Abstract
Sodium Picosulphate (SPL) is a synthetic drug, widely used for thorough evacuation of the bowel, usually for patients who are preparing to undergo a colonoscopy. Cyclodextrins (CDs) are chiral, truncated cone shaped, cyclic oligosaccharides that can encapsulate a variety of drug molecules into inclusion complexes, thereby increasing their stability and solubility. 1H NMR spectroscopic studies showed the inclusion complexation between β-CD and SPL, based on the upfield shift changes in the β-CD cavity protons (H-3′ and H-5′) and downfield shift changes in the guest (SPL) protons. The structure of inclusion complexes was determined by 2D ROESY spectral data. The 1:1 stoichiometry and overall association constant (Ka) were determined by using Scott’s plot method to be 450 M−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
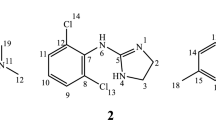
Sodium picosulfate (4, 4′-(2-pyridylmethylene) biphenyl bis (hydrogen sulfate) disodium) (SPL) is a medicine known as a stimulant laxative (Fig. 1a). After being taken by mouth, it is activated by the bacteria naturally present in the large intestine. It then stimulates nerve endings in the intestinal wall. These nerves contract the muscles in the intestine and the rectum more often and with more force, a process known as peristalsis. This moves the contents of the intestine along so that the bowel can be emptied, and hence relieves constipation. Sodium picosulfate is also used to stimulate the emptying of the bowel before surgery, childbirth or medical investigation of the gut [1, 2].
Cyclodextrins (CD) are cyclic (α-1, 4)-linked oligosaccharides of α-d-glucopyranose containing a relatively hydrophobic central cavity and hydrophilic outer surface (Fig. 1b) [3, 4]. CDs can encapsulate various pharmaceutical compounds to enhance the solubility, stability and bioavailability of drug molecules. For example, Itraconazole [5], an antifungal agent, is insoluble in water, can be solubilised by complexing it with 2-hydroxypropyl-β-CD. Similarly, a 16-fold increase in the water solubility of felodipine and threefold increase in water solubility of amlodipine was reported on complexation with methyl-β-CD [6]. The dissolution characteristics of glibornuride remained unchanged, when it was stored for 1 year in the form of β-CD complex [7]. Furthermore, many poorly water soluble drugs such as kynostatin, steroids, cinnarizine, naproxen and miconazole etc. can be solubilized by sulfobutyl-β-CD [8]. No covalent bonds are formed or broken during drug-cyclodextrin complex formation and in aqueous solutions the complexes are readily dissociated [4, 9].
Drug-cyclodextrin complexation can be regarded as molecular encapsulation, i.e. encapsulation of drug at the molecular level. The CD can insulate a labile compound from a potentially corrosive environment and, in this way, reduce or even prevent drug hydrolysis, oxidation, steric rearrangement, racemization, and other forms of isomerization, polymerization, and even enzymatic decomposition of drugs [10–14]. Furthermore, In order to use CDs as drug carrier systems it is necessary to understand from the thermodynamics and kinetics points of view how the drug molecule interacts with the CD cavity.
1H NMR spectroscopy is one of the most common useful techniques for investigating the stability and stoichiometry of the CDs complexes, particularly in the solution [15]. NMR spectra obtained from most CD complexes represent concentration weighted averages since exchanged between the free and complexed guest molecule was usually fast in the NMR time scale. Its main advantage consists in the possibility to use several independent signals for the evolution of the association constants so being less prone to misinterpretations caused by minor impurities [15, 16].
2D NMR spectroscopy has become an important tool for the investigation of the interactions between CDs and guest molecules. According to the relative intensities of these cross peaks, it is possible to estimate the orientation of the guest molecule within the CD cavity. The relative intensities of cross peaks depend on the spaces between the corresponding protons [16].
The aim of this work was to explore the use of CDs to form inclusion complexes with sodium picosulphate (SPL) to overcome the bioavailability problems of this drug. In continuation of our study, [17–19], we investigated the complexation of SPL with β-CD in aqueous solution by detailed NMR spectroscopic study and report herein our results.
Experimental
Material and instrumentation
All the NMR spectra were recorded on an INOVA-500 MHz NMR instrument. The 1H NMR spectra of SPL, in the absence as well as in the presence of β-CD, were recorded in D2O at 296.1 K. To determine the stoichiometry and association constant of the complex, the chemical shift change data (Δδ) for the β-CD protons were obtained by keeping the concentration of β-CD constant at 10 mM while varying the concentration of SPL from 1.47 to 6.65 mM. For resonance assignment, 1H–1H COSY experiment was performed for 1:1 SPL/β-CD mixture in DMSO using standard pulse sequences. ROESY spectrum was recorded by INOVA-500 instrument in D2O at 293.1 K, with a mixing time 0.600 s and acquisition time 0.158 s and under spin lock conditions. The SPL/β-CD molar ratios were calculated by direct integration of appropriate signals. As, there was no separate peak for free as well as SPL/β-CD complex, it is assumed to undergo rapid exchange between free and complexed form on NMR time scale. The chemical shift values reported in δ (ppm) were calculated with reference to solvent (D2O) resonance at 4.740 throughout this work.
Preparation of SPL/β-CD inclusion complex
The SPL/β-CD complex was prepared by the co-precipitation method. SPL (4.99 g) was dissolved in a minimum quantity of water and was added dropwise to a solution of β-CD (11.35 g) in a minimum quantity of water at 25 °C with continuous stirring. Stirring was maintained for 1 h at the same temperature. Then, a precipitate of the complex was obtained, which was filtered, dried and stored at room temperature.
Result and discussion
1H NMR chemical shift change data of β-CD
The resonance assignment of β-CD protons was made on the basis of their specific shapes, 1H NMR, 2D COSY spectral data. All the protons of β-CD shows upfield shift changes. The H-3′ and H-5′ cavity protons shows significant upfield shift in comparison to H-1′, 2′, 4′, 6′ protons. Figure 2 shows the expansion of a part of 1H NMR spectra showing the upfield shift in β-CD protons in two different [β-CD]/[SPL] molar ratios. The upfield shift of β-CD cavity protons was mainly due to magnetic anisotropy affects in the β-CD cavity, arising due to the inclusion of a π electron-rich group. Such a group in SPL molecule being a phenyl ring indicates its inclusion in the β-CD cavity. Furthermore, the magnitude of shift changes of these β-CD protons increased with the increase in concentration of SPL. The chemical shift (δ) data of β-CD protons with different [β-CD]/[SPL] molar ratios is given in Table 1.
Stoichiometry and association constant (Ka) of the SPL/β-CD complex
The stoichiometry of the SPL/β-CD complex was determined by using the Scott equation [20] for the 1:1 complex
The 1H NMR chemical shift change data for the β-CD protons was obtained in the presence of SPL by keeping the concentration of β-CD constant while varying the concentration of SPL. The plot of ΔδH-3′ and ΔδH-5′ against [SPL] in the form of [SPL]/ΔδH-3′ and [SPL]/ΔδH-5′ versus [SPL] gave a linear fit (Fig. 3a, b) confirming the 1:1 stoichiometry for the SPL–β-CD complex. The slope of the plot is thus equal to 1/Δδmax, with the intercept with the vertical axis to Δδmax/Ka, allowing the estimation of Ka to be 450 M−1. Although 450 M−1 binding constant of inclusion complex is not very high, but many complexes of pharmaceutical compounds with different CD derivatives has already been prepared and are being used, which has lesser value of binding constant than the observed binding constant in case of SPL–β-CD complex [21, 22].
1H NMR assignment of SPL
An unambiguous resonance assignment of SPL protons is required to ascertain which one of the ring/s or all the aromatic rings are involved in complexation. The assignment of SPL protons was made with the help of 2DCOSY, ROESY and HMQC spectral data. The protons of SPL and β-CD does not interfere with each other in any of the SPL/β-CD mixture spectra, which make is very convenient to assign each proton of SPL which is very much required to ascertain the structure of inclusion complex of SPL with β-CD. The protons of pyridine ring were found downfield as compared to aromatic rings. One doublet at δ = 8.273, one triplet at δ = 7.624 and another triplet at δ = 7.098 were assigned for H-13, H-11 and H-12 proton of pyridine ring, respectively, totally integrating for three protons and showed the cross correlation peaks in 1H–1H COSY spectra. The peak of H-10 proton of pyridine ring was found merged with aromatic signal at δ = 7.111–7.043 showing cross co-relation peak with H-11 in COSY spectrum. The aromatic protons were found as two doublet centered at δ = 7.098 for protons (H-2, 3, 6, 7) and δ = 7.052 for protons (H-1, 4, 8, 9), integrating totally for eight protons. Figure 4 shows an expended part of COSY spectrum showing all the cross co-relation peaks.
1H NMR chemical shift change data of SPL
In presence of β-CD, all the aromatic protons of SPL show significant downfield shift changes while H-5 shows insignificant shift changes. Figure 5 shows the changes in the aromatic region of the spectrum of SPL in the presence of β-CD compared to that for pure SPL. The upfield shift of β-CD cavity protons and downfield shift changes in aromatic protons of guest SPL, is a clear indication of SPL/β-CD inclusion complex in analogy to our previous studies [13–15], but it is difficult to know which part of aromatic guest penetrates into β-CD cavity.
ROESY spectral data for SPL/β-CD complex
Since the 1H NMR data was not conclusive of structure of inclusion complex. It gave only the information of formation of inclusion complex but it is difficult to know which part of aromatic guest penetrates into the β-CD cavity by 1H NMR. So, we performed the 2D ROESY spectral studies for confirming our results. It helped us to establish the structure of SPL/β-CD inclusion complex in solution. Expansion of a part of 2D ROESY spectral data showing 1H–1H cross connection peaks between host β-CD and guest SPL is shown in Fig. 6.
ROESY spectral data shows that all the aromatic protons of the guest SPL are close in space to cavity protons (H-3′ and H-5′) of β-CD, although H-12 and H-13 does not show any interaction with H-3′/H-5′ of β-CD, perhaps. This suggests that both the aromatic rings of SPL are included in the hydrophobic cavity of β-CD, one at a time. Moreover, a strong cross correlation peak between H-5 of SPL and H-3′ of β-CD (located towards the wider rim side) is also observed, which gave a clear indication of inclusion of aromatic rings of SPL from wider rim side of β-CD cavity. Furthermore, H-11 of pyridine ring also shows a cross correlation peak with H-3′ of β-CD, but no peak was observed with H-5′ of β-CD cavity proton, proving the shallow penetration of the pyridine ring into the β-CD cavity from the wider side of the cavity.
On the basis of ROESY spectral data, it can be supposed that SPL forms two 1:1 SPL/β-CD inclusion complexes in aqueous solution. The structures of the two 1:1 SPL/β-CD inclusion complexes are proposed as shown in Fig. 7.
Conclusion
The inclusion complex formation between SPL and β-CD the stoichiometry and the association constant were assessed by NMR in aqueous solution. ROESY spectral data proved the formation of two 1:1 SPL/β-CD inclusion complex, by penetrating the two aromatic rings deeply in β-CD cavity from wider rim side and shallow penetration of pyridine ring into the β-CD cavity from wider rim side. The association constant of SPL/β-CD complex was determined by Scott’s method and was estimated to be 450 M−1. This study also proved the pertinence of NMR spectroscopy as an important tool for studying drug: CD interactions with a better discussion of supramolecular assemblies in solution and also to characterize structurally the inclusion compounds formed in aqueous solution.
References
Kienzle-Horn, S., Vix, J.M., Schuijt, C., Peil, H., Jordan, C.C., Kamm, M.A.: Comparison of bisacodyl and sodium picosulphate in the treatment of chronic constipation. Curr. Med. Res. Opin. 23, 691–699 (2007)
Hoy, S.M., Scott, L.J., Wagstaff, A.J.: Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs 69, 123–136 (2009)
Liu, Y., You, C.-C., Zhang, H.Y., Zhao, Y.L.: Enantioselective recognition of aliphatic amino acids by β-cyclodextrin derivatives bearing aromatic organoselenium moieties on the primary or secondary side. Eur. J. Org. Chem. 8, 1415–1422 (2003)
Pandit, V., Gorantla, R., Devi, K., Pai, R.S., Sarasija, S.: Preparation and characterization of pioglitazone cyclodextrin inclusion complexes. J. Young Pharm. 3, 267–274 (2011)
Putterman, W., Caers, J., Masens, J., Peters, J.: Abstract International Conference of Pharmaceutical Applications of Cyclodextrin Conference, Lawrence (1997)
Mielcarek, J., Czernielewska, A., Czarczynska, B.: Inclusion complexes of felodipine and amlodipine with methyl-β-cyclodextrin. J. Incl. Phenom. Mol. Recogn. 54, 17 (2006)
Torres-Labandeira, J.J., Blanco-Mendez, J., Vila-jato, J.L.: Biopharmaceutical stability of the glibornuride/bet-cyclodextrin inclsuion complex after one year of storage. S. T. P. Pharm. Sci. 4, 235 (1994)
Thompson, D.O.: Cyclodextrins as enabling excipients: their present and future use in pharmaceuticals. CRC Crit. Rev. Ther. Drug. Carrier Syst. 14, 1 (1997)
Fromming, K.H., Szejtli, J.: Cyclodextrins in Pharmacy. Kluwer, Dordrecht (1994)
Fermeglia, M., Ferrone, M., Lodi, A., Pricl, S.: Host–guest inclusion complexes between anticancer drugs and β-cyclodextrin: computational studies. Carbohydr. Polym. 53, 15–44 (2003)
Brun, H., Paul, M., Razzouq, N., Binhas, M., Gibaud, S., Astier, A.: Cyclodextrin inclusion complexes of the central analgesic drug nefopam. Drug Dev. Ind. Pharm. 32, 1123–1134 (2006)
Tiwari, G., Tiwari, R., Rai, A.K.: Cyclodextrins in delivery systems: applications. J. Pharm. Bioallied Sci. 2, 72–79 (2010)
Jones, R.A.Y.: Physical and Mechanistic Organic Chemistry, pp. 227–235. Cambridge University Press, London (1979)
Helm, H., Muller, B.W., Waaler, T.: Complexation of dihydroergotamine mesylate with cyclodextrin derivatives: solubility and stability in aqueous solution. Eur. J. Pharm. Sci. 3, 195–201 (1995)
Schneider, H.-J., Hacket, F., Rudiger, V., Ikeda, H.: NMR studies of cyclodextrins and cyclodextrin complexes. Chem. Rev. 98, 1755–1786 (1998) and references cited therein
Dodziuk, H.: Cyclodextrins and their complexes chemistry, analytical methods, applications. Wiley, London (2006)
Maheshwari, A., Sharma, M., Sharma, D.: Investigation of the binding of roxatidine acetate hydrochloride with cyclomaltoheptaose (β-cyclodextrin) using IR and NMR spectroscopy. Carbohydr. Res. 346, 1809–1813 (2011)
Maheshwari, A., Sharma, D.: A comparative study of inclusion complexes of flunarizine with alpha (α-CD) and beta-cyclodextrin (β–CD). J. Incl. Phenom. Macro. Chem. 68, 453–459 (2010)
Ali, S.M., Maheshwari, A.: 1H NMR spectroscopic study of complexation of citalopram with β-cyclodextrin in aqueous solution. Mag. Res. Chem. 45, 253–256 (2007)
Scott, R.L.: Some comments on the Benesi-Hildebrand equation. Recl. Trav. Chim. Pays-Bas 75, 787–789 (1956)
Kahle, C., Holzgrabe, U.: Determination of binding constants of cyclodextrin inclusion complexes with amino acids and dipeptides by potentiometric titration. Chirality 16, 509–515 (2004)
Acosta, G., Linares, D., Olsina, R., Martínez, L.D., Gomez, M.R.: Determination of the binding constant of indomethacin-beta-cyclodextrin complex by capillary electrophoresis: experimental optimization and temperature study. Pharmazie 62, 847–852 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maheshwari, A., Sharma, M. & Sharma, D. Complexation of sodium picosulphate with beta cyclodextrin: NMR spectroscopic study in solution. J Incl Phenom Macrocycl Chem 77, 337–342 (2013). https://doi.org/10.1007/s10847-012-0251-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0251-4