Abstract
The synthesis of series of chromogenic di-substituted azocalix[4]arene derivatives is described. A ketone moiety as a different chelating agent is grafted on the lower rim of p-tert-butylcalix[4]arenes. Eight novel azocalix[4]arenes (1–8) are prepared by linking 2-, 3- and 4-nitroaniline, 4-phenylazoaniline, 3- and 4-chloroaniline or 2- and 4-methylaniline to 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)calix[4]arene through a diazo-coupling reaction, and characterized by UV–Vis, FT-IR and 1H-NMR spectroscopic techniques and elemental analysis, consecutively. The absorption spectra of the di-substituted azocalix[4]arenes are discussed, according to effect of varying pH and solvent upon the absorption ability of azocalix[4]arenes. The colors of these azocalix[4]arenes are compared with respect to nature of their aromatic rings and substituents there in. Concentration effects on the visible absorption maxima of these compounds are also reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calix[n]arenes are metacyclophanes bounded between phenolic and methylene units [1]. These are very attractive and excellent chromophores, because they provide a platform for the attachment of suitable binding groups to invent host molecules, mainly for the attraction of simple cations [2], anions [3] and neutral molecules [4]. The cup-shaped macrocycles possess hydrophobic cavities, capable of binding organic molecules. Calix[n]arenes can bind to small organic molecules in solid state [5]. The binding and solubility properties of calix[n]arenes in organic and aqueous solutions can be tailored by modification of the upper (arene) and lower (hydroxyl) rims. Functionalized calix[n]arenes have been received wide attention with applications, e.g., recovery of uranium, accelerations for instant adhesives, ionophoric electronic devices, and phase transferring agents [6]. Chemical modification of the upper and lower rim with alkyl groups leads amphiphilic calixarenes [7].
Shimuzu et al. synthesized a chromogenic calix[4]arene which has a aryl triester moiety as a metal binding site and an azophenol moiety as a coloration site [8]. Morita et al. and Shinkai et al., were studied diazo-coupling reactions of calix[4]arene by using the resulting NMR spectra [9].
The response of chromophoric calix[n]arenes to the presence of triester groups has been described [10]. Azocalix[4]arenes have been rendered through chromophoric reactions with diazonium salts to generate azo compounds. Previous reports of chromophoric phenylazocalix[n]arenes include an illustration of the autocatalytic nature of azo bond formation [11], their selective binding to metals [12], spectral properties of the azo-phenol and quinine-hydrazone tautomerization [13], and detection of amines with a chromophoric calix[8]arene [14].
In our recent works [15–20], various azocalix[n]arenes were synthesized by reacting carbocyclic and heterocyclic amines with calix[n]arenes and were characterized. The absorption spectra of novel azocalix[n]arenes were discussed, with respect to the effects of varying pH and solvent upon the absorption ability of these azocalix[n]arenes.
Present study was aimed to improve 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)calix[4]arene by using selective di-substituted azo-coupling reaction. By this way, modified azocalix[n]arene can be used as a calix[n]arene dye, providing colour with different absorption characteristics.
Experimental
General
All solvents and compounds are commercially graded reagents and they have been used without further purification. Melting points were measured using an Electrothermal IA9100 digital melting point apparatus in capillaries sealed under nitrogen and were uncorrected. 1H NMR spectra were referenced to tetramethylsilane (TMS) at 0.00 ppm as internal standard solution and recorded on a Bruker 400 MHz spectrometer at room temperature (25 ± 1 °C). IR spectra were recorded by a Mattson 1000 FTIR spectrometer as KBr pellets. UV–Vis spectra were obtained by a Shimadzu 1601 UV–Visible recording spectrophotometer. The elemental analyses were performed in the TUBITAK (The Scientific and Technological Research Council of Turkey) Laboratory.
Crystallization solvent was retained in some of the analytical samples and affected the elemental analysis. In such cases, best fits between the analytical values and appropriate fractional increments of solvents were used.
Preparation of the ligands
p-tert-Butylcalix[4]arene [21], 25,27-diacetonyloxy-26,28-dihydroxy-5,11,17,23-tetra-(tert-butyl)calix[4]arene [22] and 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)calix[4]arene [23] were synthesized as described previously.
Preparation of di-phenylazocalix[4]arenes (1–8)
Diazotization of the various carbocyclic amines is affected with conc. HCl. A typical procedure that described below is used for 2-nitroaniline. 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(3-nitrophenylazo)calix[4]arene (2), 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-nitrophenylazo)calix[4]arene (3), 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-phenylazophenylazo)calix[4]arene (4), 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(3-chlorophenylazo)calix[4]arene (5), 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-chlorophenylazo)calix[4]arene (6), 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(2-methylphenylazo)calix[4]arene (7) and 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-methyl phenylazo)calix[4]arene (8) are obtained using the same method in 50–87% yield. The obtained compounds are purified by crystallization using DMF–H2O and analyzed, consecutively.
All of the azocalix[4]arenes 1–8 are soluble in EtOH, diethyl ether, acetic acid, benzene, acetone, CHCl3, DMSO, but insoluble in water, 10% HCl and 10% NaOH.
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(2-nitrophenylazo)calix[4]arene (1)
A solution of 2-nitrophenyldiazonium chloride, which is prepared from 2-nitroaniline (0.46 g, 3.40 mmol), sodium nitrite (0.23 g, 3.40 mmol) and conc. HCl (0.85 mL) in water (3 mL), is added slowly to a cold (5 °C) solution of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)calix[4]arene (1.0 g, 1.54 mmol) and sodium acetate trihydrate (0.62 g, 5.10 mmol) in DMF–H2O (13 mL, 8:5, v/v) to give a dark brown suspension. After keeping the suspension at room temperature for 2 h, it is acidified with aqueous HCl (50 mL, 0.25%). The resulting mixture was then warmed up to 60 °C keep at that temperature for 30 min to give 1 (yield, 1.27 g, 77%) as a pale brown solid, which than is filtered, washed with water and MeOH. A sample for analysis was obtained as follows: 1 is dissolved in 100 mL of hot aqueous NaHCO3 (4.2 g) solution; to this solution is added activated charcoal (1 g). After the charcoal is filtered, the filtrate is cooled down to at room temperature and acidified with conc. HCl (1 or 2 mL). The solution is heated up to 60 °C and kept at there before cooling down for 30 min. The resulting solid is filtered, washed with water, and dried, respectively. Recrystallization from DMF/H2O mixture gave us a pale brown product (yield, 1.05 g (70%), m.p. 178 °C). [Found: C: 68.72%; H: 5.97%; N: 8.35%]; C54H54N6O10 requires C: 68.48%; H: 5.74%; N: 8.87%. IR (KBr) υ: 3350 cm−1 (–OH), 1730 cm−1 (–C=O), 1490 cm−1 (–N=N), 1200 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.2 (18H, s, t-bu), 2.2 (6H, s, CO–CH3), 3.8–4.3 (8H, d, J = 13.2 Hz, Ar–CH2–Ar), 4.8–5.1 (4H, s, –CH2–CO), 6.8–7.2 (8H, m, Ar–H), 7.3–8.1 (8H, m, ArH–NO2), 9.1–10.0 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(3-nitrophenylazo)calix[4]arene diketone (2)
Azocalix[4]arene 2 is prepared as described above, using 3-nitroaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us a pale brown product (yield, 0.88 g (60%), m.p. 169 °C). [Found: C: 68.53; H: 5.83; N: 8.45]; C54H54N6O10 requires C: 68.48; H: 5.74; N: 8.87. IR (KBr) υ: 3400 cm−1 (–OH), 1750 cm−1 (–C=O), 1490 cm−1 (–N=N), 1200 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.2 (18H, s, t-bu), 2.3 (6H, s, CO–CH3), 3.9–4.0 (8H, d, J = 13.1 Hz, Ar–CH2–Ar), 4.8–5.0 (4H, s, –CH2–CO), 7.0–7.4 (8H, m, ArH), 8.1–8.2 (8H, m, ArH–NO2), 9.0–9.8 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-nitrophenylazo)calix[4]arene (3)
Azocalix[4]arene 3 is prepared as described above, using 4-nitroaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us a pale brown product (yield, 0.72 g (50%), m.p. 140 °C). [Found: C: 68.52; H: 5.77; N: 8.65]; C54H54N6O10 requires C: 68.48; H: 5.74; N: 8.87. IR (KBr) υ: 3450 cm−1 (–OH), 1730 cm−1 (–C=O), 1490 cm−1 (–N=N), 1200 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.2 (18H, s, t-bu), 2.3 (6H, s, CO–CH3), 4.0–4.4 (8H, d, J = 13.4 Hz, Ar–CH2–Ar), 4.7–4.9 (4H, s, –CH2–CO), 6.9–7.3 (8H, m, Ar–H), 8.0–8.2 (8H, m, ArH–NO2), 9.1–9.9 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-phenylazophenylazo)calix[4]arene (4)
Azocalix[4]arene 4 is prepared as described above, using 4-phenylazoaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us a dark brown product (yield, 1.42 g (87%), m.p. 127 °C). [Found: C: 74.11; H: 6.27; N: 9.97]; C66H64N8O6 requires C: 74.41; H: 6.05; N: 10.51. IR (KBr) υ: 3450 cm−1 (–OH), 1710 cm−1 (–C=O), 1480 cm−1 (–N=N), 1230 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.3 (18H, s, t-bu), 2.4 (6H, s, CO–CH3), 4.0–4.4 (8H, d, J = 12.8 Hz, Ar–CH2–Ar), 4.7–4.9 (4H, s, –CH2–CO), 6.8–7.7 (8H, m, Ar–H), 7.8–8.0 (10H, m, Ar–H), 8.0–8.2 (8H, m, ArH), 9.2–10.2 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(3-chlorophenylazo)calix[4]arene (5)
Azocalix[4]arene 5 is prepared as described above, using 3-chloroaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us a pale yellow product (yield, 0.71 g (50%), m.p. 122 °C). [Found: C: 70.72; H: 5.87; Cl: 6.20; N: 7.55]; C54H54Cl2N4O6 requires C: 70.05; H: 5.88; Cl: 6.05; N: 7.66. IR (KBr) υ: 3410 cm−1 (–OH), 1725 cm−1 (–C=O), 1483 cm−1 (–N=N), 1200 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.3 (18H, s, t-bu), 2.3 (6H, s, CO–CH3), 3.8–4.4 (8H, d, J = 12.9 Hz, Ar–CH2–Ar), 4.8–5.2 (4H, s, –CH2–CO), 6.5–7.7 (8H, m, Ar–H), 7.8–8.1 (8H, m, ArH–Cl), 9.1–10.0 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-chlorophenylazo)calix[4]arene (6)
Azocalix[4]arene 6 is prepared as described above, using 4-chloroaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us a dark orange product (yield, 0.98 g (70%), m.p. 120 °C). [Found: C: 70.23; H: 5.81; Cl: 6.65; N: 7.72]; C54H54Cl2N4O6 requires C: 70.15; H: 5.86; Cl: 6.01; N: 7.61. IR (KBr) υ: 3450 cm−1 (–OH), 1740 cm−1 (–C=O), 1490 cm−1 (–N=N), 1190 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.2 (18H, s, t-bu), 2.2 (6H, s, CO–CH3), 3.8–4.4 (8H, d, J = 12.8 Hz, Ar–CH2–Ar), 4.8–5.2 (4H, s, –CH2–CO), 6.8–7.5 (8H, m, Ar–H), 7.8–8.2 (8H, m, ArH–Cl), 9.2–9.9 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(2-methylphenylazo)calix[4]arene (7)
Azocalix[4]arene 7 is prepared as described above, using 2-methylaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us an ivory product (yield, 0.75 g (56%), m.p. 119 °C). [Found: C: 75.23; H: 6.81; N: 6.65]; C56H60N4O6 requires C: 75.99; H: 6.83; N: 6.33. IR (KBr) υ: 3340 cm−1 (–OH), 1740 cm−1 (–C=O), 1490 cm−1 (–N=N), 1240 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.1 (18H, s, t-bu), 1.7 (6H, s, Ar–CH3), 2.2 (6H, s, CO–CH3), 4.0–4.4 (8H, d, J = 13.0 Hz, Ar–CH2–Ar), 4.7–4.9 (4H, s, –CH2–CO), 6.9–7.3 (8H, m, ArH), 7.9–8.1 (8H, m, CH3–ArH), 9.0–9.9 (2H, broad, OH/NH).
The synthesis of 25,27-diacetonyloxy-26,28-dihydroxy-11,23-di-(tert-butyl)-5,17-(4-methylphenylazo)calix[4]arene (8)
Azocalix[4]arene 8 is prepared as described above, using 4-methylaniline in water/HCl and obtained which is filtered, washed with water and MeOH, and dried, respectively. The resulting solid is recrystallized from DMF/H2O mixture that gave us an ivory product (yield, 0.70 g (52%), m.p. 128 °C). [Found: C: 75.43; H: 6.88; N: 6.55]; C56H60N4O6 requires C: 75.95; H: 6.50; N: 2.74. IR (KBr) υ: 3430 cm−1 (–OH), 1720 cm−1 (–C=O), 1480 cm−1 (–N=N), 1200 cm−1 (C–O). 1H-NMR (DMSO-d6, 25 °C) δH: 1.1 (18H, s, t-bu), 1.7 (6H, s, Ar–CH3), 2.1 (6H, s, CO–CH3), 4.2–4.9 (8H, d, J = 13.1 Hz, Ar–CH2–Ar), 4.7–4.8 (4H, s, –CH2–CO), 7.0–7.2 (8H, m, ArH), 7.8–8.1 (8H, m, ArH–CH3), 9.1–10.0 (2H, broad, OH/NH).
Results and discussion
Synthesis and characterization
Generally it has been accepted that calix[n]arene based ionophores show much more ion selectivity over diazo-coupling-based ionophores. In some cases, this is enhanced by the use of appropriate functionalities. Our previous azocalix[n]arene based studies were mostly focused on derivatives with functionalities appended to the lower rim [17–20]. The main of purpose of this work is to design new selective substituted azocalix[4]arenes with functionalities appended to the upper rim. The desired azocalix[n]arenes are easily synthesized. They have effective binding properties for a particular set of cation/anions, and can potentially be useful for multiple applications such as clinical, environmental and industrial applications.
Calix[n]arenes have been widely used as three-dimensional building blocks for the construction of artificial molecular receptors which are capable of recognizing neutral molecules [4], cations [2] and, more recently, anions [3]. Herein, we report the synthesis of a novel azocalix[4]arene bearing a di-acetone azocalix[4]arenes moiety at the lower rim and chromogenic azo groups or nitro-, azo-, chloro- and methyl-groups at the upper rim. This approach could provide an efficient methodology for improving non-linear optical (NLO) materials and for biologically and/or chemically important cations and amines.
The synthesis of azocalix[4]arenes 1–8 is based on the previously reported procedures [15]. In this study, eight new azocalix[4]arene derivatives were synthesized from 2-, 3- and 4-nitroaniline, 4-phenylazoaniline, 3- and 4-chloroaniline or 2- and 4-methyl aniline. These di-substituted azocalix[4]arenes 1–8 are shown in Scheme 1.
To achieve the desired goal, we have synthesized p-tert-butylcalix[4]arene as a starting material through the base catalyzed condensation reaction [21]. The synthesis policy has been designed to enable its derivatization following the strategies outlined in Scheme 2. Firstly, p-tert-butylcalix[4]arene has been treated with chloroacetone in acetone in the K2CO3. Then, diketone derivative of p-tert-butylcalix[4]arene has been treated with anhydrous AlCl3 in toluene with the presence of phenol to yield the calix[4]arene [22]. The diazo coupling reaction is known to be a very effective derivatization tool at the para position (upper rim) of the calix[4]arene [9, 15]. Therefore, diketone calix[4]arenes have been treated with NaNO2 and HCl/H2O in the presence of various carbocyclic amine derivatives DMF/CH3OH to yield azocalix[4]arenes (1–8). These compounds have been recrystallized from DMF/H2O mixture and obtained in 50–87% yield.
All new azocalix[4]arenes were characterized by the combination of UV–Vis, IR, 1H-NMR spectroscopic techniques and elemental analyses. The conformational characteristics of azocalix[4]arenes were conveniently estimated by the splitting pattern of the ArCH2Ar methylene protons in the 1H-NMR spectroscopy [1]. 1H-NMR data showed that these azocalix[4]arenes 1–8 have a cone conformation, a typical AB pattern for the methylene bridge protons (ArCH2Ar) of the calix[4]arene moiety appeared in two sets of doublets covering a range of δ 3.8 and 4.9 ppm in the 1H-NMR (J AB = 12.8–13.4 Hz). Selective di-substituted azocalix[4]arenes 1–8 have been used as precursors for the synthesis of diazo coupled calix[4]arene by electrophilic substitution reactions.
As we reported before the di-substituted azocalix[4]arenes may exist in six possible tautomeric forms [24], namely the azo-phenol form (A) and the quinine-hydrazone forms (B and C), and azo-phenolat anion (D) and quinine-hydrazone anions (E and F) as shown in Scheme 3. The IR spectra of all the azocalix[4]arenes (in KBr) showed intense azo (–N=N–) bands at 1480–1490 cm−1. It can be suggested that these di-substituted azocalix[4]arenes exist as azo-phenol forms in solid state. The IR spectra also reported a band at 3340–3450 cm−1, which can be assigned to a hydroxyl group (OH). The other νmax values of 1190–1240 cm−1 (etheric–O–), 1720–1740 cm−1 (C=O) were also recorded.
The 1H-NMR spectra measured in DMSO-d6 at 25 °C showed singlets at 1.1–1.3 ppm (t-butyl), 2.1–2.4 ppm (CO-CH3), 4.7–5.2 ppm (–OCH2–), multiplets at 6.8–8.0 ppm (Ar–H, calix ring), a multiplet from 7.3 to 8.2 ppm for aromatic protons (Ar–H), a broad peak at 9.0–10.2 ppm for tautomeric hydroxide (OH) and hydrazone (NH) protons. These results show that the di-substituted azocalix[4]arenes may exist as a mixture of tautomeric forms in DMSO. The spectral analyses of the azocalix[4]arenes and the resulting values are in accordance with those of the previously reported azocalix[4]arenes [17–21].
The present paper describes the synthesis of di-substituted azocalix[4]arene and their azo-phenol and quinine-hydrazone groups bearing both azo and substitute groups such as nitro-, azo-, chloro- and methyl-groups for the first time.
Absorption properties
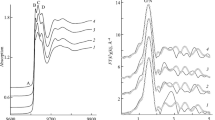
In order to evaluate the intermolecular forces between solvents and solute molecules and determine the best indicator azocalix[4]arene, a preliminary study of the absorption spectra was carried out for azocalix[4]arene 4 in selected solvents of different solvatation properties (DMSO, DMF, acetonitrile, chloroform, methanol and acetic acid). We found that azocalix[4]arene 4 shows the greatest shift in wavenumber maxima (Δλmax = 332 nm). Therefore, azocalix[4]arene 4 was submitted to a full solvatochromic study involving six solvents (Fig. 1).
Color chemistry studies have demonstrated that the coupling moiety of an azocalix[4]arene ring is a less substituted aromatic ring in typical donor-acceptor chromogen, such as azo-dyes, results in a significant bathochromic shift of the visible absorption spectra. This red shift with nitro-, azobenzene-, chloro- and methyl-groups suggest an increase in molecular hyperpolarizability according to theoretical NLO studies. Experimental data confirmed this positive effect, particularly for azobenzene ring. In accordance with other solvatochromic studies for azocalix[4]arenes, the increase of the electron-withdrawing strength on the substituent of the diazo component and/or the increase of the electron-donating strength of the coupling moiety were found to cause pronounced bathochromism. In general, red shifts in absorption were accompanied by positive solvatochromic shifts. This reasoning has been pronounced in earlier studies [25, 26].
Solvent effects
UV–Visible absorption spectra were recorded using an ATI-Unicam UV-100 spectrophotometer in the wavelength range 300–700 nm. Absorption spectra of azocalix[4]arenes 1–8 were recorded in various solvents at a concentration of 10−6–10−8 M and all of them were determined at different concentrations (Table 1). The pH value of all solutions was in the range between acidic and basic. The visible absorption spectra of the di-substituted azocalix[4]arenes did not show regular variation with the polarity of solvents.
The azocalix[4]arenes showed single or double absorbance in all used solvents. It can be suggested that the azocalix[4]arenes may exist as a mixture of tautomeric forms in various solvents. The absorption spectra of the azocalix[4]arene 4 in various solvents show a shift with respect to the absorption spectra of all solvents but methanol (e.g. λmax is 336 nm in CHCl3, 347 nm in DMSO, 352 nm in DMF) (Fig. 1).
It was also observed that the absolute absorption spectrum of the azocalix[4]arene 4 was sensitive to pH (Fig. 2). The λmax of the azocalix[4]arenes showed hypsochromic shifts when 0.1 M KOH was added to each of the azocalix[4]arene solutions in methanol. The absorption spectra of azocalix[4]arene 4 in methanol also showed bathochromic shifts when 0.1 M HCl was added. A typical example for the azocalix[4]arene 4 is shown in Fig. 2. The absorption spectra of azocalix[4]arenes 1–8 in methanol did not significantly change when 0.1 M HCl was added.
The effects of azocalix[4]arene concentration and temperature on absorption maxima were also examined. The λmax values of azocalix[4]arenes 1–8 did not significantly change with azocalix[4]arene concentration in all the solvents used. The λmax values of azocalix[4]arene in methanol showed a red shift with decreasing concentration. When solutions of the azocalix[4]arenes in DMSO and DMF were examined over the temperature range from 25 to70 °C, the λmax values of azocalix[4]arenes 1–8 did not show a significant alteration.
Substituent effects
As shown in Table 1, the introduction of electron-withdrawing nitro-group into the aromatic ring results in bathochromic shifts in DMSO and DMF (for azocalix[4]arene 1 Δλmax = 46 nm relative to azocalix[4]arene 3 for the spectra in DMSO) while they end up hypsochromic shifts in acetonitrile, methanol, acetic acid and chloroform. The electron-donating chloro-group in the aromatic ring produces bathochromic shifts in all solvents (for azocalix[4]arene 6 Δλmax = 8 nm relative to azocalix[4]arene 5 for spectra in chloroform). The electron-donating azo-group in the aromatic ring turns out bathochromic shifts in all solvents (for azocalix[4]arene 4 Δλmax = 77 nm relative to azocalix[4]arene 1 for spectra in chloroform). It was also observed that the introduction of electron-donating methyl-group in the aromatic ring (for azocalix[4]arene 8 Δλmax = 47 nm relative to azocalix[4]arene 7 for spectra in acetic acid) results in bathochromic shifts in all solvents.
Concentration effects
The effect of concentration on absorption maxima of the compounds 1–8 is given in Table 1. All azocalix[4]arenes have concentration independent λmax values of compounds indicating that azocalix[4]arenes may exist in their tautomeric form in all solvents. The λmax of all compounds did not change with compound concentration.
Conclusions
In conclusion, we have synthesized and characterized eight new chromogenic azocalix[4]arenes for applications in ionic and NLO sensors. Based on the presented evidences, the potential use of these new azocalix[4]arenes for extractions of cations, anions and neutral molecules warrants further investigations. We are currently working towards developing various analogues of di-substituted azocalix[4]arenes with the aim of accomplishing more selective anion sensing structures.
References
Gutsche, C.D.: Calixarenes Revisited: Monographs in Supramolecular Chemistry. The Royal Society of Chemistry, London (1998)
Arnaud-Neu, F., Collins, E.M., Deasy, M., Ferguson, G., Harris, S.J., Kaitner, B., Lough, A.J., McKervey, M.A., Marques, E., Ruhl, B.L., Schwing-Weill, M.J., Sewart, E.M.: Synthesis, X-ray crystal structures, and cation-binding properties of alkyl calixaryl esters and ketones, a new family of macrocyclic molecular receptors. J. Am. Chem. Soc. 11, 8681–8691 (1989)
Lang, K., Curinova, P., Dudic, M., Proskova, P., Stibor, I., Stastiny, V., Lhotak, P.: Unusual stoichiometry of urea-derivatized calix[4]arenes induced by anion complexation. Tetrahedron Lett. 46, 4469–4472 (2005)
Mohammed-Ziegler, I., Grün, A.: Complex formation between aliphatic amines and chromogenic calix[4]arene derivatives studied by FT–IR spectroscopy. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 62, 506–517 (2005)
Böhmer, V.: Calixarenes, macrocycles with (almost) unlimited possibilities. Angew. Chem. Int. Ed. Engl. 34, 713–745 (1995)
Perrin, R., Lamartine, R., Perrin, M.: The potential industrial applications of calixarenes. Pure Appl. Chem. 65, 1549–1559 (1993)
Tyson, J.C., Moore, J.L., Hughes, K.D., Collard, D.M.: Amphiphilic cup-shaped [(4-alkylphenyl)azo]-substituted calixarenes: self-assembly and host-guest chemistry at the air–water interface Langmuir 13, 2068–2073 (1997)
Shimuzu, H., Iwamoto, K., Fujimoto, K., Shinkai, S.: Chromogenic calix[4]arene. Chem. Lett. 20, 2147–2150 (1991)
Morita, Y., Agawa, T., Nomura, E., Taniguchi, H.: Syntheses and NMR behavior of calix[4]quinone and calix[4]hydroquinone. J. Org. Chem. 57, 3658–3662 (1992)
McCarrick, M., Wu, B., Harris, S.J., Diamond, D., Barrett, G., McKervey, M.A.: Chromogenic ligands for lithium based on calix[4]arene tetraesters bearing nitrophenol residues. J. Chem. Soc. Perkin Trans. 2, 1963–1968 (1993)
Shinkai, S., Araki, K., Shibata, J., Tsugawa, D., Manabe, O.: Autoaccelerative diazo coupling with calix[4]arene: substituent effects on the unusual co-operativity of the OH groups. J. Chem. Soc. Perkin Trans. 1, 3333–3335 (1990)
Nomura, E., Taniguchi, H., Tamura, S.: Selective ion extraction by a calix[6]arene derivative containing azo groups. Chem. Lett. 18, 1125–1126 (1989)
Shinkai, S., Araki, K., Shibata, J., Tsugawa, D., Manabe, O.: Diazo-coupling reactions with calix[4]arene. pKa determination with chromophoric azocalix[4]arenes. Chem. Lett. 18, 931–933 (1989)
Chawla, H., Srinivas, K.: Molecular diagnostics: synthesis of new chromogenic calix[8]arenes as potential reagents for detection of amines. J. Chem. Soc. Chem. Commun. 2593–2594 (1994)
(a) Deligöz, H., Ercan, N.: The synthesis of some new derivatives of calix[4]arene containing azo groups. Tetrahedron 58, 2881–2884 (2002); (b) Deligöz, H., Çetişli, H.: The synthesis and properties of some novel azo group containing calix[n]arene derivatives. J. Chem. Res. 10, 427–429 (2001)
Deligöz, H.: (Review), Azocalixarenes: synthesis, characterization, complexation, extraction, absorption properties and thermal behaviours. J. Incl. Phenom. Macrocycl Chem. 55, 197–218 (2006)
Karcı, F., Şener, I., Deligöz, H.: Azocalixarenes. 1: synthesis, characterization and investigation of the absorption spectra of substituted azocalix[4]arenes. Dyes Pigments 59, 53–61 (2003)
Karcı, F., Şener, I., Deligöz, H.: Azocalixarenes. 2: synthesis, characterization and investigation of the absorption spectra of azocalix[6]arenes containing chromogenic groups. Dyes Pigments 62, 131–140 (2004)
Şener, I., Karcı, F., Kılıç, E., Deligöz, H.: Azocalixarenes. 3: synthesis and investigation of the absorption spectra of hetarylazo disperse dyes derived from calix[4]arene. Dyes Pigments 62, 141–148 (2004)
Şener, I., Karcı, F., Kılıç, E., Deligöz, H.: Azocalixarenes. 4: synthesis, characterization and investigation of the absorption spectra of hetarylazo-substituted calix[6]arenas. Dyes Pigments 62, 149–157 (2004)
Gutsche, C.D., Iqbal, M.: p-tert-Butylcalix[4]arene. Org Synthesis 68, 234–238 (1990)
Gutsche, C.D., Lin, L.G.: Calixarenes 12: the synthesis of functionalized calixarenes. Tetrahedron 42, 1633–1640 (1986)
Gutsche, C.D., Iqbal, M., Stewart, D.: Calixarenes. 19. Syntheses procedures for p-tert-butylcalix[4]arene. J. Org. Chem. 51, 742–745 (1986)
Karcı, F., Ertan, N.: Hetarylazo disperse dyes derived from 3-methyl-1-(3′,5′-dipiperidino-s-triazinyl)-5-pyrazolone as coupling component. Dyes Pigments 55, 99–108 (2002)
Yen, M.S., Wang, I.J.: A facile syntheses and absorption characteristics of some monoazo dyes in bis-heterocyclic aromatic systems: part II: syntheses of 4-(p-substituted) phenyl-2-(2-pyrido-5-yl and 5-pyrazolo-4-yl)azo-thiazole derivatives. Dyes Pigments 63, 1–9 (2004)
Raposo, M.M.M., Sousa, A.M.R.C., Fonseca, A.M.C., Kirsh, G.: Thienylpyrrole azo dyes: synthesis, solvatochromic and electrochemical properties. Tetrahedron 61, 8249–8256 (2005)
Acknowledgements
This work was supported in part by a grant from the Scientific Research Projects Council of Pamukkale University (PA.U. BAP, 2003/023). The authors would like to express their thanks to Prof. Alaattin ŞEN for his critical reading of draft manuscript and making many helpful suggestions and corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karakuş, Ö.Ö., Deligöz, H. Azocalixarenes.8: synthesis and investigation of the absorption spectra of di-substituted azocalix[4]arenes containing chromogenic groups. J Incl Phenom Macrocycl Chem 61, 289–296 (2008). https://doi.org/10.1007/s10847-008-9421-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9421-9