Abstract
Presence of heavy metals is one of the major sources of environmental contamination. The widespread pollution of highly poisonous heavy metals can endanger our ecosystem thus posing a great threat to human health. Selective sensing and quantification of these toxins are fascinating areas of current research. In this regard, present work highlights the synthesis of 5,11,17,23-tetra-tert-butyl-25,27-di((2-amido(1-anthracene)ethyl)amidomethoxycalix[4]arene (3) and exploration of its selective chromogenic behavior toward toxic Hg2+. Complexation study of (3) was carried out by examining the effect of various metal ions including Li+, Na+, K+, Cs+, Ag+, Ba2+, Ca2+, Mn2+, Mg2+, Sr2+, Ni2+, Cd2+, Co2+, Cu2+, Hg2+, Pb2+, Zn2+, Fe2+, Fe3+ and Al3+ using UV–visible spectroscopy. Selective chromogenic ability of ligand (3) shows a remarkable sensitivity for Hg2+ even in the presence of various co-existing ions. The stoichiometric analysis, i.e. Job’s plot reveals that (3) forms a 1:1 complex with Hg2+. The selective chromogenic response for Hg2+ was confirmed by FT-IR spectroscopy.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmental pollution by the adulteration of heavy metals has prodigious concerns on water bodies across the world because their increasing expulsion creates toxicity and other hostile effects to ecosystem. Mercury is one of the most ubiquitous toxic heavy metal and persistent toxin that is non-recyclable and could be easily found in ambient air, soil, water and even food [1–3]. A variety of natural as well as anthropogenic activities are responsible for the entrance of this deadly poison into environment and bring about severe effects to flora and fauna [4]. Toxic mercury can easily permeate through biological membranes, inhibit catalytic activity of different enzymes, and lead to damages to the brain, nervous system, serious cognitive and motion disorders, and Minamata disease [5, 6]. The dominant problems of Hg2+ are bioaccumulation into food chain [7]. Consequently, it harms DNA, diminishes the ligand–receptor interactions, disables normal functions of liver and endocrine system of the kidneys, disrupts the immune system homeostasis and even leads to death [8]. As Hg2+ is toxic, carcinogenic and non-biodegradable in nature, the World Health Organization has set its permissible limit in drinking water as 1 μg L−1 [9]. Scientists have devoted considerable effort for the development of simple, selective, rapid, convenient and cost-effective methods for the sensing of Hg2+ in contaminated natural water sources. In this regard, they contributed in the formulation of selective and well-organized fluorogenic and chromogenic synthetic receptors those responds worthily toward Hg2+. This endeavor has realized and drawn a great attention as a reason to minimize the usage of sophisticated instrumentation as compared to other analytical techniques including atomic absorption/emission spectroscopy (AAS/AES), inductively coupled plasma mass spectrometry (ICP-MS) [10], cold-vapor atomic fluorescence spectrometry (CV-AFS) [11] and X-ray absorption spectroscopy [12]. However, these methods require sophisticated instrumentation along with complicated sample preparation processes, time-consuming, relatively high cost and complexation to run [13]. But comparatively optical methods based on chromogenic and fluorogenic molecular chemosensors have replaced and overcome the inadequacy because they are simple to operate, highly selective, adaptable and cost-effective. In this view, much work has devoted regarding to Hg2+ recognition by synthesized fluorogenic/chromogenic sensors [14–17].
Selective sensing of heavy metal ions is a very important topic for the detection and treatment of the toxic metal ions in various chemical systems. Calixarenes [18] based molecular receptors have marvelous position among supramolecular systems and more appropriately have been described as hosts with potential unlimited possibility for functionalization [19]. Multivariate functionalization at either rims can craft highly selective coordination with various cations, anions and neutral molecules [20–23]. Moreover, introduction of different functional groups at phenolic-OH rings of calixarene determines its ‘‘cone’’ conformation and hence produces metal selective cation receptors [24–26]. A number of calix[4]arene-based receptors for Hg2+ have been reported and proposed that nitrogen, oxygen and sulfur atoms containing functional groups like SH, CN, NH2, COOH and CONH2 present in the ionophores can promote the best selective coordination with Hg2+ [27–31]. For example: Bingol et al. [32] synthesized a novel benzothiazole-based azocalix[4]arene chromogenic sensor for Hg2+. Othman et al. [33] have reported calix[4]arene-based Hg2+-induced FRET chemosensor. Wang et al. [34] have developed calix[4]arene framework-based ratiometric chemosensor for Hg2+. Ho research group [35] has also synthesized p-methoxyphenylazocalix[4]arenes chromogenic sensor for Hg2+ ion. Densylcarboxyamide-based calix[4]arene fluorogenic chemosensor for Ti+ and Hg2+ was first time introduced by Talanov et al. [36]. Lee et al. [37] have explored pyrene and rhodamine-functionalized excimer-based calix[4]arene FRET chemosensor for Hg2+. Our research group has also reported chromogenic sensor based on calixarene framework for selective recognition of Hg2+ [38]. However, still there is lot of prospects macromolecules that provide the exploration of chromogenic behavior for selective and sensitive recognition of metal ions.
Present work is the extension of our previous studies [39–42] regarding exploration of chromogenic properties of calix[4]arene derivatives for toxic metal ions. In this study, we synthesized a new 5,11,17,23-tetra-tert-butyl-25,27-di((2-amido(1-anthracene)ethyl)amidomethoxycalix[4]arene (3) with chromogenic properties and explored its selective complexation efficiency toward Hg2+ ion.
Experimental section
Chemicals and instrumentation
All starting materials and reagents used were of standard analytical grade purchased from Alfa Aesar (Germany), Merck (Darmstadt, Germany) and were used without further purification. All aqueous solutions were prepared with deionized water that had been passed through a Millipore milli Q Plus water purification system. 1H and 13C NMR spectra were recorded with a Varian 400-MHz spectrometer in CDCl3. Melting points were determined on a Gallenkamp (UK) apparatus in a sealed capillary tube. FT-IR spectroscopic measurement was carried out through Thermo Nicollet AVATAR 5700, whereas CHNS instrument model Flash EA 1112 elemental analyzer was used for elemental analyses. Analytical thin-layer chromatography (TLC) was performed on pre-coated silica gel plates (SiO2, PF254 type Merck).
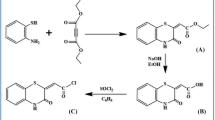
Synthesis of (II)
To a solution of anthracene carboxylic acid (2.2 g, 4.07 mmol) in dry THF (200 ml), thionyl chloride (2.5 ml) in dry THF (100 ml) was added dropwise. The reaction mixture was then refluxed under nitrogen atmosphere for 3.5 h. Solvent and residual amount of thionyl chloride were removed under reduced pressure. White solid of (I) was obtained in quantitative yield. The product was used in subsequent reaction without purification. To a solution of compound (I) containing (1.0 gm, 4.15 mmol) in dry THF (50 ml), pyridine (2 ml) and 1, 2-ethylenediamine (1.4 ml, 10.35 mmol) in excess were added dropwise for 2 h with continuous stirring and mixture was stirred for further 2 h at room temperature under nitrogen atmosphere. The reaction contents evaporated at reduced pressure untill dried as white powder of (II). The residue was diluted with water (150 ml) and neutralized with 0.1 M HCl followed by filtration and washing with water. Yield 61 % 0.56 gm (0.24 mmol), Anal. Calc. for C17H16N2O; C, 77.77; H, 0.06; N, 0.10. Found: C, 77.69; H, 0.06; N, 0.99.
5,11,17,23-tetra-tert-butyl-25,27-di((2-amido(1-anthracene)ethyl)amidomethoxycalix[4]arene (3)
Calix[4]arene derivatives (1) and (2) were prepared as reported in literature [43, 44]. Compound (3) was synthesized as follows. Compound (2) (0.2 gm, 2.52 mmol) was dissolved in 50 ml of toluene/methanol (1:3) and stirred for 30 min at room temperature. Solution of (II) (1.33 gm 5.04 mmol) in methanol added dropwise for 2 h with continuously stirring at room temperature. The reaction was refluxed and light pale yellow color precipitates were formed. Solvent was evaporated at reduced pressure and resultant solid mass washed thoroughly with 0.5 L of water. Yield 48 % 0.14 gm (0.12 mmol); m.p. 321 °C, FT-IR (KBr):3353 cm−1 (OH), 1651 cm−1 (C = O), 1601 cm−1, 1480, 1361 and 1232 cm−1 (N–H), 1190 cm−1 (C–N); 1H NMR (400 MHz, CDCl3, ppm): δH: 1.03 (18 H, s, t-Bu), 1.24 (18 H, s, t-Bu), 3.45 (4H, d, ArCH 2 Ar, 2 J = 13.2 Hz), 3.6 (4 H, m, NHCH2CH2NHOCAnth), 3.9 (4 H, m, NHCH2CH2NHOCAnth), 4.30 (4H, s, OCH2CO), 4.6 (4H, d, ArCH2Ar, J = 14 Hz), 6.92 (4H, s, ArH), 6.95 (4H, s, ArH), 7.1 (2H, s, Ar-OH), 7.2 (8 H, t, Ar Anth), 7.4 (4 H, d, Ar Anth), 7.5 (2 H, s, Ar Anth), 7.8 (4 H, s, Ar Anth), 8.0 (2 H, d, NHCH2CH2NHOCAnth), 8.4 (2 H, d, NHCH2CH2NHOCAnth). 13C NMR (400 MHz, CDCl3, ppm): δC: 31.3, 31.5. 32.2, 32.5, 36.0, 39.8, 52.8, 72.3, 124.9, 125.4, 125.7, 126.9, 127.1, 132.4, 142.7, 148.3, 148.7, 149.2, 149.5, 168.7, 169.4. Anal. Calc. for C40H48N4O6; C, 70.56; H, 7.10; N, 8.22. Found: C, 70.45; H, 7.03; N, 8.20 (Scheme 1).
Synthesis of 3–Hg2+ complex
According to stoichiometric ratio, 3–Hg2+ complex was prepared in CH2Cl2:MeOH (3:7) by mixing the nitrate salt of metal with receptor in a round-bottomed flask. The mixture was stirred at room temperature for 24 h after that it was filtered off and finally poured on a Petri dish. Solvent was evaporated at room temperature and complex was dried under vacuum.
General procedure for UV–visible studies
UV–visible behavior of (3) toward different metal ions was monitored at a specific concentration of both ligand and metals. Stock solution of ligand (2.6 × 10−3 M) was prepared in 25 mL of CH2Cl2:MeOH (3:7) and followed by dilution to 2.6 × 10−5 M in 100 ml of solvent. Cation binding property of receptor (3) was investigated using titration experiments in a binary solvent system comprising CH2Cl2:MeOH + H2O (1:1 v/v); 10 molar equivalent of the appropriate metal nitrate salt was measured using a 1-cm absorption cell. In 10-ml test tubes, 2 ml of ligand (2.6 × 10−5 M) and 2 ml of metal salt (2.6 × 10−4 M) were mixed together. UV–visible response of receptor before and after addition of metal salt solution was recorded. For the interfacial study of co-existing ions, 10 equivalent of other metals (2.6 × 10−4 M) were used in the same solvent system containing 3–Hg2+ complex.
Determination of complex stoichiometry
Continuous variation method, i.e. Job’s plot [45] was applied for the determination of stoichiometric ratio between (3) and Hg2+ in CH2Cl2:MeOH + H2O as a binary solvent system. For this method, equimolar solutions (2.6 × 10−5 M) of both host and guest, i.e. ligand and metal were mixed under the condition that sum of the ligand–metal concentration remains constant. The absorbance was measured at 220 nm.
Results and discussion
Perspective of (3) as a chemosensor
Keeping in view the toxic nature of some heavy metal ions such as Cu2+, Hg2+, Pb2+, and Cd2+; it is indispensable and is a need to discover more selective and efficient chemosensors for such toxic heavy metals especially from the environmental and economic viewpoint. Calixarenes [33–38] have been used as very efficient receptors for soft nature (borderline) metal ions. Furthermore, their basic molecular framework with selective binding sites assumed its structural rigidity, various conformations and facile introduction of fluorogenic/chromogenic center [46]. Additionally, the presence of soft nature donor atoms/molecules such as N and O along with macrocyclic characteristics proved them as a promising candidate for stronger complex formation and selective sensing with soft/borderline metal cations such as Cu2+, Hg2+ and Pb2+. In this connection herein, we report the newly synthesized anthracene functionalized 5,11,17,23-tetra-tert-butyl-25,27-di((2-amido(1-anthracene)ethyl)amidomethoxycalix[4]arene as a selective chromogenic for Hg2+ with several parameters were examined and chromogenic investigations confirmed and characterized by FT-IR spectroscopy.
Complexation affinity of (3)
To explore the binding as well as selectivity of 3 for selected metal ions such as Li+, Na+, K+, Cs+, Ag+, Ba2+, Ca2+, Mn2+, Mg2+, Sr2+, Ni2+, Cd2+, Co2+, Cu2+, Hg2+, Pb2+, Zn2+, Fe2+, Fe2+ and Al3+, titration experiments were carried out by adding (10 eq.) of each metal ion. Initial observations from UV–visible spectra revealed that free receptor (3) exhibited characteristic three bands. Two of them at 280 and 255 nm which are comparatively smaller absorption bands than the band at 221 nm. These two absorption bands may be attributed due to the π–π* and n–π* transitions, respectively (Fig. 1a). After the addition of each metal ions (2.58 × 10−4 M) into ligand solution of (2.58 × 10−5 M), there were nominal changes in the absorption intensities that were observed by the contact of different metal ions (Fig. 1a). But on addition of Hg2+ a prominent change in the absorption behavior of (3) was noticed as hyperchromic shift, i.e. the band intensities was surprisingly enhanced from 1.0 to 2.5 (Fig. 1b). Such spectral response of Hg2+ complex at 221, 280 and 255 nm could be assigned to π-electrons and lone pair of electrons present on carbonyl group and oxygen/nitrogen–metal charge transfer absorption upon complexation with Hg2+ [47]. The response suggests very strong and selective affinity of (3) toward Hg2+. The presence of soft donor binding site for soft metal ion reflects the selectivity. Besides this thermodynamic stability, ionic radii, cavity size as well as geometry of ligand and metal ion are also important aspects for specificity which confers the affinity of ligand toward metal ion.
Concentration effect
In an attempt to have further insight into quantitative binding characteristics, chromogenic behavior of receptor (3) for Hg2+ was determined by increment of metal ion concentration. Spectral variations noticed by gradual enhancement in metal ion concentration, which inferred a linear relationship between absorption intensity and Hg2+ ion concentration (Fig. 2a, c), can be visualized in plot of change in absorbance intensity of (3) at 221 nm as a function of Hg2+ concentration (Fig. 2b).
a Influence of the addition of increasing amounts (0 → 10 eq.) of Hg2+ on the absorption spectra (3) (2.6 × 10−5 M) in CH2Cl2:MeOH + H2O, b absorbance intensity of (3) at 221 nm as a function of Hg2+ concentration. c Influence of the addition of increasing amounts (0 → 10 eq.) of Hg2+ on the absorption spectra (3) (2.6 × 10−5 M) in CH2Cl2: MeOH + H2O
For this complexation effect, binding constant of receptor (3) for Hg2+ was also calculated by applying Benesi–Hildebrand equations [48, 49]. From the plot of the ratio of Ao/(A − Ao) against 1/[Hg2+] as in Fig. 3, the value of log K was calculated as 5.71 having R 2 = 0.98 for 3–Hg2+ complex.
For the evaluation of stoichiometric ratio of 3–Hg2+ complex continuous variation method, i.e. Job’s plot experiment was performed. Figure 4 is the representation of typical job’s plot of 3–Hg2+ complex; a plot of absorbance against mole fraction shows a maximum absorbance reaching a mole fraction 0.6 that clearly indicates 1:1 stoichiometric formation of 3–Hg2+ complex.
According to Pearson’s “hard and soft acids and bases” concept [50], soft binding site preferentially coordinates with soft metal ions. Since receptor (3) containing amide functionality which is one of the soft binding sites and comprising two soft donating ligating atoms N and O shows a high binding tendency toward more polarizable metal ion, i.e. Hg2+. On the basis of these results, the proposed interaction of (3) with Hg2+ is shown in Fig. 5.
Stability of complex
Determination of complex stability is an important information about selectivity as well as strength of interaction of any host molecule to the particular guest specie with respect to passage of time in specific solvent. It was intended to examine the stabilities of 3–Hg2+ complex with respect to time. Therefore, UV–visible spectra of complex in CH2Cl2:MeOH + H2O were recorded with continuous UV irradiation with the passage of time from 0–120 min with an interval of 10 min. From results, it was deduced as there were no spectral variations observed with the passage of time which confirm the stability of 3–Hg2+ complex in CH2Cl2:MeOH + H2O (Fig. 6). Absorption values at 220 nm plotted against increasing time show a straight line as shown in Fig. 6 (inset), which reflects the stability of 3–Hg2+ complex.
Effect of foreign ions
To examine the interaction or complexation nature of receptor (3) for Hg2+, an important parameter the effect of foreign metal ions was also obtained. Different metal ions were mixed with 3–Hg2+ as interfering agents. The experiments were performed at fixed concentration, i.e. 2.6 × 10−4 M, and change in absorption intensity was monitored before and after addition of interfering metal ions. The UV–visible spectra (Fig. 7) clearly indicate that no healthy change in behavior of the complex was noticed after the addition of each metal ion. The results reveal that ligand shows high and selective affinity for Hg2+ ion even in the presence of other cations. This remarkable selectivity of receptor (3) can be supported by change in ratiometric behavior of complex absorption (A/Ao) in the presence of other interfering metal ions. From the bar graph, Fig. 8 clearly indicates that (3) shows incredible selectivity for Hg2+ in the presence of other foreign metal ions as they did not cause any fruitful change in (A/Ao) response of 3–Hg2+ complex.
FT-IR study
FT-IR spectroscopy is an important characterization technique which gives information about structure, helps in deeper insight and observes the chemical structural changes in host as a result of complexation. Hg2+ chromogenic behavior of (3) was further confirmed by FT-IR spectral analysis. Receptor (3) shows prominent signals for various functionalities as in spectrum (Fig. 9a). A broad band at 3353 cm−1 represents υ (O–H) stretching and the bands 3100–2800 cm−1 due to the symmetric and antisymmetric C–H stretching modes belong to methyl and methylene groups of aliphatic as well as aromatic nature. Presence of sharp and medium bands were observed for example 1651 cm−1 indicates the C=O amide functional group, 1601 cm−1 indicates the C=C of calixarene rings, 1480 cm−1 N–H for amide, 1361 and 1232 cm−1 aromatic N–H, 1190 cm−1 C–N, 1124 cm−1 C–O–C of calix[4]arene rings and 1051 cm−1 for N–H/O–H. After complexation the spectrum of (3) shows distinctive variations in terms of shifting, disappearance and appearance of new bands as in Fig. 9b. The band vibrations at 3353 and 1651 cm−1 slightly blue-shifted to 3379 and 1636 cm−1, respectively, because of the interaction of carbonyl group of amide with Hg2+. Strong interaction of receptor (3) with Hg2+ can be realized by the disappearance of some important bands like at 1232 and 800–650 cm−1 for C–N–H stretching and bending vibrations of amide functionality in free ligand. On complexation a broader intense band appeared at 1268 cm−1 and other bands like 817, 762, 725, 682 and 661 cm−1 were replaced by some small but new bands at 806 and 710 cm−1. Furthermore, intensity of bands at 1480 and 1361 cm−1 also decreased. Such changes are informative sign due to metal–nitrogen stretching vibration that gives clear indication for involvement of nitrogen and oxygen donors with Hg2+. The stronger evidence for complexation as shifting, disappearance and appearance of various bands of specific functional groups occurred as a result of Hg2+ introduction into receptor cavity.
Conclusion
Chromogenic behavior of newly synthesized 5,11,17,23-tetra-tert-butyl-25,27-di((2-amido(1-anthracene)ethyl)amidomethoxycalix[4]arene (3) toward series of selected different metal ions is shown in this study. Experimental data clarify that (3) exhibited selective chromogenic behavior toward Hg2+. Receptor (3) forms a 1:1 stoichiometric complex with Hg2+. Moreover, selective behavior of (3) toward Hg2+ was characterized and confirmed using FT-IR spectroscopy. Since the geometry and ideal soft binding sites containing N and O in four amide groups possessing conformity in size, nature and lodging with Hg2+, from results it can be concluded that both host and guest having soft nature help in supramolecular systems to detect and determine this toxic metal in aqueous media.
References
H.H. Harris, I.J. Pickering, G.N. George, Science 301, 1203 (2003)
T.W. Clarkson, L. Magos, G.J. Myers, N. Engl, J. Med. 349, 1731 (2003)
R. Eisler, Environ. Geochem. Hlth. 25, 325 (2003)
J.F. Zhanga, C.S. Lima, B.R. Choa, J.S. Kim, Talanta 83, 658 (2010)
T. Takeuchi, N. Morikawa, H. Matsumoto, Y. Shiraishi, Acta Neuropathol. 2, 40 (1962)
M. Harada, Crit. Rev. Toxicol. 25, 1 (1995)
F.M.M. Morel, A.M.L. Kraepiel, M. Amyot, Annu. Rev. Ecol. Syst. 29, 543 (1998)
J.J. Gutknecht, Membr. Biol. 61, 61 (1981)
Guidelines for drinking-water quality, 3rd edn. World Health Organization: Geneva, 2004 p 188
N.H. Bings, A. Bogaerts, J.A.C. Broekaert, Anal. Chem. 78, 3917 (2008)
K.E. Lorber, Waste Manage. Res. 4, 3 (1986)
A. Bernaus, X. Gaona, J.M. Esbrí, P. Higueras, G. Falkenberg, M. Valiente, Environ. Sci. Technol. 40, 4090 (2006)
W. Xue-Fen, M. Qiu-Juan, W. Xiao-Jie, H. Yi-Min, Z. Xin, Sensors. Actuators. B. 183, 565 (2013)
S.H. Kim, J.S. Kim, S.M. Park, S.-K. Chang, Org. Lett. 8, 371 (2006)
N.J. Youn, S.-K. Chang, Tetrahed. Lett. 46, 125 (2005)
S.M. Park, M.H. Kim, J.I. Choe, K.T. No, S.-K. Chang, J. Org. Chem. 72, 3550 (2007)
K.C. Song, M.H. Kim, H.J. Kim, S.K. Chang, Tetrahed. Lett. 48, 7464 (2007)
C.D. Gutsche, Acc. Chem. Res. 16, 161 (1983)
V. Bohmer, Angew. Chem. Int. Ed. Engl. 34, 713 (1995)
O. Ediz, M. Tabakci, S. Memon, M. Yilmaz, D.M. Roundhill, Supramol. Chem. 16, 199 (2004)
V. Stastny, P. Lhotak, V. Michlova, I. Stibor, J. Sykora, Tetrahedron 58, 7207 (2002)
H. Deligoz, M. Yilmaz, J. Polym. Sci., Part A: Polym. Chem. 33, 2851 (1995)
G.U. Akkuş, S. Memon, M. Sezgin, M. Yilmaz, Clean: Soil Air. Water. 37, 109 (2009)
S. Wenger, Z. Asfari, J. Vicens, Pure Appl. Chem. 67, 1037 (1995)
J.W. Caldwell, P.A. Kollman, J. Am. Chem. Soc. 117, 4177 (1995)
O. Struck, L.A.J. Chrisstoffels, R.J.W. Lugtenberg, W. Ver-boom, G.J. van Hummel, S. Harkema, D.N. Reinhoudt, J. Org. Chem. 62, 2487 (1997)
S. Memon, G. Uysal, M. Yilmaz, Sep. Sci. Technol. 35, 1247 (2000)
G. Uysal, S. Memon, M. Yilmaz, React. Funct. Polym. 50, 77 (2001)
J.H. Kim, A.R. Hwang, S.K. Chang, Tetrahed. Lett. 45, 7557 (2004)
Q.-Y. Chen, C.-F. Chen, Tetrahed. Lett. 46, 165 (2005)
J.A. Hayes, Eds. Pelikan: Cambridge 5, 227–237 (1983)
H. Bingol, E. Kocabas, E. Zor, A. Coskun, Talanta 82, 1538 (2010)
A.B. Othman, J.W. Lee, J.S. Wu, J.S. Kim, R. Abidi, P. Thuéry, J.M. Strub, A.V. Dorsselaer, J. Vicens, J. Org. Chem. 72, 7634 (2007)
N.-J. Wang, C.-M. Sun, W.-S. Chung, Tetrahedron 67, 8131 (2011)
I.-T. Ho, G.-H. Lee, W.-S. Chung, J. Org. Chem. 72, 2434 (2007)
V.S. Talanov, E.D. Roper, N.M. Buie, G.G. Talanova, Tetrahed. Lett. 48, 8022 (2007)
Y.H. Lee, M.H. Lee, J.F. Zhang, J.S. Kim, J. Org. Chem. 75, 7159 (2010)
M.A. Qazi, I. Qureshi, S. Memon, J. Fluoresc 21, 1231 (2011)
Ü. Ocak, M. Ocak, K. Surowiec, X. Liu, R.A. Bartsch, Tetrahed. London. 65, 7038 (2009)
M.A. Qazi, Ü. Ocak, M. Ocak, S. Memon, Anal. Chim. Acta 761, 157 (2013)
M.A. Qazi, I. Qureshi, S. Memon, J Fluoresc. 21, 1703 (2011)
M.A. Qazi, Ü. Ocak, M. Ocak, S. Memon, I.B. Solangi, J. Fluoresc. 23, 575 (2013)
C.D. Gutsche, M. Iqbal, D. Stewart, J. Org. Chem. 51, 742 (1986)
D. Maity, A. Chakraborty, R. Gunupuru, P. Paul, Inorg. Chimi. Acta. 372, 126 (2011)
D.C. Harris, 4th edn (W.H. Freeman & Company, New York, 1995)
H. I-Ting, L. Gene-Hsiang, C. Wen-Sheng, J. Org. Chem. 72, 2434 (2007)
S.B. Maamar, N. Jadambaa, F. Vocanson, F. Meganem, C. Felix, I.D. Bonnamour, Supramol Chem. 21, 450 (2009)
J. Shao, H. Lin, H. Lin, Talanta 77, 273 (2008)
C.F. Chow, M.H.W. Lam, W.Y. Wong, Inorg. Chem. 43, 8387 (2004)
Z. Liang, Z. Liu, Y. Gao, Spectrochim. Acta A. 68, 1231 (2007)
Acknowledgments
We thank the National Center of Excellence in Analytical Chemistry, University of Sindh, Jamshoro, Pakistan and Scientific and Technological Research Council of Turkey (TUBITAK, B.02.1.TBT.0.06.01-216.01/895–6391) for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Memon, S., Bhatti, A.A., Bhatti, A.A. et al. Synthesis and chromogenic behavior exploration of a new calix[4]arene derivative. J IRAN CHEM SOC 12, 1739–1746 (2015). https://doi.org/10.1007/s13738-015-0648-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0648-2