Abstract
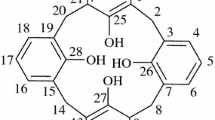
Four new azocalix[4]arenes {5,11,17,23-tetrakis[(2-hydroxy-5-tert-butylphenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (1), 5,11,17,23-tetrakis[(2-hydroxy-5-nitro phenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (2), 5,11,17,23-tetrakis[(2-amino-5-carboxylphenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (3) and 5,11,17,23-tetrakis[(1-amino-2-hydroxy-4-sulfonicacidnapthylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (4)} have been synthesized from p-tert-butylphenol, p-nitrophenol, p-aminobenzoic acid and 1-amino-2-hydroxy-4-sulphonic acid by diazo coupling reaction with p-aminocalix[4]arene. The resulting ligands (1–4) were treated with three transition metal salts (e.g., CuCl2·2H2O, NiCl2·6H2O or CoCl2·6H2O). Cu(II), Ni(II) and Co(II) complexes of the azocalix[4]arene derivatives were obtained and characterized by UV-vis, IR, 1H-NMR spectroscopic techniques and elemental analysis. All the complexes have a metal:ligand ratio of 2:1. The Cu(II) and Ni(II) complexes of azocalix[4]arenes are square-planar, while the Co(II) complexes of azocalix[4]arenes are octahedral with water molecules as axial ligands. The solvent extraction of various transition metal cations from the aqueous phase to the organic phase was carried out by using azocalix[4]arenes (1–4). It was found that, azocalix[4]arenes 1, 2 and 3 examined selectivity for transition metal cations such as Ag+, Hg+ and Hg2+. In addition, the thermal stability of metal:azocalix[4]arene complexes were also reported.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Calixarenes are a class of macrocyclic compounds of fundamental interest and they are growing technical importance in various areas. Functionalised (either at the “lower rim” oxygens or at the “upper rim” carbons) and parent calix[4]arenes have received intense attention over the past 20 years [1, 2]. Most of this interest has been focussed on the calix[4]arene series (which contain four phenolic residues) and these are the subject of our present contribution. From an organometallic and organometallic-related standpoint, dianionic di-O-alkyl functionalised calix[4]arenes have been served as novel and versatile O-donor ligand platform (tri- and tetra-anionic homologues have also received attention) and much new transition metals [2, 3] and post-transition metal [4] chemistry have emerged. Among these substituents, for instance, additional donor groups such as amide moieties, show greater efficiency for metal ion complexation than oxo, ester and either groups [5, 6], and have been grafted onto the lower rims of calixarenes for complexation of alkali [7, 8] and alkaline earth metal ions [9, 10] and transition metal ions [11, 12]. In contrast the incorporation of carbocyclic agents such as 4-n-butylaniline, 4-(phenylazo)aniline, 4-aminoacetanilide, N′-2-thiazol-2-ylsulfonyl amide and 2-aminothiazol to the upper rim of calix[4]arene [13] have a special interest in the complexation of transition metal cations.
Although quite large number of reports exist on the azocalix[4]arenes synthesis, those on the azocalix[n]arenes applications are still limited.
Until now, all synthesized studies have described the preparation of the new azo calixarenes that were synthesized using calix[n]arenes and phenols with various amines [14, 15]. Our previous studies have confirmed that in presence of Cu2+, Ni2+, Co2+ and Fe3+ ions can be extracted selectively using calix[n]arenes. We have also examined both the selective extraction of Fe3+ ion from the aqueous phase into the organic phase [16] and the liquid-liquid extractions of transitions metal cations [17] using diazo-coupled calix[n]arenes.
Recent works by the author have synthesized the series of twelve aromatic azocalix[4]arenes and azocalix[6]arenes or the series of seven heterocyclic azocalix[4]arenes and azocalix[6]arenes. These substances have been investigated for their absorption, chromogenic and ionophoric properties [18–21]. Herein, we report the synthesis, complexation, extraction and thermal behaviour of four new azocalix[4]arene with metal:ligand ratio, solvent extraction properties and thermal stability of complexes.
Results and discussion
Synthesis and characterization
Azocalix[4]arenes have been widely used as three-dimensional building blocks for the construction of artificial molecular receptors capable of recognizing neutral molecules, cations, anions and thermal behaviours. Thus, having chosen the p-tert-butylcalix[4]arene as the basis for derivatives, a synthetic scheme had to be developed to enable the derivatization of the molecule. Such a synthetic route is shown in Scheme 1. The chromogenic azocalix[4]arenes reported here were designed to take advantage of the well-established binding interactions of chromogenic molecules and transition metal cations. The synthesis of azocalix[4]arenes 1–4 were based on the previously published procedures [13] while reaction steps leading from 1 to 4 are reported for the first time.
The synthetic utility of the azocalix[4]arene is well known and can be bridged across the upper rim. Briefly, calix[4]arene is prepared by debutylation of p-tert-butylcalix[4]arene. The coupling reaction of calix[4]arene with 4-carboxyphenyldiazonium chloride in aqueous THF give p-(4-carboxyphenylazo) calix[4]arene in 85% yield. It was confirmed by the appearance of an azo band at 1470 cm−1 in the IR spectra of this compound.
The cleavage of an azocalix[4]arene in presence of zinc dust and ammonium formate or formic acid was completed within 30 min. The disappearance of a strong absorption 1400–1500 cm−1 due to –N = N– group and appearance of a strong absorption band between 3500–3000 cm−1 due to the –NH2 stretching that clearly showed the p-aminocalix[4]arene.
In this study, we have synthesized four new diazo coupling calix[4]arene containing two groups in its structure for the recognition of diazo (–N = N–) and neighbouring groups (–OH for 1, 2 and 4, –NH2 for 3). These groups enables, azocalix[4]arenes 1, 2, 3 and 4 to be converted to its diazo derivative with phenol derivatives (p-tert-butylphenol, p-nitrophenol, p-aminobenzoic acid and 1-amino-2-hydroxy-4-sulphonic acid) into acetic acid in the presence of NaNO2/H2SO4 easily. After 12 h stirring, azocalix[4]arenes 1–4 were isolated in 67–90% yield, and these four new azocalix[4]arenes were characterized by a combination of UV-vis, IR, 1H NMR and elemental analysis.
The results of the elemental analysis for nitrogen agree well with the calculated values indicating formation of the coupling product. The similarity of the reaction yields with literature values supports this [13].
The IR spectra, the stretching vibrations of the azocalix[4]arenes 1 and 2 appear at 3143–3160 cm−1 (–OH), 1652–1650 cm−1 (arom. –C = C–) and 1483–1481 cm−1 (–N = N–), azocalix[4]arene 3 have characteristic IR absorption bands of 3359 cm−1 (–NH2), 1682 cm−1 (–C = O) and azocalix[4]arene 4 have characteristic strong IR absorption bands of 3340 cm−1 (–NH2), 3160 cm−1 (–OH) and 1034 cm−1 (–SO3H), respectively.
1H NMR spectroscopy is a versatile tool for the identification of calixarene conformations. The 1H NMR data showed that all azocalix[4]arenes 1–4 exist in a cone conformation due to the appearance of ArCH2Ar as a typical AB protons signal at 3.2–4.8 ppm.
The lower field signals of the hydroxyl group of the two azocalix[4]arenes (1 and 2) resonate at ca. 9.2–10.8 ppm and 8.2–10.2 ppm, respectively and these are typical for intramolecular hydrogen bonding protons as reported in the literature [14]. It was observed that the tert-butyl protons of azocalix[4]arene 1 resonate at 1.3 ppm. Although the compounds have two aromatic rings in different environments, they give a multiple peak due to overlapping. Also, the phenyl protons were also observed as a multiple at 6.5–7.9 ppm. The peaks of aromatic protons of the azocalix[4]arenes are complicated. The azocalix[4]arene 3 shows peaks located in the range of 5.2 ppm, which are attributed to the –NH2 groups. The lower field signals of the carboxyl groups of this compound resonate at ca. 11.0 ppm. On the other hand, the azocalix[4]arene 4 was obtained as a singlet at 5.1 ppm having to the –NH2 group such as the azocalix[4]arene 3. It was observed that the –SO3H protons of azocalix[4]arene 4 resonate at 13.8 ppm.
Metal complexation
The ultraviolet spectral behaviours of the azocalix[4]arenes 1–4 were investigated in chloroform. Comparing data of the UV spectra, it was found that all of the spectra show a strong absorption maximum in the 285–298 nm range with high extinction coefficients. As can be seen from Table 1, the azocalix[4]arene 1–4 give two absorption bands (π − π* and n − π* transitions) (Fig. 1).
The electronic spectra of the complexes exhibit intense charge-transfer bands around 314–394 nm, but weak d–d transitions are observed only for the Cu(II), Ni(II) and Co(II) complexes with 1 at 538, 527, 529 nm, with 2 at 550, 417, 407 nm, with 3 at 438, 606, 608 nm and with 4 at 450, 517, 603 nm, respectively (Table 1).
The azocalix[4]arenes which are synthesized by diazo-coupling reaction have been defined as molecular design of chromogenic phenolic compounds in the literature [16].
The metal-ligand ratio in all these complexes is 2:1. The presence of coordinated or lattice water in the Cu(II) and Ni(II) complexes are indicated by the presence of broad bands in the 3420–3430 cm−1 region of the IR spectra of these complexes and described to O-H of water. The band in the IR spectra of the Cu(II) and Ni(II) complexes disappears after heating at 100 °C for 6 h. This shows that water molecules are held in the crystal lattice of these complexes.
Consequently, both a square-planar structure for the Cu(II) and Ni(II) complexes of azocalix[4]arenes and an octahedral structure with water molecules as axial ligands for the Co(II) complexes of azocalix[4]arenes are proposed as shown in Scheme 2.
The resulting ligands (1–4) synthesized by the chelation between different metal ions (Cu2+, Ni2+ and Co2+). These compounds were confirmed by elemental analysis, FT-IR spectra. In the FT-IR spectra of all the complexes, there were new bands observed in the region 650–420 cm−1, which were absent in the spectrum of the free ligand. The bands observed at 649–603 cm−1 (M–O) and 487–425 cm−1 (M–N) provided conclusive evidence concerning the bonding of nitrogen and oxygen to the metal ions. Azo groups bands of azocalix[4]arenes (1–4) exhibits at 1483–1473 cm−1 and on complexations these bands disappears. The appearance of these bands gives the evidence that azo groups were involved in chelation with Cu2+, Ni2+ and Co2+. The free 4 ligand exhibits bands at 3340, 3160, 2970, 1620, 1473, 1186, 1034 cm−1 due to different modes of vibrations of –NH2 group. On complexations, these bands were absent. It is in agreement with previous literature results [22].
Extraction studies
Extraction behaviour for transition metal ions were also studied for azocalix[4]arenes. Extraction efficiencies of the azocalix[4]arenes 1–4 have been carried out by the two phase solvent extraction of transition metal picrates (Ag+, Hg+, Hg+2, Co2+, Ni2+, Cu2+, Cd2+, Zn2+, Al3+, Cr3+ and La3+) into chloroform under neutral conditions. The results are given in Table 2. These data have been obtained by using chloroform solution of these azocalix[4]arene compounds 1–4 to extract metal picrates from an aqueous phase. The equilibrium concentration of picrate in aqueous phase was then determined spectrophotometrically (Fig. 2).
From the data in Table 2 it can be seen that, azocalix[4]arenes 1, 2 and 3 are very effective selectivity for transition metal cations such as Ag+, Hg+ and Hg2+. Those azocalix[4]arenes show higher selectivity toward Hg+ and Hg2+ than the azocalix[4]arene 4. The above phenomena can be explained by the (hard–soft) acid—base principle as follows: the azocalix[4]arenes 1 and 2 contain electron-donating and electron-withdrawing groups, respectively. Azocalix[4]arene 1 containing electron-donating groups (tert-butyl) is a harder base and prefers the Hg2+ cation (91.0%). Azocalix[4]arene 2 containing electron-withdrawing groups (–NO2) is a softer base and prefers the Hg+ cation (68.5%).
Interestingly, it was observed that azocalix[4]arene 3 showed remarkable change in the extraction of transition metals, especially in the case of Co2+ (23.4%), Zn2+ (38.7%) and Cr3+ (21.8%) due to the presence of neighbouring –NH2 group sites in the –N = N– groups. The effectiveness of azocalix[4]arene 3 in transferring transition metals rather than others indicate that, in this case, bridging amine (–NH2) groups appeared to be operative and play an important role at water-chloroform interphase, since the metal ions could possibly be interacted with these soft ligating sites. This is in agreement with our previous results [17]. The other reason for this high binding ability displayed by azocalix[4]arene 3 may be due to the preorganization and fine tuning of the cation binding sites in the lower rim of calixarene moiety, which is immobilized in cone conformation possibly provide such an environment for the complexation of metal cations.
In the light of our previous experience [17], the metal binding properties of azocalix[4]arenes were investigated and showed high selectivity for Ag+, Hg+ and Hg2+ ions, promoting us to elaborate on its structure so that it could be incorporated into different types of azocalix[n]arenes. Thus calix[4]arenes was diazotisated on the NaNO2/H2SO4 to yield the azo derivatives (1–4). The aim was to synthesize neighbour groups (–OH for 1, 2 and 4, –NH2 for 3) to diazo (–N = N–) group, which may be help in most of the solvent extraction.
The azocalixarene compounds are effective extractants of towards transition metal cations. In our previous study [23], we observed that compounds with phenolic –OH groups were also effective in the extraction of Fe3+ at low pH values. However, this conclusion is not new and has been previously reported in the literature [16]. Those azocalix[4]arenes, which are very effective in extracting the transition metal cations, particularly Ag+, Hg+ and Hg2+, do not extract the alkaline metal cations to any significant extent, as reported by Nomura et al. [24], who used p-phenylazocalix[6]arene as the ligand.
Thermal behaviour
Thermal analysis plays an important role in the study of the structure and stability of calix[n]arenes. The applicability of some azocalix[4]arenes for special uses and determining the thermal stabilities of them are also very important. All thermal analysis measurements in this work were performed under exactly the same experimental conditions. Four azocalix[4]arene different compounds (1–4) involving diazo groups in upper rim were investigated.
The TG and DTA curves of azocalix[4]arenes (1–4) in flowing dry nitrogen atmosphere are illustrated in Fig. 3. The amount of volatile pyrolysis products for azocalix[4]arenes and thermoanalytical results obtained from TG, DTA curves are also given in Table 3.
The thermal stability of azocalix[4]arene 2 was determined by thermal gravimetric analysis (TGA) in a nitrogen atmosphere. The temperature at which 13% weight loss occurred was 167–269 °C. Figure 3 gives the TGA curves of the metal(II)—azocalix[4]arene complexes. From the data in Table 3 and Fig. 3 it can be seen that the TGA curves of the copper(II) and nickel (II) azocalix[4]arene complexes exhibit a sharp decomposition temperature at about 101 and 84 °C, respectively, while the cobalt(II)—azocalix[4]arene complex exhibits a progressive decomposition temperature above 80 °C. These data show that the cobalt(II)—azocalix[4]arene complex is thermally more stable than copper(II) and nickel(II)—azocalix[4]arene complexes and possible to fabricate a small and sharp recording mark edge due to its high and sharp thermal decomposition threshold. It is in agreement with our previous results [25, 26].
Conclusions
In summary, the synthesis and complexation ability of four new azocalix[4]arene based on neighbour receptors (1–4) were studied. The spectroscopic results of all the compounds (1–4) revealed that these compounds do exist in cone conformation. The complexation studies show that azocalix[4]arene 1, 2 and 3 are excellent receptors for Ag+, Hg+ and Hg2+ metal cations as compared to azocalix[4]arene. Moreover, the extraction property of azocalix[4]arene 4 is not enhanced in the acidic medium due to its protonation.
It has been suggested that, by the introduction of appropriate functions and/or bridges or by choosing a particular conformation, the calixarene based receptors could be proved to find remarkable applications in the design of chemical sensors, using an electrochemical transductions, as conventional ion-selective electrodes (ISE) and solid-state sensors (ISFETs).
Based on the above results, we conclude that ligand groups circularly arranged on the upper rim of the calix[n]arene cavity construct, which is a novel cyclic metal receptor for selective extraction of transition metal cations.
The resistance to heat at elevated temperatures is one of the main properties required for azocalix[4]arene used in high temperature processes such as the dyeing of textile fibres, ink-jet printing and photocopying and in high technology areas lasers and electro-optical devices.
Experimental
All reagents used were purchased from Merck or Carlo-Erba and were chemically pure. The drying agent employed was anhydrous magnesium sulphate. Melting points were measured using an Electrothermal IA 9100 digital melting point apparatus in capillaries sealed under nitrogen and are uncorrected. 1H-NMR spectra were referenced to tetramethylsilane (TMS) at 0.00 ppm as internal standard and were recorded on a Bruker 400-MHz spectrometer at room temperature (25 ± 1 °C). IR spectra were recorded on a Mattson 1000 FT-IR spectrometer as KBr pellets. UV-vis spectra were obtained on a Shimadzu UV-1601 UV-visible recording spectrophotometer. The elemental analysis was performed in the TUBITAK Laboratory (Center of Science and Technology Research Turkey).
Thermal stability was measured over 50–800 °C using a Shimadzu thermogravimetric analyser (Model: DTA-60H). During the measurement, dry nitrogen gas punged at a flow rate of 100 cc/min and a ramping rate of 10 °C/min was employed.
Solvent crystallization was retained in some at the analytical samples and attached the elemental analysis in such cases, best fits between the analytical values and appropriate fractional increments of solvents were used. All aqueous solutions were prepared with deionised water that had been passed a Human Power I Plus I + UV water purification system.
Preparation of the ligands
p-tert-Butylcalix[4]arene, calix[4]arene, 5,11,17,23-tetrakis[(p-carboxyphenyl)azo]-25,26,27, 28-tetrahydroxycalix[4]arene and 5,11,17,23-tetraamino-25,26,27,28-tetrahydroxycalix[4]arene were synthesized as described by a previously reported method [27–30].
Preparation of p-phenylazocalix[4]arenes {5,11,17,23-tetrakis[(2-hydroxy-5-tert-butyl phenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (1), 5,11,17,23-tetrakis[(2-hydroxy-5-nitrophenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (2), 5,11,17,23-tetrakis[(2-amino-5-carboxylphenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (3) and 5,11,17,23-tetrakis[(1-amino-2-hydroxy-4-sulfonicacidnapthylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (4)]} were obtained 67–90% yield. The obtained compounds were purified by crystallization using the some solvent (DMF/H2O) and were than analysed.
Synthesis of 5,11,17,23-tetrakis[(2-hydroxy-5-tert-butylphenylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (1)
General procedure: [31] A solution of diazonium salt of 5,11,17,23-tetraamino-25,26,27,28-tetrahydroxycalix[4]arene was prepared by adding an aqueous solution of sodium nitrite (0.12 g, 0.17 mmol) dropwise into homogeneous mixture of 0.10 mL sulphuric acid and 1 mL glacial acetic acid. The mixture was stirred at 0 °C for 5 min. This diazonium salt solution was added dropwise into a solution of p-tert-butylphenol (0.25 g) in 3 mL of acetone at 0 °C. The solution was stirred for an additional 12 h at 0 °C. CHCl3 (25 mL) and water (25 mL) added the organic layer was separated and dried over MgSO4. Removal of the organic solvent in vacuo afforded a brownish solid, which was filtered and washed with water and MeOH, and dried. The resulting solid was recrystallized from DMF/H2O mixture that gave a dark brown product. Yield, 0.14 (67%), mp. 223 °C. [Found: C 72.11; H 6.68; N 9.74]; C68H72N8O8 requires C 72.32; H 6.43; N 9.92. IR (KBr) υ: 3143 cm−1 (–OH), 2960 cm−1 (−CH alif.), 1652 cm−1 (–C = C), 1483 cm−1 (–N = N), 1191 cm−1 (C–O). 1H-NMR (CDCl3, 25 °C) δH: 1.3 (36H, s, –C(CH3)), 3.2–4.1 (8H, s, J = 13.2 Hz, Ar–CH2–Ar), 6.7–7.5 (20H, m, Ar–H), 9.2 (4H, s, OH), 10.8 (4H, s, OH).
This azocalix[4]arene 1 was soluble in EtOH, acetone, acetic acid, benzene, CHCl3, DMSO, 10% NaOH and slightly soluble in diethyl ether, 10% HCl and insoluble in water.
Synthesis of 5,11,17,23-tetrakis[(2-hydroxy-5-nitrophenylazo)]-25,26,27,28-tetra hydroxycalix[4]arene (2)
Azocalix[4]arene 2 was prepared as described above, using p-nitrophenol in acetone and obtained as a dark brown solid, which was filtered and washed with water and MeOH, and dried. The resulting solid was recrystallized from DMF/H2O mixture that gave a brown product. Yield, 0.42 g (89%), mp. 277 °C; [Found: C 57.36; H 3.67; N 15.12]; C52H36N12O16 requires C 57.57; H 3.34; N 15.49. IR (KBr) υ: 3160 cm−1 (–OH), 2950 cm−1 (–CH alif.), 1650 cm−1 (–C = C), 1481 cm−1 (–N = N), 1208 cm−1 (C–O). 1H-NMR (CDCl3, 25 °C) δH: 3.4–4.4 (8H, s, J = 13.2 Hz, Ar–CH2–Ar), 6.5–7.2 (20H, m, Ar–H), 8.2 (4H, s, OH), 10.2 (4H, s, OH).
Azocalix[4]arene 2 was soluble in EtOH, acetone, acetic acid, benzene, CHCl3, DMSO, 10% NaOH and slightly soluble in diethyl ether, 10% HCl and insoluble in water.
Synthesis of 5,11,17,23-tetrakis[(2-amino-5-carboxylphenylazo)]-25,26,27,28-tetra hydroxycalix[4]arene (3)
Azocalix[4]arene 3 was prepared as described above, using p-aminobenzoic acid in acetone and obtained as a dark orange solid, which was filtered and washed with water and MeOH, and dried. The resulting solid was recrystallized from DMF/H2O mixture that gave a dark orange product. Yield, 0.41 g (90%), mp. 280 °C; [Found: C 62.19; H 4.23; N 15.73]; C56H44N12O12 requires C 62.45; H 4.12; N 15.61. IR (KBr) υ: 3359 cm−1 (–NH2), 3171 cm−1 (–OH), 2970 cm−1 (–CH alif.), 1682 cm−1 (–C = O), 1604 cm−1 (–C = C), 1473 cm−1 (–N = N), 1200 cm−1 (C–O). 1H-NMR (CDCl3, 25 °C) δH: 3.8–4.6 (8H, s, J = 13.2 Hz, Ar–CH2–Ar), 5.2 (8H, s, NH2), 6.8–7.1 (20H, m, Ar–H), 8.5 (4H, s, OH), 11.0 (4H, s, COOH).
Azocalix[4]arene 3 was soluble in EtOH, acetic acid, benzene, DMSO, 10% HCl and slightly soluble in diethyl ether, acetone, CHCl3,10% NaOH and insoluble in water.
Synthesis of 5,11,17,23-tetrakis[(1-amino-2-hydroxy-4-sulphonicacidnapthylazo)]-25,26,27,28-tetrahydroxycalix[4]arene (4)
Azocalix[4]arene 4 was prepared as described above, using 1-amino-2-napthol-4-sulphonic acid in 10% NaHCO3 and obtained as a dark red solid, which was filtered and washed with water and MeOH, and dried. The resulting solid was recrystallized from DMF/H2O mixture that gave a dark red product. Yield, 0.41 g (70%), mp. 327 °C; [Found: C 54.71; H 3.75; N 11.08; S 8.77]; C68H52N12O20S4 requires C 54.98; H 3.53; N 11.31; S 8.63. IR (KBr) υ: 3340 cm−1 (–NH2), 3160 cm−1 (–OH), 2970 cm−1 (–CH alif.), 1620 cm−1 (–C = C), 1473 cm−1 (–N = N), 1186 cm−1 (C–O), 1034 cm−1 (–SO3H). 1H-NMR (CDCl3, 25 °C) δH: 3.6–4.8 (8H, s, J = 13.2 Hz, Ar–CH2–Ar), 5.1 (2H, s, NH2), 6.8–7.9(24H, m, Ar–H), 8.9 (4H, s, OH), 13.8 (4H, s, OH).
Azocalix[4]arene 4 was soluble in EtOH, acetic acid, benzene, CHCl3, DMSO, and slightly soluble in diethyl ether, acetone,10% HCl, 10% NaOH and insoluble in water.
Preparation of the transition metal complexes at 1–4
A solution of 0.25 mmol metal salt (43.0 mg of CuCl2·2H2O, 59.5 mg of NiCl2·6H2O and 59.5 mg of CoCl2·6H2O) in a sufficient amount of ethanol (ca. 5 mL) was added to a solution 1–4 (0.20 g) in 20 mL of ethanol-THF (4:1) by stirring and the mixture was boiled on a water both for 30 min and subsequently was allowed to stirring at room temperature for 1 h. The solvents were then removed in vacuo and the crude product was washed with ethanol, then diethylether. Yields, colours and spectral data of the complexes are given in below.
-
1.Cu: 0.15 g (65%) as a light green solid; IR (KBr) υ: 639 cm−1 (M–O) and 425 cm−1 (M–N)
-
1.Ni: 0.17 g (75%) as a green solid; IR (KBr) υ: 605 cm−1 (M–O) and 443 cm−1 (M–N)
-
1.Co: 0.15 g (63%) as a green solid; IR (KBr) υ: 628 cm−1 (M–O) and 467 cm−1 (M–N)
-
2.Cu: 0.13 g (58%) as a green solid; IR (KBr) υ: 603 cm−1 (M–O) and 443 cm−1 (M–N)
-
2.Ni: 0.14 g (75%) as a violet solid; IR (KBr) υ: 610 cm−1 (M–O) and 439 cm−1 (M–N)
-
2.Co: 0.16 g (67%) as a violet solid; IR (KBr) υ: 617 cm−1 (M–O) and 487 cm−1 (M–N)
-
3.Cu: 0.18 g (81%) as a light blue solid; IR (KBr) υ: 647 cm−1 (M–O) and 478 cm−1 (M–N)
-
3.Ni: 0.16 g (76%) as an orange solid; IR (KBr) υ: 637 cm−1 (M–O) and 444 cm−1 (M–N)
-
3.Co: 0.16 g (69%) as an orange solid; IR (KBr) υ: 635 cm−1 (M–O) and 438 cm−1 (M–N)
-
4.Cu: 0.10 g (48%) as a light blue solid; IR (KBr) υ: 636 cm−1 (M–O) and 454 cm−1 (M–N)
-
4.Ni: 0.12 g (56%) as a green solid; IR (KBr) υ: 639 cm−1 (M–O) and 463 cm−1 (M–N)
-
4.Co: 0.12 g (54%) as an orange solid; IR (KBr) υ: 649 cm−1 (M–O) and 436 cm−1 (M–N)
Solvent extraction
A chloroform solution (10 mL) of ligand (1 × 10−3 M) and an aqueous solution (10 mL) containing 2 × 10−5 M picric acid and 1 × 10−2 M metal nitrate were shaken at 25 °C for 1 h contact time. After the two phases were allows to settle for 1 h, an aliquot of the aqueous solutions was taken and the ultraviolet spectrum was recorded. A similar extraction was performed in the absence of picrate ion in the aqueous solutions. The extractability of the metal cations expressed by means of following equation:
where A0 and A are the absorbancies in the absence and presence of ligand, respectively.
References
Gutsche, C.D.: Calixarenes. Royal Society of Chemistry, Cambridge (1989); Gutsche, C.D.: Calixarenes Revisited. Royal Society of Chemistry, Cambridge (1998)
(a) Deligöz, H.: Azocalixarenes: synthesis, characterization, complexation, extraction, absorption properties and thermal behaviours. J. Incl. Phenom. Macrocyclic Chem. 55, 197–218 (2006); (b) Floriani, C., Floriani-Moro, R.: The M-C bond functionalities bonded to an oxo-surface modeled by Calix[4]arenes. Adv. Organomet. Chem. 47, 167–233 (2001)
Wieser, C., Dieleman, C.B., Matt, D.: Calixarene and resorciarene ligands in transition metal chemistry. Coord. Chem. Rev. 165, 93–161 (1997)
Radius, U.: Shaping the cavity of the macrocyclic ligand in metallocalix[4]arenes: The role of the ligand sphere. Inorg. Chem. 40, 6637–6642 (2001)
Böhmer, V., Vicens, J.: Calixarenes; A Versalite Class of Macracylic Compounds. Kluwer Academic Publishers, Dordrecht, 149–171 (1991)
Arnaud-Neu, F., Barboso, S., Berny, F., Castani, A., Muzet, N., Pinalli, A., Ungaro, R., Schwing-Weill, M.-J., Wipff, G.: Modulation of cation binding in calix[4]arene amides: Synthesis, complexation and molecular modelling studies. J. Chem. Soc. Perkin Trans. 2, 1727–1738 (1999)
Arduini, A., Ghidini, E., Pochini, A., Ungaro, R., Andreetti, G.D., Ugozzoli, F.: Molecular inclusion in functionalized macromolecules.15. para-tert-Butylcalix[4]arene tetra-acetamide – A new strong receptor for alkali cations. J. Incl. Phenom. 6, 119–134 (1988)
Arnaud-Neu, F., Schwing-Weill, M.J., Ziat, K., Cremin, S.J., Harris, S.J., McKervey, M.A.: Selective alkali and alkaline-earth cation complexation by calixarene amides. New J. Chem. 15, 33–37 (1991)
Shimuzu, H., Iwamoto, K., Fujimoto, K., Shinkai, S.: Chromogenic calix[4]arene. Chem. Lett. 2147–2150 (1991)
Arnaud-Neu, A., Barboso, S., Fanni, S., Schwing-Weill, M.-J., McKee, V., McKervey, M.A.: Alkali and alkaline earth ion complexation and x-ray crystal structure of p-tert-Butylcalix[4]arene tetraethylamide. Ind. Eng. Chem. Res. 39, 3489–3492 (2000)
Beer, P.D., Drew, M.G.B., Leeson, P.B., Ogden, M.I.: Versatile cation complexation by a Calix[4]arene tetraamide(L) – Synthesis and crystal-structure of [ML][CLO4](2)center-dot-nmecn(M=FE-II, NI-II, CU-II, ZN-II or PB-II) . J. Chem. Soc. Dalton Trans. 1273–1279 (1995)
Van der Veen, N.J., Egberink, R.J.M., Engbersen, J.F.J., Van Veggel, F.C.J.M., Reinhoudt, D.N.: Conformationally flexible calix[4]arene chromoionophores: Optical transduction of soft metal ion complexation by cation-pi interactions. Chem. Commun. 8, 681–982 (1999)
Deligöz, H., Ercan, N.: The synthesis of some new derivatives of calix[4]arene containing azo groups. Tetrahedron 58, 2881–288 (2002)
Deligöz, H., Çetişli, H.: The synthesis and properties of some novel azo group containing calix[n]arene derivatives. J. Chem. Res. 43, 285–289 (2001)
Çetişli, H., Karakuş, M., Erdem, E., Deligöz, H.: Synthesis, metal complexation and spectroscopic characterization of three new azo compounds. J. Incl. Phenom. Macrocylic Chem. 42, 187–191 (2002)
Deligöz, H., Erdem, E., Kocaokutgen, H.: Solvent extraction of Fe3+ cation by diazo-coupling calix[4]arenes. Turk. J. Chem. 24, 157–163 (2000)
Akdoğan, A., Deniz (Tavaslı), M., Cebecioğlu, S., Şen, A., Deligöz, H.: Liquid-liquid extraction of transition metal cations by nine new azo derivatives calix[n]arene. Sep. Sci. Technol. 37, 973–980 (2002)
Karcı, F., Şener, I., Deligöz, H.: Azocalixarenes.1: Synthesis, characterization and investigation of the absorption spectra of substituted azocalix[4]arenes. Dyes Pigm. 59, 53–61 (2003)
Karcı, F., Şener, I., Deligöz, H.: Azocalixarenes.2: Synthesis, characterization and investigation of the absorption spectra of azocalix[6]arenes containing chromogenic groups. Dyes Pigm. 62, 131–140 (2004)
Şener, I., Karcı, F., Kılıc, E., Deligöz, H.: Azocalixarenes.3: Synthesis, characterization and investigation of the absorption spectra of substitue azocalix[4]arenes. Dyes Pigm. 62, 141–148 (2004)
Şener, I., Karcı, F., Kılıc, E., Deligöz, H.: Azocalixarenes.4: Synthesis, characterization and investigation of the absorption spectra of azocalix[6]arenes containing heterocyclic groups. Dyes Pigm. 62, 149–157 (2004)
Huang, F., Wu, Y., Gu, D., Gon, F.: Synthesis of blue-violet light wavelength metal(ii)-azo complexes and their absorption and thermal properties. Mater. Lett. 2461–2465 (2004)
Deligöz, H., Tavaslı, M., Yılmaz, M.: Selective extraction of Fe3+ by a polymeric calix[4]arene. J. Pol. Sci. Part A: Polym. Chem. 32, 2961–2964 (1994)
Nomura, E., Taniguchi, H., Tamura, S.: Selective ion extraction by a calix[6]arene derivative containin azo groups. Chem. Lett. 1125–1126 (1989)
Masoud, S.M., Abou El-Enein, S.A., Ayad, E.M., Gohe, S.A.: Spectral and magnetic properties of phenylazo-6-aminouracil complexes. Spectrochimica Acta Part A 60, 77–87 (2004)
Deligöz, H., Özen, Ö., Çılgı, G.K., Çetişli, H.: A study on the thermal behaviours of parent calix[4]arenes and some azocalix[4]arene derivatives. Thermochemica Acta 426, 33–38 (2005)
Gutsche, C.D., Igbal, M.: p-tert-Butylcalix[4]arene. Org. Synth. 68, 234–238 (1990)
Gutsche, C.D., Igbal, M., Steward, D.: Calixarenes.18. Synthesis procedures for data para-tert-butylcalix[4]arene. J. Org. Chem. 51, 742–745 (1986)
Morita, Y., Toshio, A.: Synthesis and NMR behavior of calix[4]quinone and calix[4]hydroquinone. J. Org. Chem. 57, 3658–3662 (1992)
(a) Gowda, S., Abiraj, K., Gowda, C.D.: Reductive cleavage of azo compounds catalyzed by commercial zinc dust using ammonium formate or formic acid. Tetrahedron Lett. 43, 1329–1331 (2002); (b) Deligöz, H., Ak, M.S.: The synthesis of ester and ketone derivatives of azocalix [4]arene containing chromogenic groups. J. Incl. Phenom. Macrocylic Chem. 55, 223–228 (2006)
Kim, J.S., Shon, O.J., Lee, J.K., Lee, S.H., Kim, J.Y., Park, K.-M., Lee, S.S.: Chromogenic azo-coupled calix[4]arenes. J. Org. Chem. 67, 1372–1375 (2002)
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Mustafa Yılmaz on the occasion of his 50th birthday
Rights and permissions
About this article
Cite this article
Ak, M.S., Deligöz, H. Azocalixarenes. 6: Synthesis, complexation, extraction and thermal behaviour of four new azocalix[4]arenes. J Incl Phenom Macrocycl Chem 59, 115–123 (2007). https://doi.org/10.1007/s10847-007-9300-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-007-9300-9