4]arene (5b)
Azocalix[4]arene 5b was prepared as described for 5a using phenol, AlCl3 and toluene and obtained which was filtered, washed with water, precipitated with MeOH, crystallized with CHCl3/CH3OH (50 mL, 2:3 v/v). The resulting solid was a dark orange product (yield, 0.71 g (83 %), m.p. (295 °C (dec.)). Found: C: 76.85 %; H: 5.79 % N: 8.07 %; C44H40N4O4 requires C: 76.72 %; H: 5.85 % N: 8.13 %. IR (KBr) υ: 1,458 cm−1 (–N=N). 1H-NMR (CDCl3, 25 °C) δH: 1.26 (t, J = 7.53 Hz, 6H, CH2 CH 3 ), 2.70 (q, J = 7.45 Hz, 4H, CH 2 CH3), 3.69 (s, 4H, ArCH 2Ar), 4.31 (s, 4H, ArCH 2Ar), 6.80 (t, J = 7.54 Hz, 2H, ArH), 7.19 (d, J = 7.57 Hz, 4H, ArH), 7.28 (d, J = 8.22 Hz, 4H, ArH), 7.69 (s, 4H, ArH), 7.74 (d, J = 8.27 Hz, 4H, ArH), 10.23 (s, 4H, ArOH).
25,26,27,28-Tetrahydroxy-5,17-(4-nitrophenylazo)calix[4]arene (5c)
Azocalix 4arene 5c was prepared as described for 5a using phenol, AlCl3 and toluene and obtained which was filtered, washed with water, precipitated with MeOH, crystallized with CHCl3/CH3OH (50 mL, 2:3 v/v). The resulting solid was a dark brown product (yield, 0.59 g (68 %), m.p. (312 °C (dec.)). Found: C: 66.55 %; H: 4.09 % N: 11.52 %; C40H30N6O8 requires C: 66.48 %; H: 4.18 % N: 11.63 %. IR (KBr) υ: 1,458 cm−1 (–N=N). 1H-NMR (CDCl3, 25 °C) δH: 3.75 (s, 4H, ArCH 2Ar), 4.37 (s, 4H, ArCH 2Ar), 6.86 (t, J = 7.59 Hz, 2H, ArH), 7.23 (d, J = 7.61 Hz, 4H, ArH), 7.78 (s, 4H, ArH), 7.94 (d, J = 9.08 Hz, 4H, ArH), 8.36 (d, J = 9.08 Hz, 4H, ArH), 10.23 (s, 4H, ArOH).
Result and discussion
The molecular structure of bisazocalix[4]arenes 5a–c given in Scheme 1 were in agreement with the data obtained from micro analysis. Besides the structures of all these azocalix[4]arenes 5a–c were also certified by traditional organic spectroscopic identification (1H-NMR and FTIR spectra). They confirmed the structures to be in cone conformations.
The 1H NMR spectrum of the azocalix[4]arene 5a exists in a cone conformation was deduced from the presence of two set of characteristic AB systems at 3.67 and 4.31 ppm respectively. On the basis of spectroscopic evidence, molecular structure of compounds 5a is within cone conformation (Fig. 1).
1H NMR data all of the bisazocalix[4]arenes 5a–c showed that a peak due to the presence of aromatic protons (phenylazo) were observable at the chemical shift δ = 6.95–7.80 ppm for methoxy-, 7.28–7.74 ppm for ethyl- and 7.94–8.36 ppm for nitro-, respectively. However, the appearance of a peak within the range of δ = 6.79, 7.18 and 7.65 ppm were due to the presence of proton of core calixarene aromatic group for azocalix[4]arene 5a.
In the 1H NMR spectra of the bisazocalix[4]arenes 5a–c, the singlets in the 10.21 and 10.23 ppm area can be attributed to the proton of the –OH groups. In addition, the 4-ethyl moiety of azocalix[4]arene 5b are shown at 1.26 ppm methyl and 2.70 ppm methylene protons as a triplet and quarted, respectively. The methoxy (–OCH3) groups of 5a is shown at 3.85 ppm as a singlet too (Fig. 1). The OH peaks disappear together with addition of the D2O.
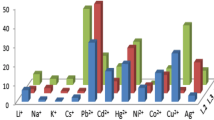
The ionophoric properties of the bisazocalix[4]arenes 5a–c towards the metal cations were investigated by the picrate extraction method [15–17]. The results expressed as a percentage of cation extracted (E%) are collected in Table 1 and shown graphically in Fig. 2.
The extractions of these cations (Sr2+, Ag+, Hg+, Hg2+, Co2+, Ni2+, Cu2+, Zn2+, Cr3+ and Al3+) with bisazocalix[4]arenes 5a–c have been performed using previously reported method in same experimental conditions. Unlike the calix[4]arene used in the previously studies contained –N=N– functional groups, in this study the bisazocalix[4]arene derivatives containing electron-donating and withdrawing groups such as methoxy-, ethyl- and nitro-groups have been selected. The reason why these bisazocalix[4]arenes are selected. Because, these functional groups are containing the electron-donating and electron-withdrawing groups. This situation increases or decrease the efficiency of extraction. This is mainly due to the positive or negative effect of these functional groups on electron density leading higher efficiencies of extraction. Besides a comparison with our data is corrected, some remarks can be made. While the extraction levels [35.8 % Hg+ and 40.3 % Hg2+] for 5b are very superior to the extraction levels [30.7 % Hg+ and 39.8 % Hg2+] and [29.6 % Hg+ and 38.1 % Hg2+] for 5a and 5c respectively. Compound 5b contain electron-donating group which preferres superior to soft metal (Hg2+) cation than compounds 5a and 5c. It can be explained by the (hard–soft) acid–base (HSAB) principle.
On the other hand, the complexation ability of azocalix[4]arens toward transition metal cations has been reported in the literature [25]. We titrated 0.01 g of bisazocalix[4]arene 5c in CHCl3–CH3OH–H2O (200 mL, 1:1:8 v/v)] with dilute aqueous HCl/NaOH (1.25 × 10−4 M) at room temperature. The observed color change may be ascribed to the protonation or deprotonation of the phenol, –N=N–, –NO2 moiety (Fig. 3).
The visible color changes of bisazocalix[4]arene 5c upon different pH values were also observed and depicted. The color of 5c solution turned orange to dark red in the presence of acid–base solution. In this unique point of view with visual color changes, it is surprising that only we can consequently demonstrate the screening process of the unknown pH (about 2 and 12) with following two steps and simple screening of its color change with ‘naked eye’ is given in Fig. 3.
The color change of the solution has been observed for the very popular phenylazo bisazocalix[4]arene 5c when dilute aqueous HCl/NaOH had separately been added in excess. These observations imply that there is a subtle balance between in base-induced release of protons from the azophenols to the quinone tautomer. Moreover, the nitro-substituents of the phenylazo groups must have strong influence on the tautomerism of azo/hydrazo, thus showing very diverse λ max shifts. A base-induced tautomerism of the bisazocalix[4]arene 5c is shown in Scheme 2.
Conclusion
In summary, we have successfully synthesized and investigated compounds 5a–c as mercury-ion selective sensor based on metal picrate extraction, pH change, 1H NMR and FTIR spectra. The present paper demonstrated that we have developed a new bisazo calix[4]arene sensor with bisazo and core cavity as the metal ion binding sites as the signal transduction unit, which showed selective of Hg2+ cation. The bisazocalix[4]arene 5c gives rise to a large bathochromic shift in the absorption spectrum with different pH values (from yellow to dark red), which is clearly visible to the naked eye. NMR spectral analysis also verifies the formation of intended synthesis.
References
Halouani, H., Dumazet-Bonnamour, I., Lamartine, R.: Synthesis of novel chromogenic bi- and tri-functionalized calix[4]arenes. Tetrahedron Lett. 43, 3785–3788 (2002)
Kim, J.Y., Kim, G., Kim, C.R., Lee, S.H., Lee, J.H., Kim, J.S.: UV band splitting of chromogenic azo-coupled calix[4]crown upon cation complexation. J. Org. Chem. 68, 1933–1937 (2003)
Chawla, H.M., Singh, S.P., Upreti, S.: Synthesis of calix[4]arene(amido)monocrowns and their photoresponsive derivatives. Tetrahedron 62, 9758–9768 (2006)
Shinkai, S., Araki, K., Shibata, J., Manabe, O.: Autoaccelerative diazo coupling with calix[4]arene—unusual co-operativty of the calixarene hydroxy-groups. J. Chem. Soc. Perkin Trans. 1, 195–196 (1989)
Deligöz, H.: Azocalixarenes: synthesis, characterization, complexation, extraction, absorption properties and thermal behaviours. J. Incl. Phenom. Macrocycl. Chem. 55, 197–218 (2006)
Buhlmann, P., Pretsch, E., Bakker, E.: Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors. Chem. Rev. 98, 1593–1687 (1998)
Gunnlaugsson, T., Leonard, J.P., Murray, N.S.: Highly selective colorimetric naked-eye Cu(II) detection using an azobenzene chemosensor. Org. Lett. 6, 1557–1560 (2004)
Jiang, P., Guo, Z.: Fluorescent detection of zinc in biological systems: recent development on the design of chemosensors and biosensors. Coord. Chem. Rev. 248, 205–229 (2004)
Lehn, J.-M.: Toward self-organization and complex matter. Science 295, 2400–2403 (2002)
Alfieri, C., Dradi, E., Pochini, A., Ungaro, R., Andreetti, G.D.: Synthesis, and X-ray crystal and molecular-structure of a novel macrobi-cyclic ligand—crowned para-tert-butyl-calix[4]arene. J. Chem. Soc. Chem. Commun. 19, 1075–1077 (1983)
Casnati, A., Ungaro, R., Asfari, Z., Vicens, J.: In Calixarenes 2001, Asfari, Z., Böhmer, V., Harrowfield, J., Vicens, J (eds.), pp. 365–384. Kluwer Academic Publishers, Dordrecht (2001)
Oueslati, I.: Calix(aza)crowns: synthesis, recognition, and coordination. A mini review. Tetrahedron 63, 10840–10851 (2007)
Deligöz, H., Yılmaz, M.: Selective complexation of Na+ by polymeric calix[4]arene tetraesters. J. Polym. Sci. Part A: Polym. Chem. 33, 2851–2853 (1995)
Deligöz, H., Yılmaz, M.: Synthesis of polymer-supported calix[4]arenes and selective extraction of Fe3+. React. Funct. Polym. 31, 81–88 (1996)
Yılmaz, M., Deligöz, H.: Selective extraction of Fe3+ cation by callxarene-based cyclic ligands. Sep. Sci. Technol. 31, 2395–2402 (1996)
Deligoz, H., Yilmaz, M.: Liquid–liquid-extraction of transitionmetal cations by calixarene based cyclic ligands. Solvent Extr. Ion Exch. 13, 19–26 (1995)
Qazia, M.A., Ocak, Ü., Ocak, M., Memon, S.: An excellent copper selective chemosensor based on calix[4]arene framework. Anal. Chim. Acta 761, 157–168 (2013)
Oueslati, I., Thuéry, P., Shkurenko, O., Suwinska, K., Harrowfield, J.M., Abidi, R., Vicens, J.: Calix[4]azacrowns: self-assembly and effect of chain length and O-alkylation on their metal ion-binding properties. Tetrahedron 63, 62–70 (2007)
Rostovtsev, V.V., Green, L.G., Fokin, V.V., Sharpless, K.B.: A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 41, 2596–2599 (2002)
Gutsche, C.D., Iqbal, M.: Para-tert-butylcalix[4]arene. Org. Synth. 68, 234–238 (1990)
Dalbavie, J.-O., Regnouf-de-Vains, J.-B., Lamartine, R., Lecocq, S., Perrin, M.: Complexation of cobalt(II) at the upper rim of two new calix[4]arene/bipyridine-based podands. Eur. J. Inorg. Chem. 4, 683–691 (2000)
Gutsche, C.D., Iqbal, M., Stewart, D.: Calixarenes. 18. Synthesis procedures for p-tert-butylcalix[4]arene. J. Org. Chem. 51, 742–745 (1986)
Elçin, S., Deligöz, H.: Di-substituted azocalix[4]arenes containing chromogenic groups: synthesis, characterization, extraction, and thermal behavior. Tetrahedron 69, 6832–6838 (2013)
Jin, C.M., Lu, G.Y., Liu, Y., You, X.Z., Wang, Z.H., Wu, H.M.: Synthesis of (p-substituted phenyl)azocalix[4]arenes. Chin. J. Chem. 22, 1080–1087 (2002)
Leilei, L., Zhigang, R., Hongxi, L., Hai, S., Jianping, L.: 5,11,17,23-Tetrakis[(p-carboxyphenyl)azo]-25,26,27,28-tetrahydroxy calix[4]arene: crystal structure and pH sensing properties. Chin. J. Chem. 28, 1829–1834 (2010)
Acknowledgments
The authors would like to express their thanks to Dr. Şevki Arslan for this critical reading of draft manuscript and making many helpful suggestions and corrections.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elçin, S., Deligöz, H. Synthesis and metal extraction studies of a novel chromogenic 5,17-bisazocalix[4]arenes. J Incl Phenom Macrocycl Chem 80, 337–343 (2014). https://doi.org/10.1007/s10847-014-0408-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-014-0408-4