Abstract
A number of chlorine-substituted azomethines of 2-hydroxybenzaldehydes and their zinc(II) complexes were synthesized. Structure of azomethines and zinc complexes was established by elemental analysis, IR, 1H NMR, and X-ray spectroscopy data. Complexes ZnL2 have a tetrahedral structure. The complexes exhibit weak photoluminescent properties in methylene chloride, whereas the photoluminescence quantum yields of the solid complexes are 100 times higher. Biological activity of the azomethines and zinc complexes was studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Chelating azomethine compounds and metal complexes based on them attract constant attention of researchers [1–12]. This is caused by their relative synthetic availability, great variability of structures, and a number of practically important properties. Azomethines and their metal complexes have a wide spectrum of biological activity [13–18], including antifungal [19, 20], antibacterial [21, 22], antimalarial [23–25], anticancer [26–29], and antiviral [30, 31], which makes them popular in medicine, veterinary medicine, and agriculture.
Complexes of metals with Schiff bases are used in homogeneous and heterogeneous catalysis. For example, copper complexes with N-(2-hydroxybenzylidene)aryl(alkyl)amines are used as catalysts for epoxidation and oxidation of olefins [32–34]. Zinc, cadmium, beryllium, etc. complexes with azomethine ligands exhibiting photoluminescent properties can be used in manufacturing electroluminescent devices, for example, as OLED emission layers [35, 36–38]. They have thermal stability, high electron transport properties, and easily sublimate during the formation of films. The most in demand are luminescent compounds emitting in the 400–450 nm range—the main components of blue, red, and white emitters in optoelectronics [39–41]. OLED devices of various configurations were made on the basis of zinc complexes with N-(2-hydroxybenzylidene)arylamines, which demonstrated changes in brightness characteristics depending on the structure of the complexes [35, 36–38].
Earlier, a series of photoluminescent zinc complexes, λfl 465‒541 nm, with bidentate azomethine ligands, N-[2-hydroxy-3-methoxy(methyl)benzylidene]-2,4,6-trimethylimines, was obtained [42]. On their basis, three different OLED devices were made, one of which had a maximum brightness of about 8000 cd/m2 at 17 V, and for the other two, the brightness was 2500 and 3000 cd/m2, respectively. The synthesis of new azomethine zinc complexes with photo- and electroluminescent properties is still relevant.
We have obtained a series of chlorine-substituted azomethines and their zinc(II) complexes and studied the photoluminescent properties and biological activity of these compounds. Azomethines 1а–1d were obtained by condensation of substituted 2-hydroxybenzaldehydes and amines in glacial acetic acid (Scheme 1).
Azomethines 1а–1d are fine-crystalline substances, from light yellow to orange in color, with mp from 122 to 172°C. Their structure was established by elemental analysis and IR, 1H NMR spectroscopy. The IR spectra of compounds 1а–1d contain absorption bands ν(СH=N) in the range of 1615‒1620 cm–1 and ν(Ph‒O) in the range of 1277‒1278 cm–1. The 1H NMR spectra of azomethines 1a, 1b, and 1d contain signals of protons corresponding to their structures. The signals of the protons of the phenolic OH groups appear as a singlet in the range 12.36‒ 13.57 ppm, and the signals of the protons of the CH=N groups in the range 8.52‒8.92 ppm. In the 1H NMR spectrum of azomethine 1b, along with the signal of the proton of the OH group at 12.70 ppm and the signal of the CH=N group proton at 8.91 ppm, there are the proton signal of the quinoid form of the NH group at 14.11 ppm and the signal at 9.00 ppm of the proton at the carbon atom in the СН‒NН group. As is known [43, 44], for N-(2-hydroxybenzylidene)arylimine benzoid-quinoid tautomerism is possible (Scheme 2), due to the proton transfer from the oxygen atom to the nitrogen atom. Thus, the appearance in the 1H NMR spectrum of the proton signals of the OH, CH=N, and CH‒NH groups and the analysis of their integral intensities suggest that a mixture of benzoic (A) and quinoid (B) tautomers of azomethine 1c exists in a solution in DMSO in the ratio 1: 1.
In order to confirm this assumption and determine the relative stability in DMSO of two tautomeric forms A and B of azomethine 1c, quantum-chemical calculations by the density functional method were carried out. These calculations showed that the energy of the quinoid form of the tautomer B is only 0.66 kcal/mol lower than the energy of the benzoic form A, which points to the existence of the tautomeric equilibrium of complex 1c in DMSO.
Bischelate zinc complexes 2а–2d were synthesized by refluxing a mixture of azomethine 1а–1d with methanol–chloroform (1 : 1) and a methanolic solution of zinc acetate dihydrate in a 2 : 1 molar ratio (Scheme 1). Complexes 2а–2d are yellow fine-crystalline substances with high melting points from 262 to >290°C. According to the elemental analysis, zinc complexes 2а–2d have the composition ZnL2. In the IR spectra of the zinc complexes, absorption bands ν(С=N) are observed at 1599‒1606 cm–1, which are shiftedg to the low-frequency region by 9‒16 cm–1 in comparison with the initial azomethines 1а–1d, whereas the absorption bands ν(Ph‒O) are shifted to the high-frequency region by 25‒42 cm–1 up to 1301‒1319 cm–1.
The signals of OH groups protons of azomethines 1а–1d disappear from the 1H NMR spectra of complexes 2а–2d, whereas the signals of the СH=N groups protons are slightly shifted by 0.06‒0.48 ppm in a strong field in comparison with azomethines and appear at 8.46‒ 8.53 ppm. Changes observed in the spectra of complexes 2а–2d in comparison with the initial azomethines are characteristic of the formation of chelate structures [19, 20, 42, 45, 46].
The signals of the OH and NH groups protons of ligand 1c disappear from the 1H NMR spectrum of zinc complex 2c, and the coordination of the zinc atom, as in other complexes 2a, 2b, and 2d, occurs with the benzoid form, as evidenced by the shape and nature of the 1H NMR and IR spectra.
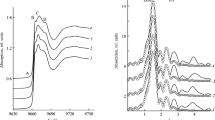
The local atomic structure of the nearest atomic environment of zinc ions in complexes 2а–2d was established by the X-ray spectroscopy from the analysis of XANES (X-Ray Absorption Near Edge Structure) and EXAFS (Extended X-Ray Absorption Fine Structure) of Zn absorption K-edges. The normalized XANES spectra and the corresponding EXAFS Fourier transform modules (MFT) for the compounds obtained are shown in Fig. 1. It can be noted that the position and shape of the Zn absorption K-edges of complexes 2а–2d are very close, which points to the same environment of zinc ions in these compounds. In the XANES spectra (Fig. 1a) of complexes 2a–2d, there is no pre-edge peak A due to the filled 3d-orbital of Zn(II). The presence of several maxima B, C, and D usually indicates a mixed composition of the nearest environment of zinc ions (in our case, oxygen and nitrogen atoms).
The quantitative characteristics of the coordination polyhedron in complexes 2а–2d were obtained from the EXAFS analysis of Zn absorption K-edges. The MFT EXAFS of these compounds are shown in Fig. 1b. All MFT have a main peak at r 1.51–1.53 Å, which corresponds to the scattering of a photoelectron wave by the nearest coordination sphere of nitrogen and oxygen atoms of the ligands. The MFT peaks at large r values are associated with subsequent coordination spheres, including various ligand atoms, mainly carbon atoms. As a result of calculations of model EXAFS spectra, it was found that the nearest environment of zinc ions in complexes 2а–2d is the same and consists of two nitrogen atoms and two oxygen atoms with average Zn···O distances about 1.92 Å and Zn···N distances about 2.01 Å (Table 1). The obtained Debye‒Waller factors of about 0.0032 Å2 are typical for such Zn···O/N distances in structurally similar coordination compounds [47].
A comparative study of the electronic spectra of azomethines 1а–1d and zinc complexes 2а–2d, recorded at room temperature in methylene chloride and in the solid state, has been carried out. Electronic absorption spectra (EAS) of solutions of zinc complexes 2а–2d are shown in Fig. 2, and the spectral and photoluminescent characteristics of the studied compounds are given in Table 2. In the spectral range from 300 to 400 nm, the electronic absorption spectra of azomethines 1а–1d are characterized by three absorption bands similar in shape, position, and intensity (Table 2).
In the EAS of zinc complexes 2а–2d in methylene chloride, four absorption bands are observed in the region of 309–418 nm. The longest-wavelength absorption bands in the spectra of zinc complexes 2а–2d are shifted bathochromically compared to azomethines 1а–1d by 58–60 nm and are observed at 412 (2a), 418 (2b), 413 (2c), and 417 nm (2d), respectively.
Azomethines 1a–1d do not luminesce. Complexes 2а–2d in methylene chloride exhibit weak photoluminescence with insignificant photoluminescence quantum yields, φ 0.002–0.008. The photoluminescence bands of zinc complexes, in comparison with azomethines 1a–1d, undergo a bathochromic shift. The Stokes shifts (the difference between the maxima of the long-wavelength absorption spectra and fluorescence bands) were 4431 (2a), 4584 (2b), 4643 (2c), and 4903 cm–1 (2d) in methylene chloride and 4923 (2a), 5923 (2b), 6626 (2c), and 6634 cm–1 (2d) for solid complexes.
The maxima of the photoluminescence bands λfl of complexes 2а–2d in solid state are even more bathochromically shifted compared to the spectra of their solutions (by 38–41 nm, Fig. 3). The photoluminescence quantum yields of complexes in solid state are much higher (almost 100 times) than the quantum yields of their solutions in methylene chloride (Table 2). Complexes 2а and 2b have the highest quantum yields (φ 0.147 and 0.191, respectively).
Azomethines 1a–1d and zinc complexes 2a–2d were tested for antibacterial, protistocidal, and fungistatic activity (Table 3). Azomethines 1a–1d and zinc complexes 2a–2d did not show fungistatic activity against Penicillium italicum. Azomethine 1b had antibacterial activity against Staphylococcus aureus, but its activity is 2 times weaker compared to the reference drug furazolidone. Azomethine 1b was moderately active against Escherichia coli (its activity is 2.2 times weaker than that of furazolidone). Complexes 2 did not show bacteriostatic activity against Staphylococcus aureus and Escherichia coli, except for complex 2b, the activity of which against these bacteria was 2 and 2.2 times weaker compares to the reference drug furazolidone, respectively.
When studying protistocidal properties (Table 3), it was found that among azomethines, azomethine 1c is most active against Colpoda steinii, the activity of which is 4 times weaker than that of the reference drug, whereas the activity of azomethine 1d is 8 times less than that of baycox. Azomethines 1a and 1b did not show protistocidal activity. Zinc complexes 2b and 2d had the highest activity against Colpoda steinii, but their activity was 8 times weaker compared to the baycox preparation. Complexes 2a and 2c had no protistocidal activity.
Thus, a number of chlorine-substituted azomethines of 2-hydroxybenzaldehydes and their zinc(II) complexes have been obtained. The structure of the complexes was established by X-ray absorption spectroscopy. In all the complexes obtained, a tetrahedral configuration of two oxygen atoms and two nitrogen atoms is realized around zinc(II) ions. The zinc complexes in methylene chloride exhibit weak photoluminescent properties; however, in solid state, the photoluminescence quantum yields for these complexes are about 100 times higher.
The biological activity of azomethines and zinc complexes has been studied. Azomethine, which has two chlorine atoms in positions 4 and 6 of the aldehyde fragment and one chlorine atom in position 4 of the amine fragment, exhibited the highest protistocidal activity among the studied azomethines and zinc complexes. The highest antibacterial activity was shown by 2-[(E)-(3,4-dichlorophenyl)iminomethyl]-4-chlorophenol and its complex with zinc, but their activity was 2–2.2 times weaker than that of the reference drug furazolidone. The results of studying biological activity make it possible to consider the search for antiprotozoal drugs among chlorine-substituted azomethines of 2-hydroxybenzaldehyde and metal complexes based on them as promising.
EXPERIMENTAL
We used commercially available solvents, zinc acetate dihydrate (CAS no. 5970-45-6), 2-hydroxybenzaldehyde (CAS no. 90-02-8), 2-hydroxy-5-chlorobenzaldehyde (CAS no. 635-93-8), 2-hydroxy-3,5-dichlorobenzaldehyde (CAS no. 90-60-8), 2-hydroxy-3,4-dichlorobenzaldehyde, acetic acid (CAS no. 64-19-7), sodium hydroxide (CAS no. 1310-73- 2), 4-chloroaniline (CAS no. 106-47-8), aniline (CAS no. 62-53-3), and 3,4-dichloroaniline (CAS no. 95-76-1) (Alfa Aesar) without preliminary purification.
The C, H, N elemental analysis was carried out on a Carlo Erba Instruments TCM 480 analyzer. The amount of the metal was determined by the gravimetric method. Melting points were measured on a Kofler table. The IR spectra of the samples were recorded on a Varian 3100-FTIR Excalibur instrument in the range 4000–400 cm–1 by the method of disturbed total internal reflection. The 1H NMR spectra were recorded on a Varian Unity-300 instrument (300 MHz) in DMSO-d6 and CDCl3. The chemical shifts of the 1H nuclei are given relative to the residual signals of the deuterated solvent. Electronic absorption spectra for 2.0×10–5 M solutions were obtained on an Agilent 8453 spectrophotometer. Photoluminescence spectra of 5.0×10–6 M solutions were recorded on a Varian Cary Eclipse fluorescence spectrophotometer. All spectra were recorded in dichloromethane (spectroscopy grade, Acros Organics) solutions at room temperature. The fluorescence quantum yields were determined relative to 3-methoxybenzanthrone in toluene as a standard (φfl 0.1, excitation at 365 nm) [48]. The photoluminescence spectra of the compounds in the solid state were recorded using a Hamamatsu C11347-01 absolute quantum yield spectrometer. The photoluminescence absolute quantum yields were determined using an integrating sphere of a Hamamatsu C11347-01 spectrometer (excitation at 390 nm).
The X-ray Zn absorption K-edges of zinc complexes were obtained at the “Structural Materials Science” station of the Kurchatov Synchrotron Center (Moscow) [49]. The energy of the electron beam, which was used as a source of X-ray synchrotron radiation, was 2.5 GeV at an average current of 100–120 mA. The X-ray absorption spectra were processed by standard procedures for background isolation, normalization to the value of the K-edge jump, and isolation of atomic absorption μ0, after which the Fourier transformation of the extracted EXAFS (χ)-spectrum was performed in the range of photoelectron wave vectors k from 2.5 to 12– 13 Å–1 with k3 weight function. The exact parameters of the zinc ion nearest environment in the studied compounds were determined by nonlinear fitting of parameters of the corresponding coordination spheres by comparing the calculated EXAFS patterns and those extracted from the full absorption spectrum by the Fourier filtration method. This procedure was performed using the IFFEFIT software package [50]. The phases and amplitudes of the photoelectron wave scattering required for constructing the model spectrum were calculated using the FEFF7 program [51]. As the initial atomic coordinates required for calculating the phases and amplitudes of scattering and further fitting, we used X-ray structural data for single crystals of metal complexes with a similar molecular structure from the Cambridge Database. The function of the Q fit quality, minimization of which was carried out while finding the parameters of the nearest environment structure, was calculated by formula (1).
Here w(ki) is the weight function, m is the number of experimental points, χexp(Ri) and χth(Ri) are EXAFS functions in r-space.
Quantum-chemical calculations were carried out within the framework of the density functional theory using the hybrid exchange-correlation functional B3LYP [52, 53] and the 6-311++G(d,p) valence-split basis of Gaussian functions extended with polarization d-functions on heavy atoms [54]. We used the Gaussian’03 program [55]. The geometry of the molecules was optimized without restriction on symmetry, the minima of the potential energy surface being characterized by the absence of imaginary frequencies of the calculated normal vibrations. The influence of the medium was taken into account within the continuous polarizable medium model [56] using the parameters for the solvent (DMSO).
The antibacterial activity was estimated using the Staphylococcus aureus 6538 P and Escherichia coli F 50 strains (field isolates from the collection of the Rostov Regional Veterinary Laboratory) by the agar diffusion method [20, 57]. Furazolidone was used as a reference. The level of antibacterial activity was determined by the size of the growth inhibition zones.
The study of the fungistatic activity of new substances was carried out on a culture of fungi of the Penicillium genus and Penicillium italicum Wehmer (1894) species (field isolate) from the collection of micromycetes of the mycotoxicology laboratory of the North Caucasian Zonal Research Veterinary Institute according to the method [20]. Fundazol served as a reference drug.
The protistocidal activity was studied on the protozoa of the Colpoda steinii species (field isolate) from the collection of the parasitology laboratory of SKZNIVI. Protistocidal activity was studied by the method of serial dilutions according to the procedure [20, 57, 58] on the species of Colpoda steinii protozoa culture. The reference drug was baycox (2.5% solution of toltrazuril) in the form of aqueous solutions with the same concentrations as the test compounds.
General procedure for the synthesis of azomethines 1a–1d. To a hot solution of 10 mmol of chlorine-substituted aniline in 5 mL of glacial acetic acid, a hot solution of 10 mmol of chlorine-substituted 2-hydroxybenzaldehyde in 5 mL of glacial acetic acid was added. The reaction mixture was stirred for 1 h at 100°С, then cooled to room temperature, and 10 mL of ethanol was added. The precipitate was filtered off, washed with ethanol, dried in a vacuum oven at 100°C, recrystallized from glacial acetic acid, and washed with ethanol.
4-Chloro-2-[(E)-(4-chlorophenyl)iminomethyl]phenol (1а) was obtained from 1.56 g of 2-hydroxy-5-chlorobenzaldehyde and 1.27 g of 4-chloroaniline. Yield 2.07 g (78%), yellow crystals, mp 151–152°C (AcOH). IR spectrum, ν, cm–1: 1615 m (СH=N), 1276 s (Ph‒O). 1H NMR spectrum (DMSO-d6), δ, ppm: 6.99 d (1HAr, 3J 8.7 Hz), 7.41‒7.53 m (5HAr), 7.74 d (1HAr, 4J 2.7 Hz), 8.91 s (1H, CH=N), 12.70 s (1H, OH). Found, %: C 58.63; H 3.47; N 5.23. C13H9Cl2NO. Calculated, %: C 58.67; H 3.41; N 5.26.
2-[(E)-(3,4-Dichlorophenyl)iminomethyl]4-chlorophenol (1b) was obtained from 1.56 g of 2-hydroxy-5-chlorobenzaldehyde and 1.62 g of 3,4-dichloroaniline. Yield 2.4 g (80%), orange powder, mp 137‒138°C (AcOH). IR spectrum, ν, cm–1: 1620 s (СH=N), 1278 s (Ph‒O). 1H NMR spectrum (DMSO-d6), δ, ppm: 6.99 d (1НAr, 3J 9.0 Hz), 7.38‒7.47 m (2HAr), 7.68 s (1HAr), 7.71‒7.73 m (2HAr), 8.92 s (1H, CH=N), 12.36 s (1H, OH). Found, %: C 51.93; H 2.65; N 4.62. C13H8Cl3NO. Calculated, %: C 51.95; H 2.68; N 4.66.
2,4-Dichloro-6-[(E)-(4-chlorophenyl)iminomethyl]phenol (1c) was obtained from 1.91 g of 2-hydroxy-3,5-dichlorobenzaldehyde and 1.27 g of 4-chloroaniline. Yield 2.52 g (84%), orange powder, mp 121‒122°C (AcOH). IR spectrum, ν, cm–1: 1615 m (СH=N), 1277 m (Ph‒O). 1Н NMR spectrum (DMSO-d6), δ, ppm: 7.41‒7.46 m (1.5НAr), 7.49‒7.57 m (3НAr), 7.71‒7.74 m (1.5НAr), 8.91 s (0.5Н, СH=N), 9.00 s (0.5H, СH‒NH), 12.70 s (0.5H, OH), 14.11 s (0.5H, NH). Found,%: C 51.98; H 2.63; N 4.69. C13H8Cl3NO. Calculated,%: C 51.95; H 2.68; N 4.66.
2,4-Dichloro-6-[(E)-(3,4-dichlorophenyl)iminomethyl]phenol (1d) was obtained from 1.91 g of 2-hydroxy-3,5-dichlorobenzaldehyde and 1.62 g of 3,4-dichloroaniline. Yield 2.58 g (77%), orange powder, mp 171–172°C (AcOH). IR spectrum, ν, cm–1: 1615 m (СH=N), 1278 m (Ph‒O). 1H NMR spectrum (CDCl3), δ, ppm: 7.13 d. d (1HAr, 3J 8.7, 4J 2.4 Hz), 7.3 d (1HAr, 4J 2.7 Hz), 7.38 d (1HAr, 4J 2.4 Hz), 7.47 d (1HAr, 4J 2.4 Hz), 7.5 d (1HAr, 3J 8.7 Hz), 8.52 s (1H, CH=N), 13.57 s (1H, OH). Found, %: C 46.54; H 2.15; N 4.12. C13H7Cl4NO. Calculated, %: C 46.61; H 2.11; N 4.18.
General procedure for the synthesis of complexes 2а–2d. A solution of zinc acetate dihydrate (0.22 g, 1 mmol) in 5 mL of methanol was added to a boiling solution of 2 mmol of azomethine 1a–1d in 30 mL of a mixture of methanol and chloroform (1 : 1). Then, a solution of 0.08 g (2 mmol) of sodium hydroxide in 5 mL of methanol was added dropwise. The reaction mixture was boiled for 2 h, the precipitate was filtered off, washed with methanol, and dried in a vacuum oven at 100°C.
Bis{4-chloro-2-[(E)-(4-chlorophenyl)iminomethyl]phenoxy}zinc (2а) was obtained from 0.53 g (2 mmol) of azomethine 1а. Yield 0.42 g (70%), yellow powder, mp 267–268°C. IR spectrum, ν, cm–1: 1600 m (СH=N), 1301 m (Ph‒O). 1H NMR spectrum (DMSO-d6), δ, ppm: 6.67 d (2HAr, 3J 9.0 Hz), 7.27 d. d (2HAr, 3J 9.3, 4J 2.7 Hz), 7.28 d (4HAr, 3J 9.0 Hz), 7.33 d (4HAr, 3J 9.0 Hz), 7.49 d (2HAr, 4J 2.7 Hz), 8.53 s (2H, CH=N). Found, %: C 52.47; H 2.76; N 4.73; Zn 10.95. C26H16Cl4N2O2Zn. Calculated, %: C 52.43; H 2.71; N 4.70; Zn 10.98.
Bis{2-[(E)-(3,4-dichlorophenyl)iminomethyl]4-chlorophenoxy}zinc (2b) was obtained from 0.6 g (2 mmol) of azomethine 1b. Yield 0.49 g (73%), yellow powder, mp 261–262°C. IR spectrum, ν, cm–1: 1604 m (СH=N), 1312 m (Ph‒O). 1H NMR spectrum (DMSO-d6), δ, ppm: 6.62 d (2HAr, 3J 9.0 Hz), 7.24 d (2HAr, 3J 8.4 Hz), 7.34 d. d (2HAr, 3J 8.4, 4J 2.1 Hz), 7.45 s (2HAr), 7.6 d (2HAr, 3J 8.4 Hz), 7.69 s (2HAr), 8.45 s (2H, CH=N). Found, %: C 46.94; H 2.16; N 4.25; Zn 9.85. C26H14Cl6N2O2Zn . Calculated, %: C 46.99; H 2.12; N 4.22; Zn 9.84.
Bis{2,4-dichloro-6-[(E)-(4-chlorophenyl)iminomethyl]phenoxy]zinc (2c) was obtained from 0.6 g (2 mmol) of azomethine 1c. Yield 0.51 g (76%), yellow powder, mp > 290°C. IR spectrum, ν, cm–1: 1599 s (СH=N), 1319 w (Ph‒O). 1H NMR spectrum (DMSO-d6), δ, ppm: 7.24 d (2HAr, 8.1 Hz), 7.35‒7.52 m (10HAr), 8.47 s (2H, CH=N). Found, %: C 46.95; H 2.16; N 4.27; Zn 9.80. C26H14Cl6N2O2Zn. Calculated, %: C 46.99; H 2.12; N 4.22; Zn 9.84.
Bis[2,4-dichloro-6-[(E)-(3,4-dichlorophenyl)iminomethyl]phenoxy]zinc (2d) was obtained from 0.67 g (2 mmol) of azomethine 1d. Yield 0.52 g (71%), yellow powder, mp > 290°C. IR spectrum, ν, cm–1: 1606 s (СH=N), 1318 w (Ph‒O). 1H NMR spectrum (DMSO-d6), δ, ppm: 7.46‒7.49 m (6HAr), 7.61 d (2HAr, 3J 7.2 Hz), 7.93 br. s (2HAr), 8.46 s (2H, CH=N). Found, %: C 42.53; H 1.69; N 3.86; Zn 8.95. C26H12Cl8N2O2Zn. Calculated, %: C 42.58; H 1.65; N 3.82; Zn 8.91.
REFERENCES
Hernandez Molina, R. and Mederos, A., Comprehensive Coordination Chemistry II, Lever, A.B.P., Ed., Amsterdam: Elsevier-Pergamon Press, 2003, vol. 1, p. 411.
Synthetic Coordination and Organometallic Chemistry, Garnovskii, A.D. and Kharisov, B.I., Eds., New York: Marcel Dekker, 2003. https://doi.org/10.1023/B:RUCO.0000011647.15103.04
Vigato, P.A. and Tamburini, S., Coord. Chem. Rev., 2004, vol. 248, nos. 17–20, p. 1717. https://doi.org/10.1016/j.cct.2003.09.003
Vigato, P.A., Tamburini, S., and Bertolo, L., Coord. Chem. Rev., 2007, vol. 251, nos. 11–12, p. 1311. https://doi.org/10.1016/j.ccr.2006.11.016
Vigato, P.A. and Tamburini, S., Coord. Chem. Rev., 2008, vol. 252, nos. 18–20, p. 1871. https://doi.org/10.1016/j.ccr.2007.10.030
Garnovskii, A.D., Vasil’chenko, I.S., and Garnovskii, D.A., Russ. Chem. Rev., 2002, vol. 71, no. 11, p. 943. https://doi.org/10.1070/RC2002v071n11ABEH000759
Garnovskii, A.D. and Vasil’chenko, I.S., Russ. Chem. Rev., 2005, vol. 74, no. 3, p. 193. https://doi.org/10.1070/RC2005v074n03ABEH001164
Garnovskii, A.D., Vasilchenko, I.S., Garnovskii, D.A., and Kharisov, B.I., J. Coord. Chem., 2009, vol. 62, no. 2, p. 151. https://doi.org/10.1080/00958970802398178
Garnovskii, A.D., Sadimenko, A.P., Vasilchenko, I.S., Garnovskii, D.A., Sennikova, E.V., and Minkin, V.I., Adv. Heterocycl. Chem., 2009, vol. 97, p. 291. https://doi.org/10.1016/S0065-2725(08)00205-5
Darensbourg, D.J., Mackiewicz, R.M., Phelps, A.L., and Billodeaux, D.R., Acc. Chem. Res., 2004, vol. 37, no. 11, p. 836. https://doi.org/10.1021/ar030240u
Miyasaka, H., Saitoh, A., and Abe, S., Coord. Chem. Rev., 2007, vol. 251, no. 21-24, p. 2622. https://doi.org/10.1016/j.ccr.2007.07.028
Gupta, K.S. and Sutar, A.K., Coord. Chem. Rev., 2008, vol. 252, nos. 12–14, p. 1420. https://doi.org/10.1016/j.ccr.2007.09.005
Yousif, E., Majeed, A., Al-Sammarrae, K., Salih, N., Salimon, J., and Abdullah, B., Arab. J. Chem., 2017, vol. 10, p. 1639. https://doi.org/10.1016/j.arabjc.2013.06.006
Arunadevi, A. and Raman, N., J. Coord. Chem., 2020, vol. 73, p. 2095. https://doi.org/10.1080/00958972.2020.1824293
More, M.S., Joshi, P.G., Mishra, Y.K., and Khanna, P.K., Mat. Today Chem., 2019, vol. 14, p. 100195. https://doi.org/10.1016/j.mtchem.2019.100195
Loginova, N.V., Harbatsevich, H.I., Osipovich, N.P., Ksendzova, G.A., Koval’chuk, T.V., and Polozov, G.I., Curr. Med. Chem., 2020, vol. 27, p. 5213. https://doi.org/10.2174/0929867326666190417143533
Erxleben, A., Inorg. Chim. Acta, 2018, vol. 472, p. 40. https://doi.org/10.1016/j.ica.2017.06.060
Kumar, S., Dhar, D.N., and Saxena, P, N., J. Sci. Ind. Res., 2009, vol. 68, p. 181.
Vlasenko, V.G., Burlov, A.S., Koshchienko, Yu.V., Kiskin, M.A., Garnovskii, D.A., Zubavichus, Ya.V., Kolodina, A.A., Trigub, A.L., Zubenko, A.A., and Drobin, Yu.D., Inorg. Chim. Acta, 2020, vol. 510, p. 119776. https://doi.org/10.1016/j.ica.2020.119766
Burlov, A.S., Vlasenko, V.G., Koshchienko, Yu.V., Makarova, N.I., Zubenko, A.A., Drobin, Yu.D., Fetisov, L.N., Kolodina, A.A., Zubavichus, Ya.V., Trigub, A.L., Levchenkov, S.I., and Garnovskii, D.A., Polyhedron, 2018, vol. 154, p. 65. https://doi.org/10.1016/j.poly.2018.07.034
Hui, R.-H., Zhou, P., and You, Z.-L., Indian J. Chem. A, 2009, vol. 48, p. 1102.
Adhikary, C., Banerjee, S., Chakraborty, J., and Ianelli, S., Polyhedron, 2013, vol. 65, p. 48. https://doi.org/10.1016/j.poly.2013.08.019
Lopes, F., Capela, R., Goncaves, J.O., Horton, P.N., Hursthouse, M.B., Iley, J., Casimiro, C.M., Bom, J., and Moreira, R., Tetrahedron Lett., 2004, vol. 45, p. 7663. https://doi.org/10.1016/j.tetlet.2004.08.093
Savir, S., Wei, Z.J., Liew, J.W.K., Vythilingam, I., Lim, Y.A.L., Saad, H.M., Sim, K.S., and Tan, K.W., J. Mol. Struct., 2020, vol. 1211, p. 128090. https://doi.org/10.1016/j.molstruc.2020.128090
Mehta, J.V., Gajera, S.B., and Patel, M.N., Spectrochim. Acta A, 2015, vol. 136, p. 1881. https://doi.org/10.1016/j.saa.2014.10.103
Tadele, K.T. and Tsega, T.W., Med. Chem., 2019, vol. 19, p. 1786. https://doi.org/10.2174/1871520619666190227171716
Parsekar, S.U., Haldar, P., Antharjanam, P.K.S., Kumar, M., and Koley, A.P., Appl. Organomet. Chem., 2021. e6152. https://doi.org/10.1002/aoc.6152
Kargar, H., Behjatmanesh-Ardakani, R., Torabi, V., Sarvian, A., Kazemi, Z., Chavoshpour-Natanzi, Z., Mirkhani, V., Sahraei, A., Tahir, M.N., and Ashfaq, M., Inorg. Chim. Acta, 2021, vol. 514, p. 120004. https://doi.org/10.1016/j.ica.2020.120004
Ribeiro, N., Bulut, I., Cevatemre, B., Teixeira, C., Yildizhan, Y., Andre, V., Adao, P., Pessoa, J.C., Acilan, C., and Correia, I., Dalton Trans., 2021, vol. 50, p. 157. https://doi.org/10.1039/d0dt03331f
Malik, M.A., Dar, O.A., Gull, P., Wani, M.Y., and Hashmi, A.A., Med. Chem. Commun., 2018, vol. 9, p. 409. https://doi.org/10.1039/c7md00526a
Shah, S.S., Shah, D., Khan, I., Ahmad, S., Ali, U., and Rahman, A.U., Res. Appl. Chem., 2020, vol. 10, p. 6936. https://doi.org/10.33263/BRIACI06.69366963
Das, G., Shukla, R., Mandal, S., Singh, R., and Bharadwaj, P.K., Inorg. Chem., 1997, vol. 36, p. 323. https://doi.org/10.1021/ic9510371
Lu, X.-H., Xia, Q.-H., Zhan, H.-J., Yuan, H.-X., Ye, C.-P., Su, K.-X., and Xu, G., J. Mol. Catal. (A), 2006, vol. 250, p. 62. https://doi.org/10.1016/j.molcata.2006.01.055
Bunce, S., Cross, R.J., Farrugia, L.J., Kunchandy, S., Meason, L.L., Muir, K.W., O’Donnell, M., Peacock, R.D., Stirling, D., and Teat, S.J., Polyhedron, 1998, vol. 17, p. 4179. https://doi.org/10.1016/S0277-5387(98)00226-5
Chen, L., Qiao, J., Xie, J., Duan, L., Zhang, D., Wang, L., Qiu, Y., and Chen, L., Inorg. Chim. Acta, 2009, vol. 362, p. 2327. https://doi.org/10.1016/J.ICA.2008.10.016
Sano, T., Nishio, Y., Hamada, Y., Takahashi, H., Usuki, T., and Shibata, K., J. Mater. Chem., 2000, vol. 10, p. 157. https://doi.org/10.1039/A903239H
Pivovarov, A.P., Kaplunov, M.G., Yakushchenko, I.K., Belov, M.Y., Nikolaeva, G.V., and Efimov, O.N., Russ. Chem. Bull., 2002, vol. 51, no. 1, p. 67. https://doi.org/10.1023/A:1015053512033
Kaplunov, M.G., Yakushchenko, I.K., Krasnikova, S.S., Shamaev, S.N., Pivovarov, A.P., and Efimov, O.N., Russ. Chem. Bull., 2004, vol. 53, no. 10, p. 2148. https://doi.org/10.1007/s11172-005-0088-8
Ma, D.Y., Zhang, L.X., Rao, X.Y., Wu, T.L., Li, D.H., Xie, X.Q., Guo, H.F., and Qin, L., J. Coord. Chem., 2013, vol. 66, no. 18, p. 3261. https://doi.org/10.1080/00958972.2013.832230
Burlov, A.S., Vlasenko, V.G., Garnovskii, D.A., Uraev, A.I., Maltsev, E.I., Lypenko, D.A., and Vannikov, Elektrolyuminestsentnye organicheskie svetodiody na osnove koordinatsionnykh soedinenii metallov (Electroluminescent Organic Light-Emitting Diodes Based on Coordination Compounds of Metals), Rostov-on-Don: YuFU, 2015.
Pushkarev, A.P. and Bochkarev, M.N., Russ. Chem. Rev., 2016, vol. 85, no. 12, p. 1338. https://doi.org/10.1070/RCR4665
Burlov, A.S., Vlasenko, V.G., Koshchienko, Yu.V., Milutka, M.S., Mal’tsev, E.I., Dmitriev, A.V., Lypenko, D.A., Nekrasova, N.V., Kolodina, A.A., Makarova, N.I., Metelitsa, A.V., Lazarenko, V.A., Zubavichus, Y.V., Khrustalev, V.N., and Garnovskii, D.A., Appl. Organomet. Chem., 2021, vol. 35, no. 2, p. e6107. https://doi.org/10.1002/aoc.6107
Kuznetsova, L.I., Burlov, A.S., Volbushko, N.V., Korshunov, O.Yu., Zaletov, V.G., and Garnovskii, A.D., Zh. Obshch. Khim., 1998, vol. 68, no. 8, p. 1338.
Sergienko, V.S., Abramenko, V.L., and Gorbunova, Yu.E., Crystallogr. Rep., 2020, vol. 65, no. 1, p. 53. https://doi.org/10.31857/S0023476120010233
Burlov, A.S., Mal’tsev, E.I., Vlasenko, V.G., Garnovskii, D.A., Dmitriev, A.V., Lypenko, D.A., Vannikov, A.V., Dorovatovskii, P.V., Lazarensko, V.A., Zubavichus, Ya.V., and Khrustalev, V.N., Polyhedron, 2017, vol. 133, p. 231. https://doi.org/10.1016/j.poly.2017.05.045
Lysakova, T.P., Burlov, A.S., Vlasenko, V.G., Koshchienko, Yu.V., Aleksandrov, G.G., Levchenkov, S.I., Zubavichus, Ya.V., Cheprasov, A.S., Garnovskii, D.A., and Metelitsa, A.V., Russ. J. Coord. Chem., 2016, vol. 42, no. 11, p. 701. https://doi.org/10.1134/S1070328416110075
Burlov, A.S., Vlasenko, V.G., Dmitriev, A.V., Chesnokov, V.V., Uraev, A.I., Garnovskii, D.A., Zubavichus, Y.V., Trigub, A.L., Vasilchenko, I.S., Lypenko, D.A., Mal’tsev, E.I., Lifintseva, T.V., and Borodkin, G.S., Synth. Met., 2015, vol. 203, p. 156. https://doi.org/10.1016/j.synthmet.2015.02.028
Krasovitskii, B.M. and Bolotin, B.M., Organicheskie lyuminifory (Organic Phosphors), Moscow: Khimiya, 1984, p. 292.
Chernyshov, A.A., Veligzhanin, A.A., and Zubavichus, Ya.V., Nucl. Instr. Meth. Phys. Res. A, 2009, vol. 603, p. 95. https://doi.org/10.1016/j.nima.200812.167
Newville, M., J. Synchrotron Rad., 2001, vol. 8, p. 96. https://doi.org/10.1107/S0909049500016290
Zabinski, S.I., Rehr, J.J., Ankudinov, A., and Alber, R.C., Phys. Rev., 1995, vol. 52, p. 2995. https://doi.org/10.1103/PhysRevB.52.2995
Lee, C., Yang, W., and Parr, R.G., Phys. Rev. (B), 1988, vol. 37, no. 2, p. 785. https://doi.org/10.1103/PhysRevB.37.785
Becke, A.D., J. Chem. Phys., 1993, vol. 98, no. 7, p. 5648. https://doi.org/10.1063/1.464913
Ditchfield, R., Hehre, W.J., and Pople, J.A., J. Chem. Phys., 1971, vol. 54, no. 2, p. 724. https://doi.org/10.1063/1.1674902
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Vreven, Jr.T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian 03, Revision A.1, Gaussian, Inc., Pittsburgh PA, USA (2003).
Tomasi, J., Mennucci, B., and Cammi, R., Chem. Rev., 2005, vol. 105, p. 2999.
Burlov, A.S., Vlasenko, V.G., Koshchienko, Yu.V., Makarova, N.I., Zubenko, A.A., Drobin, Yu.D., Borodkin, G.S., Metelitsa, A.V., Zubavichus, Ya.V., and Garnovskii, D.A., Polyhedron, 2018, vol. 144, p. 249. https://doi.org/10.1016/j.poly.2018.01.020
Fetisov, L.N., Zubenko, A.A., Bodryakov, A.N., and Bodryakova, M.A., Abstracts of Papers, Mezhdunarodnyi parazitologicheskii simpozium “Sovremennye Problemy Obshchei i Chastnoi Parazitologii” (International Parasitological Symp. “Modern Problems of General and Private Parasitology”), 2012, p. 70.
Funding
The work was carried out with the financial support of the Russian Foundation for Basic Research within the framework of the scientific project no. 20-33-90044 “Graduate Students.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 9, pp. 1426–1437 https://doi.org/10.31857/S0044460X21090146.
Rights and permissions
About this article
Cite this article
Milutka, M.S., Burlov, A.S., Vlasenko, V.G. et al. Synthesis, Structure, Spectral-Luminescent Properties, and Biological Activity of Chlorine-Substituted Azomethines and Their Zinc(II) Complexes. Russ J Gen Chem 91, 1706–1716 (2021). https://doi.org/10.1134/S1070363221090140
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221090140