Abstract
Modified carbon paste electrodes based on amino acid (l-ornithine) or curcumin (turmeric) as ionophores and o-nitrophenyl octyl ether (o-NPOE) as solvent mediator were prepared and investigated as Cu(II) ion-selective electrodes. The reaction mechanism between Cu(II) and l-ornithine (electrode I) or turmeric (electrode II) ionophores at the sensor surface were studied using energy-dispersive X-ray analysis (EDX), scanning electron microscopy (SEM) and IR spectra measurement. These electrodes showed potentiometric response with a divalent cationic Nernstian slopes of 29.0 ± 0.04 and 30.4 ± 0.01 mV decade−1, within a working concentration ranging from 1.0 × 10−6 to 1.0 × 10−2 mol L−1 with a short response time of 10 and 7 s for electrodes I and II, respectively. The electrodes exhibited constant potentiometric response in pH range 2-6 and 3-6 for electrodes I and II, respectively. The proposed sensors exhibited adequate sensitivity to Cu(II) ions over the other metal ions. The sensors were used for estimation of Cu(II) ion concentration in real water samples with satisfactory and good reproducibility results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper has a catalytic effect on auto-oxidation, which may lead to undesirable oxidation in nutritional or other samples. The metals which are present in fats and oils may be due to a corrosion of processing equipment (Fe, Ni), hardening (Ni, Cu) and the bleaching (Fe), or natural origin. Although copper (II) ions are very important for different biological processes, their levels in some cases are signals of a trouble or malady in numerous environments or living systems.
Accumulation of copper in the liver organism is a distinctive of Wilson’s disease, which leads to psychiatric and neurological disordered. As a result, the essential element in human diet is copper, although intake of big quantities may be toxic. Copper compounds soluble in drinking water show the biggest menace to humans. Therefore, the determination of copper at low levels is of special interest in both environmental monitoring and process monitoring.
Numerous methods have been applied to detect copper ion including atomic absorption spectrometry and the solid-phase extraction of copper (II) using modified octadecyl silica membrane [1], dispersive liquid–liquid microextraction [2], spectrophotometry [3], high-performance liquid chromatography [4], adsorptive stripping voltammetry [5] and anodic stripping voltammetry [6].
Electroanalytical analysis has found a wide range of applications in many fields, such as environmental monitoring, industrial quality control and biomedical analysis. Electroanalytical techniques have importance in the laboratory applications which are achieved by electroanalytical chemistry role as a main leading force in biosensor technology, modern chemical sensor as well as microsystems technology [7].
Electroanalytical methods had certain general advantages over other types of procedures. First, electrochemical measurements were often specific for a particular oxidation state of an element. A second important advantage of electrochemical methods was that the instrumentation was relatively inexpensive. In a third feature, they provided information about activities rather than concentrations of chemical species; moreover, the potentiometric measurements do not consume the analytes, so they are favored when low concentration is to be determined. Potentiometric sensors have economic and analytical advantages such as low cost, simple instrumentation, ease of preparation, fast response, high sensitivity, application to turbid or colored solutions and wide linear dynamic ranges [8, 9].
l-ornithine is (S)-2,5-diaminopentanoic acid, and it is a nonessential amino acid, plentiful in protein foods such as dairy products, meat, eggs and fish and also found free in the body tissues. It spurs the liberation of growth hormone, which decreases the body fat and increases muscle mass [10]. Complexes of Cu(II) with amino acids have attracted a lot of attention, where l-ornithine is one of the four important amino acids having basic side chains and containing amino and carboxylic groups which is responsible for chelation with metal ions [11].
Turmeric is 1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione and considered as one of the most efficient component of the everyday use; it is known as Indian spice, and it has medicinal features since old times which was frequently used in Ayurvedic medicines and traditional Chinese from more than 4000 years [12, 13]; moreover, it is known as a blood purifier. Turmeric exhibits keto–enol tautomerism depending on nature of the solvent, but many reports clear that the enolic form of isomer is relatively more stable than the keto form due to strong intramolecular hydrogen bonding [14, 15] and suggests that turmeric exists predominantly in its enolic form in polar solvents [16], with three possible metal chelating sites: the keto–enolic moieties and the two phenol groups as the potential reactive centers, although the keto–enolic moiety is most likely to form metal chelates [17]. The turmeric moiety is sparingly soluble in water at physiological pH and affirms its hydrophobic property [18]. Its chelating capacity with diverse metal ions has been investigated which is dependable for decreasing the deposition of metal ions within the human body [19], so turmeric has become an alluring field of research for inorganic researchers. There are a number of detailed complexes which evident higher stability than the free turmeric molecule [20, 21].
So depending on the above literature survey and chelating properties of the l-ornithine and turmeric, the researcher chose them as ionophores to modify and prepare new carbon paste sensors selective for copper potentiometric determination in different real water samples and the performance characteristics of the electrodes were studied according to IUPAC recommendations.
Experimental
Instrumentation and chemicals
All potentiometric estimations were performed at 25 ± 1 °C employing advanced Hanna pH/mV meter (model 8417), the energy-dispersive X-ray analyzer and scanning electron microscope (National Research Center, Egypt) which were used. FT-IR spectra were recorded on a PerkinElmer 1650 spectrometer (4000–400 cm−1) in KBr pellets at the Micro-analytical Center, Cairo University, Egypt.
All chemicals used were of analytical grade, including l-ornithine which was purchased from Sigma-Aldrich, while turmeric from Aroma Depot. Copper sulfate and graphite fine powder were purchased from Merck (Germany), while tricresyl phosphate (TCP), dibutyl phthalate (DBP) and dioctyl phthalate (DOP) were supplied from BDH; o-nitrophenyl octyl ether (o-NPOE) was supplied from Fluka.
Paste preparation
The traditional CPEs were consisting of Teflon holder for carbon paste filling as the electrode body. The carbon paste was formed by blending 100 μL of a suitable liquid binder (DBP, TCP, DOP or o-NPOE), 250 mg of graphite powder and 5-25 mg of the proposed ionophores. This matrix was completely blended in the mortar, and the prepared paste was used to fill the electrode body. Anew carbon paste surface was got by pushing tenderly the stainless-steel screw forward. Paste reproducibility was guaranteed by carefully taking after the ideal condition of manufacture.
E.M.F Measurements
The E.M.F measurements with the plasticized modified CPEs were carried out with the following cell assemblies: Ag | AgCl | KCl || copper solution | CPE.
The calibration curve was graphically constructed with E.M.F as a function of the negative log [Cu2+], and the intersection of the two extrapolated linear segments was taken as the limit of detection. The measured potential is related straightforwardly to the distinction in potential across the phase boundary between the copper solution phase and the hydrophobic paste phase. The mobility of copper ions from the aqueous sample phase into the paste phase causes potential changes of CPE.
Determination of Cu(II) ion in real water samples
Water (samples no. 1, 2 and 3) samples were supplied from Nikla Stations (input), Nikla Stations (output) and El Hagiz Stations (input), Egypt, respectively. The water samples composition was determined and then spiked with known amounts of Cu(II) ion; finally, they were analyzed by the proposed sensors and AAS.
Results and discussion
Electrode composition
A critical prerequisite for ion-selective sensor preparation was the ionophore, which was utilized in the paste as sensing material. It should display strong affinity for a certain ion to be determined and destitute affinity for others. They should have quick exchange kinetics and sufficient complex formation constants and should be completely soluble in the paste matrix. It should have adequate lipophilicity to avoid leaching from the paste into the analyte solution.
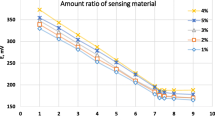
To evaluate the optimum amount of the proposed ionophores, different amounts of l-ornithine or turmeric ionophores as sensing material were used in the preparation of the modified CPEs for Cu(II) ion determination, and the potential response is listed in Table 1.
From Table 1, it was found that the sensors modified with 20 mg and 25 mg of l-ornithine (electrode I) and turmeric (electrode II), respectively, were the most effective amount which gave the best sensitivity with the best Nernstian response of 29.0 ± 0.04 and 30.4 ± 0.01 mV decade−1, over a working concentration ranging from 1.0 × 10−6 to 1.0 × 10−2 mol L−1 with adequate detection limit and a good correlation coefficient for electrodes I and II, respectively. The detection limit was decided from the crossing point of the two extrapolated segments of the calibration graph and was found to be 1.0 × 10−6 mol L−1 for each.
Also, the nature of plasticizer was anticipated to play a key role in deciding the ion selectivity characteristics of the sensor as it impacts the dielectric constant of the paste and the mobility of ions [22]. It also caused selective extraction of the target ion which made the electrochemical phase boundary potential due to thermodynamic balance at the interface. So, different plasticizers were used, namely TCP, o-NPOE, DOP and DBP, in order to study their impact on the execution of the sensors.
Among these plasticizers, the electrodes plasticized by o-NPOE provided sensitive, more stable and faster response in the concentration ranging from 1.0 × 10−6 to 1.0 × 10−2 mol L−1, as shown in Table 1. This can be attributed to its high dielectric constant and relatively high molecular weight.
In order to depict the surface morphology of the modified CPEs, scanning electron microscopy (SEM) and energy-dispersive X-ray analyzer (EDX) were utilized as shown in Figs. 1 and 2. The prepared electrodes surfaces showed a similar configuration, while incorporation of a suitable ionophore and plasticizer of high dielectric constant in the paste followed by immersion of the sensors in Cu(II) ion solution aid in the mobility of copper ions into the hydrophobic phase which were observed in filling the spaces between graphite particles as indicated by arrows in Figs. 1a and 2a, which allow the formation of a complex between the Cu(II) ions and incorporated ionophore which confirmed by the appearance of a new band of Cu(II) in EDX with percentage of 0.38%, as shown in Figs. 1b and 2b. These suggestions were supported by IR spectra of the electrodes surface as shown in Figs. 3 and 4. The paste incorporated l-ornithine as sensing material exhibits bands at 3437 and 3360 cm−1 due to υ(O–H) and υ(NH2) groups, respectively. While bands at 2966, 2927 and 3360 cm−1 may be due to the υ (CH2), then IR spectrum of the same electrode surface was carried out after immersion in copper ion solution, and a difference in the spectra is observed. The bands due to υ (CH2) were shifted to lower frequency 2958, 2931 and 2862 cm−1, while the bands due to υ(O–H) and υ(NH2) groups were disappeared or shifted to lower frequency (3300 cm−1), respectively [11, 23], recommending the complex formation by means of coordination of the l-ornithine to Cu(II) ion through the (O–H) and (NH2) groups [24].
While turmeric exhibits intensity bands at 1608 cm−1 due to υ(C = O) group, it exhibits intensity bands at 3687, 3560 and 3510 cm−1 due to υ(O–H) stretching vibrations, which was shifted to lower frequency 1570 cm−1 or disappeared, respectively, which confirm complex formation between turmeric and copper ions through (C=O) and (O–H) groups [12, 25].
Performance characteristics of the proposed electrodes according to IUPAC recommendations
pH effect
The pH dependence of the electrodes potential was examined over a range of pH of 1.0–10.0 in 1.0 × 10−2 and 1.0 × 10−4 mol L−1 solution of Cu(II) ions. The pH was adjusted by using very small volumes of NaOH and/or HCl solutions. The results showed that the electrodes potential was independent on pH within extent of 3-6 and 2-6 for electrodes I and II, respectively, as shown in Fig. 5. Exterior this range, the potential changed altogether. The increment in potential underneath pH 3.0 may be attributed to the competitive binding of protons to the ionophores on the electrode surface rather than Cu(II) ions, while at pH > 6.0, the decrease in potential can be attributed to the formation of hydroxyl complexes of Cu(II) such as Cu(OH)2 which monished its ability to combine with the ionophores [26, 27].
Response time
It is the time required for the electrode to give a stable potential reading within a range of variation of ± 1 mV depending on electrode structure and type, ion type, ionic strength and concentration. The response time was moderately short when measuring a high ion concentration after measuring low ion concentrations, while in the reverse process, a longer response time was obtained. Furthermore, at the minimum measurement limit, the response time was relatively long, being of the order of several minutes. So, it was essential to hold up until the potentiometer showed a stable reading before taking a value [28].
Response time was considered as one of the most essential factors for ion-selective electrode. The response time of the sensor was recorded experimentally by observing the potential change over a working concentration ranging from 1.0 × 10−6 to 1.0 × 10−2 mol L−1 of Cu(II). The potential readings versus time is constructed in Fig. 6. It is clear from the figures that the electrodes achieved their equilibrium responses in a very short time of 10 and 7 s over the linear concentration range for electrodes I and II, respectively, which reflect the fast kinetics exchange of complexation–decomplexation of Cu(II) ion with the proposed ionophores at the electrode surface [29].
Temperature effect
One of the important parameter which has an impact on the execution of the sensors is change in temperature of a test solution. The calibration graphs of the electrodes were constructed within the temperatures 10–60 °C. The electrodes exhibited good Nernstian behavior in the temperature range (10–50 °C) as shown in Table 2, and the slope of electrodes did not improve at higher temperature. The standard cell potentials (E 0cell ) were determined at various temperatures from the respective calibration graphs as the intercepts of these plots at pCu(II) = 0 and were utilized to estimate the isothermal temperature coefficient (dE0/dt) of the cell using the below equation [30, 31]:
Plot of E0 of the electrode versus (t − 25) gave a straight line, and the slope of this line was taken as the isothermal temperature coefficient of the Cu(II) electrodes. It amounted to be 0.518 and 0.280 V/ °C for electrodes I and II, respectively. The small values of (dE0/dt)electrode evident the high thermal stability of the electrodes within the examined temperature range.
Selectivity
Clearly, the selectivity was one of the most imperative characteristics of a sensor. It frequently decides whether a reliable measurement in the target sample was conceivable or not. Hypothetically, selectivity depiction permitted researchers to distinguish the key parameters for optimizing the execution of potentiometric sensors, e.g., by choosing various plasticizers or adjusting weighing parameters (i.e., absolute membrane concentrations) or matrices [32]. Ion-selective electrodes (ISE) have the most essential characteristic, that is, its relative response to other ions present in solution, which is expressed in terms of selectivity coefficients (K PotA,B ). Several methods can be utilized for measuring the selectivity coefficients of the electrode such as matched potential method (MPM) and separate solution (SSM) methods [33]. In the present study, SSM was used, which depends on measuring the potential of a cell comprising a reference electrode and an ISE with two separate solutions. One contains the ion of interest Cu2+ at the activity a 2+Cu (but no B), and the other contains the interfering ion B at the same activity aB = a 2+Cu (but no Cu2+). In this method, the values of the selectivity coefficient can be determined from the below equation:
EB and E 2+Cu are the potentiometric response of aB and a 2+Cu , respectively. a 2+Cu is the primary ion activity, and aB is the activity of an interfering ion [34], where a 2+Cu = aB = 1.0 × 10−3 mol L−1. S, Nernstian slope, Z 2+Cu and ZB are the charge of the copper and interfering ions, respectively. The selectivity coefficient values are recorded in Table 3. As the value of selectivity coefficient reaching 1.0 indicated equal response to both primary and interfering ions, the value of selectivity coefficient smaller than 1.0 showed that the sensor was selective to the primary ion over the interfering ions. The data given in Table 3 indicated that the electrodes (I and II) have adequate selectivity toward the Cu(II) ion rather than others; also, these results were confirmed by constructing the calibration graphs for all the interfering ions regarding to the linear range and slope values, as shown in Table 3.
According to the matched potential method (MPM) [35], the selectivity coefficient is defined as the activity ratio of the primary ion (A) and the interfering ion (B) that gives the identical potential change in a reference solution. The selectivity coefficient is determined as the following equation:
where aA is the initial primary ion Cu(II) activity, aA’ is the new activity of Cu(II) after addition of an aliquot of primary ion, and aB is the activity of interfering ions after the respective addition. The concentration of copper ions solution used, as primary ion in this study, was 1.0 × 10−4 mol L−1. At first, a known activity (aA’) of the copper ion solution is added into a reference solution that contains a fixed activity (aA) of copper ions and the corresponding potential change (ΔE) was recorded. Next, a solution of an interfering ion (aB) was added to the reference solution until the same potential change (ΔE) was reached. The change in potential produced at the constant background of the copper ion must be the same in both cases.
Analytical application
The proposed Cu(II)-selective electrodes (I and II) were successfully used for potentiometric determination of Cu(II) ions in real water samples. The composition of different collected real water samples was determined and is listed in Table 4 and then spiked with known amount of copper ions and subjected to analysis by the proposed sensors. The results obtained were also compared with those from AAS analysis. The data obtained are listed in Table 5, which recommended the successful use of the proposed electrodes (I and II) in the determination of Cu(II) ions in the presence of other ions, and there was a good agreement with those obtained by AAS method.
Method validation
Inter- and intra-day accuracy and precision
Collected real water samples spiked with distinctive concentrations of Cu(II) ion were utilized to carry out four duplicate experiments to assess the applicability and validity of the proposed method and reproducibility of the results obtained. Table 6 shows the values of the intra- and inter-day relative standard deviations for different concentrations of the samples obtained from experiments carried out within the same day (intra-day) or over a period of 4 days (inter-day). From the data obtained, the relative standard deviations were found to be small indicating sensible repeatability of the proposed sensors, so modified CPEs were effectively applied to determine Cu(II) in pure and real water samples.
Comparative study of copper determination
Table 7 shows comparison between the developed potentiometric carbon sensors and other previously reported methods for copper determination concerning working ranges of Cu(II) determination, the modifier type, Nernstian slope, pH, detection limit and response time with respect to our developed sensors. From the data listed in Table 7, we notice that most of the modifiers used need multistep of preparation or used expensive reagents, while the proposed ionophores, l-ornithine and turmeric which were considered as amino acid and Indian spice or a blood purifier, respectively, have advantages of simple, safe, harmless and natural reagents.
Conclusion
In this work, modified ion-selective electrodes carbon pastes were fabricated based on natural compounds as ionophores. The developed electrodes possessed fabulous execution in determination of Cu(II) in real water samples with high recovery in comparison with AAS method with fast response, high sensitivity, low detection limit and good selectivity with Nernstian behavior over a wide working concentration range.
References
Ö. Dalman, S. Karaböcek, A. Demirak, M. Tüfekçi, Turk. J. Chem. 27, 649 (2003)
J. Škrlíková et al., Microchem. J. 99, 40 (2011)
H.A. Panahi, M. Karimi, E. Moniri, H. Soudi, Afr. J. Pure Appl. Chem. 2, 096 (2008)
V. Kaur, A.K. Malik, Ann. Chim. 97, 1279 (2007)
S. Abbasi, H. Khani, R. Tabaraki, Food Chem. 123, 507 (2010)
B.C. Janegitz et al., Sens. Actuators B Chem. 142, 260 (2009)
rsc.org/Membership/Networking/InterestGroups/Electroanalytical/#, (2012)
H. Nam et al., Proc. IEEE 91, 870 (2003)
A. Lynch et al., Electroanalysis 10, 1096 (1998)
L.R. Bucci, J.F. Hickson, I. Wolinsky, J.M. Pivarnik, Int. J. Sport Nutr. 2, 287 (1992)
R. Kumar, S. Obrai, A. Sharma, A.K. Jassal, M.S. Hundal, J. Mitra, J. Mol. Struct. 1075, 43 (2014)
S. Wanninger, V. Lorenz, A. Subhan, F.T. Edelmann, Chem. Soc. Rev. 44, 4986 (2015)
A. Kareem, Laxmi, M. Arshad, S.A.A. Nami, N. Nishat, J. Photochem. Photobiol. B: Biology. 160, 163 (2016)
K.I. Priyadarsini, Curr. Pharm. Des. 19, 2093 (2013)
L. Nardo, R. Paderno, A. Andreoni, M. Másson, T. Haukvik, H.H. Tønnesen, Spectroscopy. 22, 187 (2008)
M. Călinescu, M. Fiastru, D. Bala, C. Mihailciuc, T. Negreanu-Pîrjol, B. Jurcă, J. Saudi Chem. Soc. 23, 817 (2019)
C.P.V. Mary, S. Vijayakumar, R. Shankar, J. Mol. Graph. Model. 79, 14 (2018)
K. Bairwa, J. Grover, M. Kania, S.M. Jachak, RSC Adv. 4, 13946 (2014)
R.S. Priya, S. Balachandran, J. Daisy, P.V. Mohanan, Univ. J. Phys. Appl. 3, 6 (2015)
D. Pucci, A. Crispini, B.S. Mendiguchia, S. Pirillo, M. Ghedini, S. Morelli, L. De Bartolo, Dalton Trans. 42, 9687 (2013)
E. Ferrari, R. Benassi, S. Sacchi, F. Pignedoli, M. Asti, M. Saladini, J. Inorg. Biochem. 139, 48 (2014)
V.K. Gupta, S. Jain, S. Chandra, Anal. Chim. Acta 486, 199 (2003)
S. Ramaswamy, M. Umadevi, R.K. Rajaram, V. Ramakrishnan, J. Raman Spectrosc. 34, 806 (2003)
C. Conato, A. Contino, G. Maccarrone, A. Magrõ, M. Remelli, G. Tabbõ, Thermochim. Acta 362, 13 (2000)
O. Vajragupta, P. Boonchoong, H. Watanabe, M. Tohda, N. Kummasud, Y. Sumanont, Free Radic. Biol. Med. 35, 1632 (2003)
X. Yuan et al., Anal. Chim. Acta 779, 35 (2013)
E.Y. Frag, R.M. Abdelhameed, Microchem. J. 144, 110 (2019)
E. Bakker, P. Bühlmann, E. Pretsch, Chem. Rev. 97, 3083 (1997)
M.R. Ganjali et al., Sens. Actuators B Chem. 98, 92 (2004)
Antropov, L. Antropov, Theoretical electrochemistry (Mir Publishers, Moscow, 1977)
S. Khalil, S. Abd El-Aliem, J. Pharm. Biomed. Anal. 27, 25 (2002)
E. Bakker, E. Pretsch, P. Bühlmann, Anal. Chem. 72, 1127 (2000)
E. Lindner, Y. Umezawa, Pure Appl. Chem. 80, 85 (2008)
S. Kamata et al., Anal. Chem. 60, 2464 (1988)
Y. Umezawa, K. Umezawa, H. Sato, Pure Appl. Chem. 67, 507 (1995)
M.J. Gismera, M.A. Mendiola, J.R. Procopio, M.T. Sevilla, Anal. Chim. Acta 385, 143 (1999)
M.J. Gismera, D. Hueso, J.R. Procopio, M.T. Sevilla, Anal. Chim. Acta 524, 347 (2004)
M. Ghaedi, S. Naderi, M. Montazerozohori, F. Taghizadeh, A. Asghari, Arab. J. Chem. 10, 2934 (2017)
T.A. Ali, G.G. Mohamed, M.M.I. El-Dessouky, S.M. Abou El-Ella, R.T.F. Mohamed, J. Solut. Chem. 42, 1336 (2013)
E.Y. Frag, M.E. Mohamed, G.G. Mohamed, E.M. Fahim, Appl. Organomet. Chem. 33, 1 (2019)
V.K. Gupta, A.K. Jain, G. Maheshwari, H. Lang, Z. Ishtaiwi, Sens. Actuators B Chem. 117, 99 (2006)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frag, E.Y., Mohamed, G.G. & Saad, M. Chemically modified copper potentiometric sensors based on curcumin and amino acid. J IRAN CHEM SOC 18, 651–660 (2021). https://doi.org/10.1007/s13738-020-02051-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-020-02051-5