Abstract
Three types of host molecules 1–3 based on a phenolphthalein (one of the most popular pH indicators) and two crown ethers were prepared for use in colorimetric recognition of linear diamines, triamines, sequence of non-protected dipeptides in methanol or water solution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A living system contains molecules of all sizes, ranging from tiny metallic ions such as Na+ and K+ to enormously large molecules like proteins, polysaccharides, and nucleic acids, and these constituents are strictly and tactfully recognized by biological receptors. This recognition is the basis for vital functions in cells. Supramolecular chemistry and/or host–guest chemistry based on molecular recognition has attracted the attention of organic chemists as well as researchers in other academic fields, and recent progress in supramolecular chemistry is remarkable. Relatively weak non-covalent forces, for example, hydrogen bonding, van der Waals interaction, and electrostatic interaction, or a combination of these weak non-covalent forces, have been utilized as key interactions in these studies. To date, studies on host–guest chemistry have revealed numerous features of guest molecules, including shapes, size, length, and chirality. If weak signals derived from such molecular interactions could be transformed into visible information, then it will be feasible to directly gather precise and abundant information about these interactions [1–5]. Some known functional dyes, which possess the ability to selectively capture metallic cations with a simultaneous color change, have been practically applied as sensors [6–8]. In this paper, we report the colorimetric recognition of guest molecules using functional phenolphthalein derivatives (hosts 1–3), which consist of a phenolphthalein skeleton and two crown rings (Fig. 1) [9–16].
Mechanism of color development of phenolphthalein
Because these host molecules inherit the properties of the mother phenolphthalein, it is prudent to discuss the mechanism of color development by phenolphthalein in the aqueous alkaline region reported by Tamura et al. (Scheme 1) [17]. They reported the following mechanism: (i) The monoanion of phenolphthalein is colorless. (ii) Two types of dianions (the colored carboxylate form and colorless lactone form) are present [18, 19], but (iii) in the strong alkaline region, a Michael addition of the hydroxide occurs to form the corresponding colorless trianion. Thus, the dianionic carboxylate form, which spreads the π-conjugated system over two phenolic rings, is sole colored species. Moreover, the equilibrium between the two types of dianions (colored carboxylate and colorless lactone) has been observed in our host molecules 1–3. Hence, this equilibrium makes visual recognition unique, but complicated, as described later.
Bidirectional recognition of sodium and potassium ions [14]
Initially the equilibrium between the colored carboxylate form and the colorless lactone form was examined. Because host–guest interactions, in general, are enhanced as the temperature decreases, our systems, which used hosts 1–3 and various guests, showed typical behaviors. However, using host 1 and changing the temperature in the presence of sodium and potassium ions resulted in interesting, but opposite behaviors for the equilibrium of the colored carboxylate form and colorless lactone. Figure 2 shows the variable temperature UV–Vis spectra and photographs of host 1 and Na+ or K+. The absorbance near 565 nm by the complex of 1 and NaOH increased as the temperature decreased (Fig. 2a). In contrast, the coloration between 1 and KOH decreased as the temperature decreased (Fig. 2b). In addition, these phenomena could be detected by the naked-eye (Fig. 2c, d).
(a, b) The variable-temperature UV–Vis spectra of host 1 with Metal-OH in methanol. [host 1] = 1.7 × 10−4 M, [Metal-OH] = 6.7 × 10−4 M. (a) for NaOH, (b) for KOH. Light path length = 10 mm. (c, d) Color development by host 1 with NaOH and KOH at (c) 0 °C and (d) 60 °C. [host 1] = 1.3 × 10−4 M, [Metal-OH] = 5.0 × 10−4 M
Because these phenomena cannot be accounted for by normal unidirectional host–guest interactions, we assumed bidirectional complexation [20]. Thus, complexation between host 1 and NaOH preferentially gives the colored carboxylate complex, while that between host 1 and KOH leads to the colorless lactone complex due to a high affinity of K+ for the pseudo phenol 18-crown-6 rings (Scheme 2).
To verify this assumption, variable temperature IR spectra were performed. Host 1 has lactone carbonyl absorption near 1,755 cm−1, while carboxylate carbonyl absorption appears near 1,655 cm−1. In Fig. 3a, in the presence of NaOH, the absorption (around 1,655 cm−1) corresponding to the carboxylate carbonyl group increased relative to the lactone carbonyl (1,755 cm−1) as the temperature decreased. In contrast, the opposite behavior was observed between host 1 and KOH. These spectral behaviors indicate the equilibrium between the colorless lactone complex and the colored carboxylate complex. Thus, we confirmed that the equilibrium relation of the dianionic colored carboxylate form and dianionic colorless lactone form exists even in host 1.
Colorimetric recognition of α,ω-diamines [9]
Researchers at Osaka University have reported a series of hybrid molecules with phenol crown ring and azobenzene, and they revealed that these phenol crown ethers can trap primary amines [21–28]. Appling this feature to our molecules, we assumed that host 1 could discriminate the length of linear diamines by the formation of a bridged-type complex. Consequently, we could “see” the molecular length using the naked-eye. Therefore, interactions of host 1 with α,ω-diamines (4–10) with various chain lengths (n = 4–10) were examined (Fig. 4).
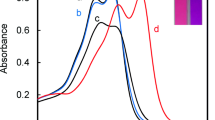
Actually, host 1 could recognize the length of diamines, and transformed this information into a purple color. The most significant color development occurred with 1 and guest diamines 8 and 9. Moreover, the degree of coloration was much smaller with either longer or shorter diamines. It is noteworthy that diamines shorter than 1,5-diaminopentane (5) did not cause a color change, which the naked-eye could detect. Next, the stoichiometry of the colored complex formed by host 1 and guest diamine 9 (n = 9) was determined by Job’s plot (Fig. 5) [29]. Job’s plot exhibited a peak at approximately 0.6, which suggests that the host–guest ratio was 1:2 or 2:3 (Fig. 5, blue dotted-line d). To clarify the stoichiometry of complex formation, Job’s plot was measured in the presence of a large excess of N-ethylpiperidine (NEP). Under these conditions, NEP acted as only a source of the counter cation for the carboxylate, and the peak of Job’s plot shifted to 0.5 (Fig. 5, blue solid line c). This behavior indicates that the component of the colored complex (host 1: guest = 1:2 or 2:3) should be shifted to that with host 1: guest: NEP = 1:1:1 (Fig. 6). Job’s plots of host 1 and diamines 7, 8, and 10 in the presence of a large excess of NEP also gave peaks at 0.5.
The apparent association constants (K app) of the complexes and molar absorption coefficients (ε) were determined by the UV–Visible titration method in the presence of a large excess of NEP (Table 1) [30, 31]. Table 1 indicates that the degree of coloration caused by the interaction between 1 and diamines is due to both the difference in the molar absorption coefficients (ε) and the difference in the association constant (K app). Although the molar absorption coefficient (ε) should be constant for a given complex, the observed ε’s changed. Thus, for this system, the change in ε reflects the equilibrium between the colored complex and the colorless complex. Hence, this is the first example of a colorimetric recognition of α,ω-diamines by artificial host molecule.
Colorimetric recognition of linear triamines [10]
Host 1 can also discriminate the length of linear triamines (11–15) in methanol (Fig. 7). Considering the bridged-type complexation between host 1 and linear amines, host 1 should be better adapted for linear triamines than α,ω-diamines. Host 1 can trap linear triamines through a three point interaction; that is, two interactions occur between the phenol crown moieties of host 1 and the terminal amines of linear triamines, and the third interactions occurs between the carboxylate of 1 and the imine part of linear triamines. Job’s plot indicates that the host–guest ratio was 1:1 without NEP (Fig. 8) and the association constants (K a ) as well as the molar absorption coefficients (ε) were determined by UV–Vis titration and analyzed by the Rose–Drago method (Table 2) [30, 31].
Job’s plots of host 1 and linear triamines (11–15) in MeOH at 25 °C. Conditions: [1] + [11] = 1.7 × 10−3 M, [1] + [12] = 1.3 × 10−3 M, [1] + [13] = 1.4 × 10−3 M, [1] + [14] = 5.1 × 10−4 M, [1] + [15] = 2.6 × 10−3 M. (a) 11 (m = n = 3), (b) 12 (m = 3, n = 4), (c) 13 (m = n = 4), (d) 14 (m = n = 5), (e) 15 (m = n = 6). Light path length = 10 mm
In these cases, variable molar absorption coefficients (ε) were also observed. Compared to triamines 14 and 15, the ε can provide a sharper discrimination than its association constant (K a ). To obtain the conformational information, the 1H-1H NOESY spectrum of host 1 and triamine 13 was measured. Intermolecular cross peaks between the aromatic proton of host 1 and the methylene protons adjacent to that of imino group of triamine 13 were observed. Based on the data, we propose a canonical structure for the colored complex where the terminal amino groups of guest 13 is bridged between two phenolic crown rings of host 1 and the protonated central imino group captures the carboxylate generated from the γ-lactone (Fig. 9).Footnote 1
Colorimetric sequence-selective recognition of non-protected dipeptides [12]
For more complicated and useful colorimetric recognition using host 1, we applied it to sequence-selective recognition of non-protected dipeptide in methanol–water system [32–36]. Because host 1 can develop purple color by recognizing the distance between an N-terminal amino group of 1,8-diaminooctane (8) or 1,9-diaminononane (9), we assumed that host 1 can distinguish AA-(l)Lys among other dipeptides. For instance, dipeptide AA-(l)Lys has eight atoms between the terminal amino groups. On the other hand, (l)Lys-AA has only five carbons between them. Thus, the color development of 1 is expected in the presence of AA-(l)Lys, but not in the presence of (l)Lys-AA, which has the reverse amino acid sequence (Fig. 10).
To screen the proper dipeptide sequence, we speculated that receptor 1 has vague recognition against bulkiness that extends from the inner carboxyl group of the guest dipeptide based on putative host–guest complex (Fig. 11). Therefore, we examined the interaction between receptor 1 and 400 polymer supported dipeptides. Figure 12 depicts the results. Strong color development was observed for only six of the 400 dipeptides (Gly-Lys, Ala-Lys, Ser-Lys, Thr-Lys, Arg-Lys, and Lys-Lys). Those six dipeptides were classified into three groups. The first group has small side chains (Gly-Lys and Ala-Lys), the second group has a hydroxy group on the side chains (Ser-Lys and Thr-Lys), and the third group has a relatively large, but remote functional group on the side chains (Arg-Lys and Lys-Lys). Moreover, Fig. 12 demonstrates several important points. One is Lys at the C-terminus is an essential factor. Because color development was observed for Gly-Lys and Ala-Lys, but Val-Lys did not produce color, color development is sensitive to the steric bulkiness of the substituent on the N-terminal.
Next, the interactions between the host 1 and dipeptides containing Lys (not polymer supported) in solution were studied. Figure 13 shows typical examples. In the methanol–water solution, color development and discrimination clearly depend on the size of the side chain at the N-terminus (Fig. 14). However, color barely developed with Ser-Lys (16) and Thr-Lys (17), although these sequences showed a purple color in the polymer supported screening (Fig. 12). These findings indicate that the hydroxy group on the side chain acts as steric repulsion rather than an attractive interaction through hydrogen bonding. Consequently, only four of the 400 dipeptides (Gly-Lys, Ala-Lys, Arg-Lys, and Lys-Lys) have a large affinity for host 1. The apparent association constants (K app) as well as the molar absorption coefficients (ε) were determined by UV–Vis titration under methanol/water = 10/1 in the presence of a large excess of NEP at 25 °C (Table 3).
Visible recognition of the sequence of dipeptides (18–37) by the host 1. The concentration of 1 was 1.0 × 10−3 M and those of dipeptides were 1.5 × 10−3 M in the presence of NEP (2.5 × 10−2 M) in methanol/water = 10/1. 1, Host 1 only; 18, Gly-Gly; 19, Lys-Gly; 20, Lys-Ala; 21, Lys-Arg; 22, Lys-Met; 23, Lys-Lys; 24, Lys-Trp; 25, Lys-Glu; 26, Lys-Pro; 27, Lys-Val; 28, Lys-Tyr; 29, Gly-Lys; 30, Ala-Lys; 31, Arg-Lys; 32, Met-Lys; 33, Trp-Lys; 34, Glu-Lys; 35, Pro-Lys; 36, Val-Lys; 37, Tyr-Lys
It is worthwhile to note that host 1 can discriminate not only a sequence of the dipeptides, but also the diastereomeric relationship of the dipeptides. Figure 15 shows a typical example. This diastereomeric recognition can be explained by the steric repulsion around the N-terminus of (d)Ala-(l)Lys (38), which is slightly larger than that of (l)Ala-(l)Lys (30).
Colorimetric recognition of α,ω-diamines in water [15]
One distinctive characteristic feature of host 1 is its full potential in methanol. From the standpoint of driving forces for complexation, a combination of hydrogen bonding and electrostatic interactions (such as Coulomb force and dipole–dipole interaction) is used, these interactions are generally disturbed and weakened by surrounding solvent molecules in protic solvent [37]. Figure 16 illustrates the color development of host 1 and spermidine (12) in a variety of mixed solvents, which consisted of different ratios of methanol and chloroform. The coloration drastically decreased as the proportion of chloroform increased. To take advantage of the feature of host 1, the quaternary ammonium part of water-soluble host 2 was introduced into host 1, and the coloration ability of the synthesized compound against various length of α,ω-diamines (5–10) in a completely aqueous medium was examined (Fig. 17).
Structure of water-soluble host 2. (a) UV–Vis spectra of host 2 with α,ω-diamines (5–10) in HEPPSO buffer. Conditions: [host 2] = 1.0 × 10−2 M, [α,ω-diamine 5–10] = 1.0 × 10−2 M. pH = 7.9. HEPPSO = 0.32 M, titrant = NaOH, T = 10 °C, Light path length = 10 mm. (b) Color development by host 2 with α,ω-diamines. [host 2] = 1.0 × 10−2 M, [α,ω-diamine 5–10] = 1.0 × 10−2 M. pH = 7.7
Figure 17a shows the UV–Vis spectra of host 2 and various lengths of diamines (5–10) at pH 7.9. Although, host 2 is slightly affected by the pH of the solution in the absence of a guest diamine, host 2 unambiguously recognized the length of α,ω-diamines with a change in the color that depended on the length of the diamine. Guest diamine 9 with host 2 produced the deepest color. Furthermore, the degree of coloration was much smaller with either a longer or shorter diamine, and these phenomena could be detected by the naked-eye (Fig. 17b). Although the sensitivity and selectivity are unsatisfactory compared to the methanol solution system as mentioned above, the molecular recognition system in a completely aqueous medium can be constructed based on hydrogen bonding and electrostatic interactions.
Development of a highly sensitive host molecule [16]
To increase the sensitivity for α,ω-diamines, we further modified host 1. After much trial and error, we created host 3 with a dimethylamino group at the meta position of the carbonyl group, which had a stronger affinity to α,ω-diamines compared to host 1. The colorations of host 3 (5 × 10−5 M) and α,ω-diamines (2.5 × 10−4 M) were almost at the same level as host 1 (2.5 × 10−4 M) and α,ω-diamines (2.5 × 10−3 M), as can be seen by comparing Figs. 18 and 4. Hence, we are currently trying to develop more sensitive host molecules based on host 3, which may be applicable for practical use, especially for rapid quantification of biogenic polyamines.
Structure of host 3. (a) UV–Vis spectra of host 3 with α,ω-diamines in methanol. Conditions: [host 3] = 5.0 × 10−5 M, [α,ω-diamine] = 2.5 × 10−4 M, T = 20 °C, Light path length = 10 mm. (b) Color development by host 3 with α,ω-diamines. [host 3] = 5.0 × 10−5 M, [α,ω-diamine] = 2.5 × 10−4 M. T = 20 °C. 39; NH2(CH2)12NH2
Conclusion
In this paper, we describe three phenolphthalein-based hosts with two crown ethers developed in our laboratory. Despite their simple structures, these hosts show various functions such as visualization of linear α,ω-diamines and triamines, and colorimetric recognition of sequences of non-protected dipeptides, etc. It should be noted that these molecular recognitions, which are derived from hydrogen bonding and electrostatic interactions, were accomplished in a protic solvent (methanol or methanol–water). Further studies to improve the sensitivity and selectivity for biogenic polyamines are underway.
Notes
In Fig. 9, the proposed colored complex between host 1 and guest 13 is shown as canonical structure. Therefore, there may be some irrational interaction between the quinoid form of the phenolic crown ether of the host and the neutral amino group of the guest. We can interpret the actual complex as follows: (i) the guest amine reacts with free host 1 to generate two kinds of dianions, (ii) with regard to “colored” complex, one anion preferentially locates on the carboxylate, and another anion spreads over the two phenolic rings, and (iii) one proton is exchanged between the corresponding two amines through surrounding methanol, and thus both amines acquire a cationic character (No coloration was observed in a protic polar solvents, such as CHCl3, DMSO, and DMF, see Fig. 16).
References
de Silva, A.P., Gunaratne, H.Q.N., Gunnlaugsson, T,. Huxley, A.J.M., McCoy, C.P., Rademacher, J.T., Rice, T.E.: Signaling recognition events with fluorescent sensors and switches. Chem. Rev. 97, 1515–1566 (1997)
McQuade, D.T., Pullen, A.E., Swager, T.M.: Conjugated polymer-based chemical sensors. Chem. Rev. 100, 2537–2574 (2000)
Wiskur, S.L., Ait-Haddou, H., Lavigne, J.L., Anslyn, E.V.: Teaching old indicators new tricks. Acc. Chem. Res. 34, 963–972 (2001)
Kuwabara, T.: Color change indicator for molecules. J. Jpn. Soc. Colour Mater. 78, 265–271 (2005)
Nguyen, B.T., Anslyn, E.V.: Indicator-displacement assays. Coord. Chem. Rev. 250, 3118–3127 (2006)
Bubnis, B.P., Pacey, G.E.: Alkali metal ion complexation with lariat ethers possessing a chromogenic group. Talanta 31, 1149–1152 (1984)
Nakamura, H., Takagi, M., Ueno, K.: Complexation and extraction of alkali metal ions by 4′-picrylaminobenzo-18-crown-6 derivatives. Anal. Chem. 52, 1668–1671 (1980)
Bubnis, B.P., Steger, J.L., Wu, Y.P., Meyers, L.A., Pacey, G.E.: Substituent effects on complexation and extraction of alkali metals with chromogenic crown ethers. Anal. Chim. Acta. 139, 307–313 (1982)
Fuji, K., Tsubaki, K., Tanaka, K., Hayashi, N., Otsubo, T., Kinoshita, T.: Visualization of molecular length of α,ω-diamines and temperature by a receptor based on phenolphthalein and crown ether. J. Am. Chem. Soc. 121, 3807–3808 (1999)
Tsubaki, K., Hayashi, N., Nuruzzaman, M., Kusumoto, T., Fuji, K.: Visual recognition of triamines by phenolphthalein derivatives: consideration of the structure of the colored complex. Org. Lett. 3, 4067–4069 (2001)
Tsubaki, K., Nuruzzaman, M., Kusumoto, T., Hayashi, N., Wang, B.-G., Fuji, K.: Visual enantiomeric recognition using chiral phenolphthalein derivatives. Org. Lett. 3, 4071–4073 (2001)
Tsubaki, K., Kusumoto, T., Hayashi, N., Nuruzzaman, M., Fuji, K.: Sequence-selective visual recognition of nonprotected dipeptides. Org. Lett. 4, 2313–2316 (2002)
Tsubaki, K., Tanima, D., Nuruzzaman, M., Kusumoto, T., Fuji, K., Kawabata, T.: Visual enantiomeric recognition of amino acid derivatives in protic solvents. J. Org. Chem. 70, 4609–4616 (2005)
Tsubaki, K., Tanima, D., Kuroda, Y., Fuji, K., Kawabata, T.: Bidirectional and colorimetric recognition of sodium and potassium ions. Org. Lett. 8, 5797–5800 (2006)
Tsubaki, K., Tanima, D., Sasamori, T., Tokitoh, N., Kawabata, T.: Colorimetric recognition of the length of α,ω-diamines in water. Tetrahedron Lett. 48, 2135–2138 (2007)
Tsubaki, K., Tanima, D., Imamura, Y., Kawabata, T. (submitted)
Tamura, Z., Abe, S., Ito, K., Maeda, M.: Spectrophotometric analysis of the relationship between dissociation and coloration, and of the structural formulas of phenolphthalein in aqueous solution. Anal. Sci. 12, 927–930 (1996)
Taguchi, K.: Transient binding of phenolphthalein-β-cyclodextrin complex: an example of induced geometrical distortion. J. Am. Chem. Soc. 108, 2705–2709 (1986)
Kuwabara, T., Takamura, M., Mastushita, A., Ikeda, H., Nakamura, A., Ueno, A., Toda, F.: Phenolphthalein-modified β-cyclodextrin as a molecule-responsive colorless-to-color change indicator. J. Org. Chem. 63, 8729–8735 (1998)
For a recent review for temperature effect on the selectivity: Buschmann, H., Scharf, H.-D., Hoffmann, N., Esser, P.: The isoinversion principle. A general selection model in chemistry. Angew. Chem. Int. Ed. Engl. 30, 477–515 (1991)
Naemura, K., Tobe, Y., Kaneda, T.: Preparation of chiral and meso-crown ethers incorporating cyclohexane-1,2-diol derivatives as a steric barrier and their complexation with chiral and achiral amines. Coord. Chem. Rev. 148, 199–219 (1996)
Kaneda, T., Sugihara, K., Kamiya, H., Misumi, S.: Synthetic macrocyclic ligands. IV. Lithium ion-characteristic coloration of a “crowned” dinitrophenylazophenol. Tetrahedron Lett. 22, 4407–4408 (1981)
Kaneda, T., Umeda, S., Ishizaki, Y., Kuo, H.S., Misumi, S., Kai, Y., Kanehisa, N., Kasai, N.: Azophenolic acerands: amine-selective coloration and crystal structure of a piperidinium saltex. J. Am. Chem. Soc. 111, 1881–1883 (1989)
Kaneda, T., Hirose, K., Misumi, S.: Chiral azophenolic acerands: color indicators to judge the absolute configuration of chiral amines. J. Am. Chem. Soc. 111, 742–743 (1989)
Naemura, K., Ueno, K., Takeuchi, S., Tobe, Y., Kaneda, T., Sakata, Y.: Azophenolic acerands having chiral 1-phenyl-cis-1,2-cyclohexanediol units: a correlation between enantiorecognitive coloration and host–guest complementarity. J. Am. Chem. Soc. 115, 8475–8476 (1993)
Hirose, K., Fuji, J., Kamada, K., Tobe, Y., Naemura, K.: Temperature dependent inversion of enantiomer selectivity in the complexation of optically active azophenolic crown ethers containing alkyl substituents as chiral barriers with chiral amines. J. Chem. Soc. Perkin Trans. 2, 1649–1657 (1997)
Ogasahara, K., Hirose, K., Tobe, Y., Naemura, K.: Preparation of optically active azophenolic crown ethers containing 1-phenylethane-1,2-diol and 2,4-dimethyl-3-oxapentane-1,5-diol as a chiral subunit: temperature-dependent enantiomer selectivity in the complexation with chiral amines. J. Chem. Soc. Perkin Trans. 1, 3227–3236 (1997)
Hirose, K., Ogasahara, K., Nishioka, K., Tobe, Y., Naemura, K.: Enantioselective complexation of phenolic crown ethers with chiral aminoethanol derivatives: effects of substituents of aromatic rings of hosts and guests on complexation. J. Chem. Soc. Perkin Trans. 2, 1984–1993 (2000)
Job, P.: Formation and stability of inorganic complexes in solution. Ann. Chim. 9, 113–203 (1928)
Rose, N.J., Drago, R.S.: Molecular addition compounds of iodine. I. An absolute method for the spectroscopic determination of equilibrium constants. J. Am. Chem. Soc. 81, 6138–6145 (1959)
Hirose, K.J.: Inclusion phenom.: a practical guide for the determination of binding constants. Macrocyclic Chem. 39, 193–209 (2001)
Still, W.C.: Discovery of sequence-selective peptide binding by synthetic receptors using encoded combinatorial libraries. Acc. Chem. Res. 29, 155–163 (1996)
Hossain, M.A., Schneider, H.-J.: Sequence-selective evaluation of peptide side-chain interaction. New artificial receptors for selective recognition in water. J. Am. Chem. Soc. 120, 11208–11209 (1998)
Tashiro, S., Tominaga, M., Kawano, M., Therrien, B., Ozeki, T., Fujita, M.: Sequence-selective recognition of peptides within the single binding pocket of a self-assembled coordination cage. J. Am. Chem. Soc. 127, 4546–4547 (2005)
Wehner, M., Janssen, D., Schaefer, G., Schrader, T.: Sequence-selective peptide recognition with designed modules. Eur. J. Org. Chem. 138–153 (2005)
Bush, M.E., Bouley, N.D., Urbach, A.R.: Charge-mediated recognition of N-terminal tryptophan in aqueous solution by a synthetic host. J. Am. Chem. Soc. 127, 14511–14517 (2005)
Israelachvili, J.N.: Intermolecular and Surface Forces. Academic Press, London (1992)
Acknowledgments
The author thanks all collaborators mentioned in the references. He especially thanks Prof. Kaoru Fuji and Prof. Takeo Kawabata for their suggestions and discussions, Dr. Daisuke Tanima, Dr. Tomokazu Kusumoto, Dr. Mohammad Nuruzzaman, Mr. Noriyuki Hayashi, and Ms. Yoko Imamura for their great contributions to this work. This study was partly supported by Grants-in-Aid for Scientific Research (Nos. 14572003 and 18390003) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and Foundation for Pharmaceutical Sciences, Japan. He also thanks Prof. Norihiro Tokitoh and Prof. Takahiro Sasamori (Kyoto Univ.) for performing the X-ray structural analysis, and Prof. Yasuhisa Kuroda (Kyoto Institute of Technology), Prof. Keiji Hirose and Prof. Yoshito Tobe (Osaka Univ.) for gifts of software for determination of binding constants. This is a paper selected for “HGCS Japan Award of Excellence 2007”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsubaki, K. Colorimetric recognition using functional phenolphthalein derivatives. J Incl Phenom Macrocycl Chem 61, 217–225 (2008). https://doi.org/10.1007/s10847-008-9419-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-008-9419-3