Abstract
A new modified carbon paste electrode for determination of Cu2+ made in our laboratory that used a new synthesized macrocycle 7,16-diaza-1-thia-4,10,13,19-tetraoxa-6,17-dioxo-2,3;20,21-dinaphtho-cyclouneicosane as modifier. This sensor exhibits a good affinity toward copper (II) ions over a wide variety of other metal ions. The electrode exhibits a Nernstian slope of 30 (±0.5) mV per decade for copper (II) ions over a wide concentration range (1.0 × 10−8–1.0 × 10−2 mol L−1), with a limit of detection of 7.0 × 10−9 mol L−1 (~0.45 ppb). It has a response time of 30 s and can be used for at least 3 months without any considerable divergence in responses. The potentiometric response of the electrode is independent of the pH of test solution in the pH range 3.5–7.5. Finally, it was successfully used as an indicator electrode for determination of copper (II) in real samples such as Karoun river and tap water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper is known as the third in abundance (after Fe3+ and Zn2+) among the essential heavy transition metals in the human body and plays an important role in various physiologic processes [1, 2]. It plays a vital part in the development and performance of the human nervous and cardiovascular systems, as well as the skin, bone, immune, and reproductive systems, including gene transcription. This element can be also poisonous and even fatal to organisms. Copper concentration in potable water is usually very low (≤20 μg/L) [3]. A concentration more than 1 μg/ml can be impart a bitter taste to water. Large oral doses can cause vomiting and may eventually cause liver damage. Moreover, Cu2+ can be toxic to biological systems when levels of this ion exceed cellular needs, and it is also able to displace other metal ions which act as cofactor in enzyme-catalyzed reactions [4, 5]. Therefore, a precise, accurate and rapid measurement of copper ion is much interest.
A number of instrumental methods such as ICP [6], atomic absorption [7], UV-vis spectrometry [8] and voltammetry [9] methods are employed for the determination of copper at low concentration levels. Most of these methods are either time consuming, involving multiple sample manipulations, or too expensive for most analytical laboratories. Potentiometric method with using an ion sensor as an indicator electrode is an alternative method for determination of copper. The development and application of ion-selective electrodes continue to be an interesting area of analytical research as they provide accurate, rapid, non-destructive and low cost methods of analysis.
Ion carrier plays main role in these sensors. A variety of ion carriers have been used in the construction of copper (II) selective electrodes. However, the poor detection limit, the serious interferences, and especially, low linear range of the prepared electrodes have necessitated the development of new copper selective electrodes.
Macrocyclic ligands have the ability to form selective and stable complexes with metal ions of compatible dimensions [10]. Due to their specific selectivity and extraction efficiency, macrocyclic crown ethers have been widely used as suitable neutral ionophores for constructing membrane-selective electrodes for alkali, alkaline earth and heavy metal cations [10–13]. The substitution of some oxygen atoms of these compounds by nitrogen and/or sulfur atoms will make them ideal candidates for use as ion carriers in the preparation of transition and heavy metal ion selective electrodes [14], where facile complexation and decomplexation of the ionophore is of vital importance.
Carbon pastes are well known as useful materials for the fabrication of various electrometric sensors for analytical purposes [15, 16]. Most of these electrodes are based on the ion-exchange mechanism of the active component incorporated into the carbon-paste matrix. The operation mechanism of such chemically modified carbon paste electrodes (CMCPEs) depends on the properties of the modifier materials used to import selectivity towards the target species. In comparison with ion-selective electrodes based on polymeric membranes, the CMCPEs possess advantages of much lower ohmic resistance and very stable response. The main attraction of using the modified electrode is that the electrode surface can be renewed after every use by squeezing a little carbon paste out of the tube and a fresh surface is smoothed on a piece of weighing paper whenever needed [17]. Although considerable attention has been given to the preparation of CMCPEs so far, the application of these CMCPEs have been mainly focused on the field of voltammetric analysis [18, 19] and only few of these types of the electrodes have been used in potentiometry. The advantages of carbon paste electrodes drew the attention of researchers in recent years where these advantages were exploited for various measurements including potentiometric [20, 21].
In recent years, we have reported on a number of new carbon paste and PVC-membrane electrodes for some transition and heavy metal ions such as Cu [22], Hg [23], Cd [24], Ag [25, 26], Co [27], Ni [28] and Fe [29] using some neutral cyclic and acyclic ligands as suitable ionophores.
In this work, a macrocyclic compound 7,16-diaza-1-thia-4,10,13,19-tetraoxa-6,17-dioxo-2,3;20,21-dinaphtho-cyclouneicosane (ligand (I)), recently synthesized in our laboratories [30] (Fig. 1), was used as a novel ionophere in carbon paste electrode for determination of sub-ppb level Cu2+ ion potentiometrically. The proposed electrode exhibits significantly good selectivity to Cu2+ ion over alkali, alkaline earth, and several transition metal ions.
Experimental
Reagents and materials
All analytical reagent grade chemicals and distilled water were used for preparing all aqueous solutions. Copper nitrate was obtained from Merck, carbon graphite powder and paraffin oil was purchased from Fluka. Salts of metal nitrates (all from Merck) were of the highest purity available and used without any further purification except for vacuum drying over P2O5.
The stock solution of Cu2+ solution (0.1 M) was prepared by dissolved an appropriate weight of copper nitrate in distilled, deionized water (~1.2 μs conductivity) and diluted to the mark in a volumetric flask.
Apparatus
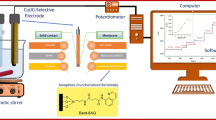
All potentiometric measurements were made with a pH/mV meter (Metrohm-827) using proposed sensor in conjunction with a double junction Ag/AgCl (Azar electrode, Iran) reference electrode.
Preparation of carbon paste modified electrode
Bare CPEs were prepared by mixing 1.20 g of graphite powder, which had been heated at 700 °C in a muffle furnace for 15 s, with 800 μL of paraffin oil with a mortar and pestle. A modified paste was prepared in a similar fashion, except that the graphite powder was mixed with a desired weight of ligand (I) to get different composition as given in Table 1. Both unmodified and modified pastes were packed into a polyethylene tube (2.5 mm diameter), the tip of which had been cut off. Electrical contact to the paste was established via inserting a copper wire thorough flank. A fresh electrode surface was obtained by squeezing more out. The surplus of paste was cut out with a glass rod and the exposed-end polished on a paper until the surface showed shiny appearance.
Electrode conditioning
Fresh modified electrode surfaces were conditioned by exposure to 1.0 × 10−4 mol L−1 Cu(NO3)2 solution for 2 h. The electrodes were, then, rinsed with de-ionized water for 30 s. Each measurement was performed on a new surface, obtained by a simple/polishing procedure.
Electrodes system and emf measurement
All emf measurements were carried out with the following cell assembly:
The proposed carbon paste electrode was connected to the pH/mV meter as an indicator electrode and the Ag/AgCl reference electrode was connected to the reference terminal of the meter.
For all measurements the two electrodes were immersed in the 50 mL beaker contain de-ionized water, and the solution was stirred using a magnetic stirrer. The CPE was allowed to equilibrate until a steady state response was achieved (about 2 min). An accurately measured volume of standard Cu(II) solution was added by microsyringe to the stirred solution and the potential was recorded after 30 s. consecutive amounts of Cu(II) were added so that a final Cu(II) concentration in the range from 1.0 × 10−10 to 1 × 10−2 mol L−1 was obtained. The potential of the electrode against the Ag/AgCl reference electrode was recorded after each Cu(II) addition, and then plotted as a logarithmic function o Cu(II) activity.
The activities of metal ions were based on the activity coefficient (λ), where calculated from the modified form of the Debye–Huckel equation [31], which is applicable to any ion
where μ is the ionic strength and Z the valency. All measurements were carried out at 25 ± 0.1 °C.
All the metal nitrate solutions were freshly prepared by accurate dilution from their stock standard solution of 0.1 mol L−1, with distilled, deionized water.
Determination of selectivity coefficients
In this work selectivity coefficients of the electrode towards different cationic species (Mn+) were evaluated by using both of the matched potential method (MPM) and the fixed interference method (FIM) [32]. According to the MPM, the activity of Cu2+ was increased from aA = 1.0 × 10−5 mol L−1 as reference solution to a′A = 5.0 × 10−4 mol L−1, and the corresponding changes in potential (∆E) were measured. Afterwards, a solution of an interfering ion of concentration aB, in the range 5.0 × 10−4–5.0 × 10−1 mol L−1, was added to a new 1.0 × 10−5 mol L−1 (reference solution) until the same potential change (∆E) was recorded. The selectivity factor, \( k_{A,B}^{MPM} \), for each interferent was calculated using the following equation:
In addition, the selectivity coefficients of interfering species were evaluated by the fixed interference method (FIM). In this manner, the CPE and the reference electrode was placed in 50.0 mL of 1.0 × 10−2 mol L−1 interference ion solution. Various volumes of 0.001, 0.01, or 0.1 mol L−1 of copper (II) nitrate solution were added by microsyringe. The solution was stirred magnetically throughout and after each addition, the cell potential was recorded. In this manner, the copper ion concentration was varied over a wide range (1.0 × 10−8–1.0 × 10−2 mol L−1) while the interference ion (Mn+) concentration was kept constant without having to transfer the electrodes to new solutions. The pHs of total test solutions were constant about 5 with 0.01 mol L−1 acetate buffer.
Results and discussion
In preliminary experiments, ligand (I) due to its sufficient insolubility in water and the presence of donating oxygen, sulfur and nitrogen atoms in its structure, ligand (I) was expected to act as a suitable ion carrier in the carbon paste electrode with respect to special transition and heavy metal ions of proper size and charge. The potential responses of the most sensitive electrodes, prepared under the same experimental conditions (except for 2 h conditioning in a 1.0 × 10−5 mol L−1 of the corresponding cations) are shown in Fig. 2. As it is seen, among different tested cations, Cu2+ with the most sensitive response seems to be suitably determined with the carbon paste electrode based on ligand (I) and the emf responses obtained for all other cations are much lower than that predicted by the Nernst equation. This is probably due to existence of N and S as soft donating atoms for interaction with copper ion. Moreover the macrocycle (I) including of diamide group exhibit a good affinity and selectivity toward Cu2+ ion over a wide variety of other cations. In the other hand cavity size of macrocycle is an important parameter for selectivity detection of metal ions. As can be seen in energy minimized structure of ligand (I) (Fig. 3), the existence of two naphthalene in neighboring each other and also two C=O groups in structure of ligand (I) caused the extra angle strain in proposed 19-membered macrocycle. Torsion of macrocycle because of this angle strain produces a distorted structure with proper cavity size and best orientation for interaction with Cu2+ ion. Therefore, it was considered as a modifier for construction of a Cu(II) selective electrode.
Composition and characteristics of the electrode
It is well known that the sensitivity, linearity and other analytical characteristics of the CPE electrodes depend significantly on the paste composition [33]. Thus, the influence of the type and amount of modifier, and amount of paraffin oil on the potential response of the electrode to Cu2+ ion activity were investigated and the results are summarized in Table 1. As seen, in the absence of ligand (I) modifier the electrode has no response or low response towards copper ion. It has been found that, increasing the amount of the modifier up to 5% has lead to a sharp increase of the electrode response. More increase in the modifier percentage from 5 to 8% has lead to a decrease again in the electrode response. This may be explained by the decrease in the conductance of the electrode material with increasing the percentage of the modifier. As seen from Table 1, the ligand (I)-modified electrode with the graphite powder/paraffin oil/ligand (I) percentage ratio of 65:30:5 was selected as the one with the optimal ingredient composition (No.6). Each composition was tested three times. After the consideration of these results, the optimized carbon paste composition was used in the further studies.
Effect of pH
The effect of pH of test solution on the determination of copper ions was studied for 1.0 × 10−4, 1.0 × 10−5 and 1.0 × 10−6 mol L−1 Cu(NO3)2 solutions in the pH range of 1.0–9.0. The pH of the solution was adjusted at the required value by addition of 1.0 mol L−1 sodium hydroxide and/or 1.0 mol L−1 nitric acid. As can be seen in Fig. 4 the potential was constant in the pH range of 3.5–7.5. At lower pH (<3.5), the increased potential observed could be due to simultaneous response of the electrode to H3O+ and Cu2+ ions, the contribution of H3O+ to the potential counteracts that of Cu2+ ion, and diminished potential in higher pH (>7.5) could be due to formation of some hydroxy complexes of Cu2+ ions in solution.
Linear range and detection limit
The potential response of the electrode at varying concentration of Cu(II) ions displays a linear response to the concentration of Cu2+ ions in a wide range of 1.0 × 10−8–1.0 × 10−2 mol L−1 (Fig. 5). The slope of calibration graph was 30 (±0.5) mV per decade of the activity of Cu2+ ions. The detection limit of the sensor, as determined from the intersection of the two extrapolated linear segments of the calibration graph was 4.0 × 10−9 mol L−1 (0.45 ppb).
Response time and reversibility of the proposed CPE
The response time is a significant factor for any ion selective electrode in analytical application. The optimum equilibration time and concentration of the conditioning solution for the electrode based on ligand (I) was ∼2 h in 1.0 × 10−4 mol L−1 copper (II) nitrate solution. After these conditions, the electrode generated stable potentials in contact with the copper (II) ion solution. The critical emf response of the electrode was assessed according to IUPAC recommendations [34]. The average time required for the mentioned electrode to reach a potential within ±1 mV of the final equilibrium value after successive immersion of Cu (II) ion solutions, each having a 10-fold difference in concentration, was investigated. Typical potential time plots in 1.0 × 10−6 and 1.0 × 10−4 mol L−1 copper (II) nitrate solution are given in Fig. 6. This potential time plot for these concentrations clearly indicates that the potentiometric response time of the electrode was ∼25 s. It should be noted that the equilibrium potentials essentially remained constant for more than 6 min, after which only a very slow divergence within the months without any measurable change in response time, slope, or detection limit. To evaluate the reversibility of the electrode [35], the emf measurements were performed in the sequence high-to-low sample concentrations. The results showed that the response of the electrode was reversible; although the time needed to reach equilibrium values (50 s) was longer than that for low-to-high sample concentrations (25 s), because residual copper will still be adsorbed on the surface of the CPE, which will lead to poor response time.
Selectivity
The potentiometric selectivity coefficient of an electrode as one the most important characteristics is defined by its relative response for the primary ion over other ions present in the solution. In this work, the potentiometric selectivity coefficients were determined graphically by the fixed interference (FIM) and matched potential (MPM) methods. The resulting selectivity coefficient values thus obtained for the proposed Cu (II) sensor (the electrode with optimized composition No. 6) are given in Table 2. As it is evident, most of the interfering ions show low values of selectivity coefficients, indicating no interference in the performance of the carbon paste electrode assembly. However, as seen, the selectivity coefficients for Ag+ and Pb2+ ions are relatively high. It was probably occur by interaction of soft S donor atoms in ligand (I) with Ag+ and Pb2+ ions.
Effect of anions
To investigate the effect of anions on the electrode’s potential responses, the cell potentials were obtained using copper nitrate, copper chloride, and copper sulfate. No significant change in the emf vs. pCu plots was observed, indicating that these anions (NO3 −, Cl− and SO −24 ) do not cause any interference.
Reusability and stability of the Cu (II) electrode
The reusability of the proposed electrode was carried out by performing periodic calibration (week to week) with standard solutions and calculating the response and slope over the range 1.0 × 10−10–1.0 × 10−2 mol L−1 Cu2+ solution and it was found that the electrode worked well over the period of more than 3 months without showing any significant divergence in concentration range, slope and response time. During non-usage, the electrode was stored in distilled water.
Comparison with other electrodes
Table 3 compared the performance characteristics of the proposed sensor with those of the best previously reported copper sensors. It is clear that its detection limit is lower than found for the other electrodes and its working range is wider than the others. Overall (or more) evaluation indicates this electrode is more useful in such applications.
Real sample analysis and analytical performance
For accessing the capability of the method for real samples with different matrices containing varying amounts of diverse ions, the method was applied to determination of copper (II) from different environmental (drinking, mineral and river water) samples. Each sample was analyzed in triplicate and the analysis by sensors was repeated under identical conditions. The data obtained with the proposed electrode for spiked copper (II) to water samples were presented in Table 4. The results of analysis of each water sample showed that the copper (II) recovery was almost quantitative.
Conclusions
On the basis of the present results, ligand (I) can be regarded as a carrier for construction of a novel carbon past electrode for copper (II) ion determination. The interferences from common ions are not very serious and the potentiometric responses of the electrode are independent of the pH of test solution in the pH range of 3.5–7.5. The proposed electrode has shown to perform with good operating characteristics (i.e., sensitivity, stability, response time, detection limit and a wide linear range). It can be easily constructed and used for determination of copper cations in real samples. The high lipophilicity of the ionophore, decreasing its leaching from the membrane to the test solution drastically, is the reason for the long life-times of the electrode based on ligand (I).
References
Mayr, T., Klimant, I., Wolfbeis, O.S., Werner, T.: Dual lifetime referenced optical sensor membrane for the determination of copper(II) ions. Anal. Chim. Acta 462, 1–10 (2002)
Gupta, V.K., Jain, A.K., Maheshwari, G., Langb, H., Ishtaiwi, Z.: Copper (II)-selective potentiometric sensors based on porphyrins in PVC matrix. Sens. Actuators B 117, 99–106 (2006)
Yamini, Y., Hassan, J., Karbasi, M.H.: Solid-phase extraction of copper with cupron on octadecyl silica cartridge and its determination with atomic absorption. Microchim. Acta 148, 305–309 (2004)
Mahendra, N., Gangaiya, P., Subramaniam, S., Narayanaswamy, R.: Investigation of a fibre optic copper sensor based on immobilized a-benzoinoxime (cupron). Sens. Actuators B 90, 118–123 (2003)
Xiang, Y., Tong, A.J., Jin, P.Y., Ju, Y.: New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org. Lett. 8, 2863–2866 (2006)
Koplik, R., Mestek, O., Fingerora, H., Suchanek, M.: Validation protocol for the determination of copper in plant samples by isotope dilution inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 14, 241–245 (1999)
Taher, M.A., Mobarakeh, S.Z.M., Mohadesi, A.R.: Determination of trace copper by FAAS after solid phase extraction and preconcentration onto amberlite XAD-2 loaded with nitroso-R salt. Turk. J. Chem. 29, 17–26 (2005)
Ghasemi, J., Shahabadi, N., Seraji, H.R.: Spectrophotometric simultaneous determination of cobalt, copper and nickel using nitroso-R-salt in alloys by partial least squares. Anal. Chim. Acta 510, 121–126 (2004)
Pacheco, W.F., Miguel, E.M., Ramos, G.V., Cardoso, C.E., Farias, P.A.M., Aucélio, R.Q.: Use of hydrogen peroxide to achieve interference-free stripping voltammetric determination of copper at the bismuth-film electrode. Anal. Chim. Acta 625, 22–27 (2008)
Izatt, R.M., Bradshaw, J.S., Nielsen, S.A., Lamb, J.D., Christensen, J.J., Sen, D.: Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 85, 271–339 (1985)
Izatt, R.M., Pawlak, K., Bradshow, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interaction with cations and anions. Chem. Rev. 91, 1721–2085 (1991)
Faulkner, S., Kataky, R., Parker, D., Teasdale, A.: Lithium selective ionophores based on pendant arm substituted crown ethers. J. Chem. Soc., Perkin Trans. 2, 1761–1770 (1995)
Kimura, K., Shono, T.: Cation Binding by Macrocycles. In: Inoue Y., Gokel G.W. (eds.) chap. 10. Marcel Dekker, New York (1990)
Umezawa, Y.: CRC Handbook of Ion-Selective Electrodes. CRC Press, Boca Raton, FL (1990)
Kalcher, K., Kaufmann, J.M., Yang, Z.: Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period. Electroanalysis 7, 5–22 (1995)
Yeom, J.S., Won, M.S., Shim, Y.B.: Voltammetric determination of the iodide ion with quinine copper (II) complex modified carbon paste electrode. J. Electroanal. Chem. 463, 16–23 (1999)
Hamidi, H., Shams, E., Yadollahi, B., Esfahani, F.K.: Fabrication of bulk-modified carbon paste electrode containing α-PW12O40 3− polyanion supported on modified silica gel: preparation, electrochemistry and electrocatalysis. Talanta 74, 909–914 (2008)
Mousavi, M.F., Rahmanifar, S.M., Golabi, M., Shamsipur, M., Sharghi, H.: Differential pulse anodic stripping voltammetric determination of lead(II) with a 1,4-bis(prop-2-enyloxy)-9,10-anthraquinone modified carbon paste electrode. Talanta 55, 305–312 (2001)
Tian, M., He, Y.N., Wang, J.L.: Subspeciation of NiS in sulfidic nickel by carbon paste electrode voltammetry. Talanta 55, 349–356 (2001)
Abu-Shawish, H.M.: Potentiometric response of modified carbon paste electrode based on mixed ion-exchangers. Electroanalysis 20, 491–497 (2008)
Gismera, M.J., Sevilla, M.T., Procopio, J.R.: Copper(II) ion-selective electrodes based on dithiosalicylic and thiosalicylic acids. Talanta 74, 190–197 (2007)
Mashhadizadeh, M.H., Eskandari, Kh., Foroumadi, A., Shafiee, A.: Copper(II) modified carbon paste electrodes based on self-assembled mercapto compounds-gold-nanoparticle. Talanta 76, 497–502 (2008)
Mashhadizadeh, M.H., Sheikhshoaie, I.: Mercury(II) ion-selective polymeric membrane sensor based on a recently synthesized Schiff base. Talanta 60, 73–80 (2003)
Shamsipur, M., Mashhadizadeh, M.H.: Cadmium ion-selective electrode based on tetrathia-12-crown-4. Talanta 53, 1065–1071 (2001)
Mashhadizadeh, M.H., Shamsipur, M.: Silver(I)-selective membrane electrode based on hexathia-18-crown-6. Anal. Chim. Acta 381, 111–116 (1999)
Mashhadizadeh, M.H., Mostafavi, A., Allah-Abedi, H., Sheikhshoai, I.: New Schiff base modified carbon paste and coated wire PVC membrane electrode for silver ion. Sens. Actuators B 113, 930–936 (2006)
Mashhadizadeh, M.H., Momeni, A., Razavi, R.: Cobalt(II)-selective membrane electrode using a recently synthesized mercapto compound. Anal. Chim. Acta 462, 245–252 (2002)
Mashhadizadeh, M.H., Sheikhshoaie, I., Saeid-Nia, S.: Nickel(II)-selective membrane potentiometric sensor using a recently synthesized Schiff base as neutral carrier. Sens. Actuators B 94, 241–246 (2003)
Mashhadizadeh, M.H., Sheikhshoaie, I., Monadi, N.: A novel ion selective membrane potentiometric sensor for direct determination of Fe(III) in the presence of Fe(II). Talanta 64, 1048–1052 (2004)
Shockravi, A., Shamsipur, M., Fattahi, H., Taghdiri, M., Heidaryan, D., Alizadeh, K., Rostami, E., Abbaszadeh, M., Yousefi, A.: Efficient synthesis and metal cations complexation of some novel dinaphthosulfide-substituted macrocyclic diamides. J. Incl. Phenom. Macrocycle Chem. 61, 153–160 (2008)
Kamata, S., Bhale, A., Fukunaga, Y., Murata, A.: Copper(II)-selective electrode using thiuram disulfide neutral carriers. Anal. Chem. 60, 2464–2467 (1988)
Umezawa, Y., Umezawa, K., Sato, H.: Selectivity coefficients for ion-selective electrodes: recommended methods for reporting \( K_{A,B}^{pot} \) values. Pure Appl. Chem. 67:507–518 (1995)
Mashhadizadeh, M.H., Pour Taheri, E., Sheikhshoaie, I.: A novel Mn2+ PVC membrane electrode based on a recently synthesized Schiff base. Talanta 72, 1088–1092 (2007)
IUPAC Analytical Chemistry Division: Recommendations for nomenclature of ion selective electrodes. Pure Appl. Chem. 48, 127–132 (1976)
Singh, A.K., Mehtab, S.: Polymeric membrane sensors based on Cd(II) Schiff base complexes for selective iodide determination in environmental and medicinal samples. Talanta 74, 806–814 (2008)
Gholivand, M.B., Rahimi-Nasrabadi, M., Ganjali, M.R., Salavati-Niasari, M.: Highly selective and sensitive copper membrane electrode based on a new synthesized Schiff base. Talanta 73, 553–560 (2007)
Abbaspour, A., Moosavi, S.M.M.: Chemically modified carbon paste electrode for determination of copper(II) by potentiometric method. Talanta 56, 91–96 (2002)
Abu-Shawish, H.M., Saadehb, S.M., Hussien, A.R.: Enhanced sensitivity for Cu(II) by a salicylidine-functionalized polysiloxane carbon paste electrode. Talanta 76, 941–948 (2008)
Javanbakht, M., Badiei, A., Ganjali, M.R., Norouzi, P., Hasheminasab, A., Abdouss, M.: Use of organofunctionalized nanoporous silica gel to improve the lifetime of carbon paste electrode for determination of copper(II) ions. Anal. Chim. Acta 601, 172–182 (2007)
Mashhadizadeh, M.H., Mostafavi, A., Razavi, R., Shamsipur, M.: Highly selective Cu(II) PVC membrane electrode based on 3, 6, 9, 14-tetrathiabicyclo[9.2.1]tetradeca-11, 13-diene as suitable neutral ionophore. Sens. Actuators B 86, 222–228 (2002)
Mittal, S.K., Kumar, A., Gupta, S.K.N., Kaur, S., Kumar, S.: 8-Hydroxyquinoline based neutral tripodal ionophore as a copper (II) selective electrode and the effect of remote substitutents on electrode properties. Anal. Chim. Acta 585, 161–170 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mashhadizadeh, M.H., Khani, H. & Shockravi, A. Used a new aza-thia-macrocycle as a suitable carrier in potentiometric sensor of copper (II). J Incl Phenom Macrocycl Chem 68, 219–227 (2010). https://doi.org/10.1007/s10847-010-9770-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9770-z