Abstract
The conservation of insects, particularly endangered species such as the Apollo butterfly, is a pressing global concern. Understanding the habitat requirements and factors influencing species occupancy is crucial for designing effective conservation strategies. We focused on investigating the habitat characteristics expected to affect the occupancy of the nationally endangered Apollo butterfly in Southwest Finland. We conducted field surveys and GIS analysis to assess the impact of larval host plant and adult nectar resources, habitat encroachment, elevation, connectivity, and spatial variation on Apollo larval occupancy in rocky outcrop habitats. We found that rocky outcrops with abundant host plants and those less isolated from nectar patches play a significant role in supporting Apollo reproduction, whereas encroachment, specifically increased tree volume, negatively affected occupancy. We additionally observed spatial variation in occupancy across different blocks within the study area. Our findings emphasise the importance of resource availability for Apollo butterflies and highlight the dynamic nature of their habitat requirements. Maintaining a network of intact rocky outcrops with suitable resources is essential for the long-term persistence of the Apollo butterfly population in the region.
Implications for insect conservation: Our research underscores the critical need to protect and restore habitats for the Apollo butterfly, particularly by addressing threats such as habitat encroachment and construction projects that pose risks to their breeding sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity conservation is currently one of the most significant challenges facing our society and ecosystems (Cardinale et al. 2012). Anthropogenic actions, climate change, and habitat loss are among the primary factors causing biodiversity loss and documented declines in terrestrial insects (Sánchez-Bayo and Wyckhuys 2019; Wagner 2020; Cardoso 2020; Ceballos et al. 2020). Conserving biodiversity and insect diversity is essential for maintaining ecosystem services, stability and functioning (Börschig et al. 2013; Potts et al. 2016; Cardoso 2020; Sollai and Solari 2022). Butterflies (Rhopalocera) are among the best-studied groups of insects and are valuable environmental indicators because they react quickly to changes in their habitat. Nevertheless, European butterflies, particularly those of the grassland species, are facing a general decline (Warren et al. 2021). Although climate change is considered a significant global threat, habitat loss is known to be the most destructive threat to biodiversity, especially for butterflies and threatened species (Newbold et al. 2015; McWilliams et al. 2019; Horváth et al. 2019; Warren et al. 2021; Hogue and Breon 2022). Hanski (2005) identified four main types of habitat loss: loss of habitat quality, loss of habitat area, loss of habitat connectivity, and loss of habitat continuity. Direct human action has transformed almost half of the land, with negative consequences for biodiversity (Fischer et al. 2007). Agricultural intensification, leading to monocultures and habitat fragmentation, further exacerbates this issue (Raven and Wagner 2021).
Like many other regions, Finland has experienced significant habitat loss and changes in land use due to human activities (Ruuska and Helenius 1996; Millennium Ecosystem Assessment 2005; Hanski 2011; Kontula and Raunio 2019; Sunde et al. 2023). The disappearance of cultural habitats created and maintained by traditional agriculture, including various kinds of meadows and pastures, is the second most significant threat to biodiversity after forestry in Finland (Hyvärinen et al. 2019). In general, in the EU (Warren et al. 2021), these open habitats suffer from overgrowth, a direct consequence of changes in agriculture such as abandoning land, reducing grazing, and reaping and burn-clearing. Eutrophication caused by fertilisers and long-range transboundary deposits, combined with a warming climate, facilitates habitat overgrowth. Regardless of habitat type, land use changes and habitat loss have caused declines in several insect species and populations, particularly butterflies (Sánchez-Bayo and Wyckhuys 2019; Wagner 2020; Cardoso 2020; Wagner et al. 2021).
This study focuses on the Apollo butterfly (Parnassius apollo, L.), a species similar to many other butterflies that are susceptible to environmental changes due to their restricted and specialised habitats (Van Swaay and Warren 1999; Crone and Schultz 2003; Wiens and Graham 2005). Although classified as "Least Concern" on the IUCN Red List due to its wide distribution and minor estimated decline worldwide (Nadler et al. 2021), the Apollo butterfly is declining worldwide, particularly in European lowlands (Nakonieczny et al. 2007; Van Swaay et al. 2010; Nadler et al. 2021). Protected under the EU Habitats Directive (Annex IV), the Apollo butterfly's habitat management is crucial for its conservation. Yet, many areas lack proper management, leading to population declines (Nadler et al. 2021). Finland represents the lowland population in the northernmost range of Apollo, and the known populations occupying only the country's southwestern corner are small and declining (Marttila et al. 1991; Hyvärinen et al. 2019). Furthermore, the species generally occurs at low densities, and the probability of colonisation is very low outside its range (Marttila et al. 1991; Fred and Brommer 2010). The conservation-driven translocation of the Apollo butterfly within Finland initially showed some success (Fred and Brommer 2015), but no long-term establishment was achieved (M. Fred pers. comm.). According to Hyvärinen et al. (2019), Apollo is considered an endangered species at the national level. The remaining strongholds of the Apollo butterfly are on the Åland Islands and the coast of Southwest Finland (Marttila et al. 1991). However, in the archipelago of Southwest Finland, the abundance and probability of occupancy of Apollo butterflies have decreased by 50% over the last two decades (Kukkonen et al. 2022).
Describing species' habitats can be challenging, but in Finland, Apollo butterflies are mainly found on rocky outcrops, where their sole host plant, orpine (Hylotelephium telephium), grows. These rock outcrops are threatened by overgrowth and construction (Kontula and Raunio 2018; Hyvärinen et al. 2019). Characterised by their chemical composition, steep topography, microclimates, proximity to water bodies, natural conditions, and various combinations (Ministry of the Environment 2017), rocky outcrops are a critical habitat. In this paper, we study a coastal population around the city of Parainen, which, based on recorded observations, has endured from the early 20th century to the present day (Häkkinen 1976; NAFI 2023). In this population, Apollo butterflies occur on rocky outcrops scattered in an agricultural landscape. Given the declining Apollo population, the threat to its habitats from construction (Nieminen and Ahola 2017, pers. obs.), and the risk of regional extinction as a sedentary habitat specialist overwintering in the egg stage (Sunde et al. 2023), conservation efforts benefit from a better understanding of which rocky outcrops Apollo uses in the landscape. In patchy populations, such as the coastal population in Parainen (Brommer and Fred 1999), many suitable outcrops exist for Apollo, but not all are equally important. Recognising the most important breeding and resting habitats for Apollo and protecting them with proper management plans is crucial, as the species does not thrive under passive protection (Nakonieczny et al. 2007). For the Apollo and many butterflies, adult and larval feeding requirements typically require different plant species, resulting in spatial decoupling (Janz 2005). For butterflies, the nectar supply is one of the primary resources determining habitat quality (Wallisdevries et al. 2012). The quantity of nectar flowers also influences the movement patterns of Apollo butterflies, affecting the next generation; female Apollo butterflies lay more eggs in suitable habitats near nectar resources (Brommer and Fred 1999; Fred and Brommer 2003; Fred et al. 2006). Additionally, the survival of the Apollo butterfly, both globally and nationally, is heavily reliant on the availability of its larval host plant (Nakonieczny et al. 2007; Fred and Brommer 2010).
In this study, we investigate the habitat requirements of the Apollo butterfly, focusing on the presence/absence of Apollo larvae on rocky outcrops. We survey Apollo larvae, as the presence of larvae indicates that the rocky outcrop is reproductively important to the population. We surveyed 327 rocky outcrops for Apollo larvae in the spring of 2022 and 2023. The surveys were carried out in four spatially separate networks of rocky outcrops that are presumably semi-independent of each other. We hypothesise that Apollo butterflies are more likely to occur in rocky outcrops that are open, well-connected, well-lit, and larger in size, with abundant orpine and close proximity to nectar plants. Using a combination of Geographic Information Survey (GIS) data and field-based mapping, we analyse the characteristics of rocky outcrops and other landscape elements that may affect the Apollo butterfly. Specifically, we consider (1) the number of Apollo butterfly host plants (orpine) on rocky outcrops, (2) the encroachment and openness of rocky outcrops, (3) the potential exposure of rocky outcrops to sunlight to describe their microclimate, (4) proximity of rocky outcrops to adult nectar resources, (5) elevation of rocky outcrops, as more highly elevated outcrops could contain fewer trees and more open areas (Macias-Fauria and Johnson 2013), (6) the distance between surveyed outcrops (connectivity), and (7) whether the survey date affects the presence of larvae, as early-season larvae may go undetected due to their small size.
By understanding these factors, we aim to inform conservation strategies that can be applied not only to the Apollo butterfly but also to other specialised butterfly species facing similar ecological challenges.
Materials and methods
Study system
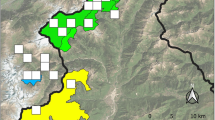
The coastal population of Southwest Finland resides on the islands that comprise the Parainen archipelago municipality. For more than 100 years, several Apollo observations have been made in this area (Häkkinen 1976; FinBIF 2023). For parts of this region, detailed surveys of Apollo larvae were carried out approximately 20 years ago and in 2020 (Fred 1998; Laaksonlaita 2023). The information on presence from the abovementioned previous studies was used to select a study area of approximately 22 km2 within the Parainen coastal archipelago (Fig. 1). The study area comprises three relatively large islands containing agricultural landscapes, forests, and human settlements. The study area was divided into four semi-independent blocks (Fig. 1), with the objective of surveying all rocky outcrops (potentially suitable habitat) in each block and thereby assessing the potential for spatial variation. Block 1 (3 km2) is located on the northernmost island, closest to the mainland. Block 2 (9 km2) is located on the southernmost island of the three islands surveyed. The middle survey island between blocks 1 and 2 was divided into two blocks, blocks 3 and 4 (both 5 km2). The central island was divided into two blocks to separate one large landowner's estate from the rest of the island. This landowner has performed small-scale restoration work for Apollo by opening meadows and rocky outcrops and, in general, favours agricultural and forestry practices that promote biodiversity and reduce the effects of climate change (P. Heikkinen, pers. comm.). For instance, fields are cultivated biologically and organically. Furthermore, Apollo's occurrence was previously recorded multiple times in the estate area (NAFI 2023).
The study area is in southwest Finland's municipality of Parainen. The survey blocks are presented with numbers (1–4) inside the islands. ©National Land Survey of Finland (NLS), the regions of Finland 2021, CC By 4.0 DEED and Natural Earth, Admin 0—Countries version 5.1.1, Free vector and raster map data @ naturalearthdata.com. Modified: Jonna M. Kukkonen 25.6.2024
The Apollo butterfly has a yearly life cycle in the northernmost part of its range, with larvae and adult butterflies present mainly from May to August (Marttila et al. 1991). The presence of larvae is a sure sign that a host-plant patch is being used for breeding (Fred et al. 2006); thus, the detection of Apollo larvae was the focus of our surveys. Apollo overwinters as an egg (Marttila et al. 1991; Fred and Brommer 2003). The larvae start to hatch in May, and after three to four weeks, they form a cocoon in the undergrowth and become pupae (Fred and Brommer 2003). Larvae occur singly but can be observed relatively effectively due to their aposematic colouration (orange and black). In addition, the grazing patterns on host plants can aid in finding larvae even when they have taken cover in the surrounding vegetation. Development to pupation is not synchronous for all larvae. Throughout most of the larval period, some individuals have not yet hatched from their eggs and others that have already pupated (Fred and Brommer 2003). This allows the detection of Apollo larvae of varying ages from the beginning of May to mid-June, when the surveyor(s) visited rocky outcrops in the study area to count Apollo larvae and the host plants. When searching for host plants, all areas were surveyed by walking through them as consistently as possible, with approximately equal searching effort per unit area. The total time spent surveying for larvae was proportional to the total amount of host plants present. The larval survey season was considered to last approximately 1.5 months (46 days). However, surveys were not conducted daily throughout this time period.
The surveys focused on areas outlined as rocky outcrops on a topographical map, i.e., habitat patches. Our sampling approach aimed to survey both documented occurrences of Apollo larvae from a previous, recent study (Laaksonlaita 2023) and nearby outcrops that were previously unsurveyed to capture habitat characteristics across the landscape comprehensively. In 2022, 124 outcrops were surveyed. The following year, 203 outcrops were explored, of which 99 were not surveyed in 2022. Consequently, 223 unique rocky outcrops were surveyed over the course of these two years. The geographical information system QGIS (version 3.22, 2022) was used to estimate the total patch area sizes of the rocky outcrops.
Apollo depends on two significant resources: larval host plants and flowering nectar plants for the adult stage (Brommer and Fred 1999; Fred and Brommer 2003; Fred et al. 2006). The larval host plant, perennial orpine, can grow one or multiple stems and can be found singularly around a rocky outcrop or in more dense patches. These patches of the host plant were marked on the map, and the number of host plants in each patch was counted. During the larval surveys and during the adult Apollo season, from July to early August, nectar plant patches were also marked on the map of the survey area. In the coastal population, most host-plant patches do not contain a nectar-plant patch (Fred et al. 2006). The Apollo butterfly is not a specialist in nectar plants (Fred 2004) but favours large, bright-coloured flowers that are relatively common species in Finland, such as Cirsium spp., Centaurea spp., Hieracium spp., Trifolium pratensis, Chamaenerion angustifolium, and Valeriana officinalis (Marttila et al. 1991; Fred 2004). For an area to be designated as a nectar site, it should contain a minimum of 10 stems with large inflorescences clustered together or a smaller group of very large and conspicuous plants, each bearing several flowers, such as Cirsium vulgare (Fred et al. 2006). In Parainen, patches of nectar-producing plants were typically extensive (Fred et al. 2006), often stretching several meters along roadsides and field margins.
We employed two isolation measures to assess the connectivity of rocky outcrops within the study area. The first measure,\(-\sum exp\left(-\alpha {D}_{in}\right)\), quantifies the isolation of rocky outcrops from nectar patches, where Din represents the Euclidean distance between outcrop i and nectar patch n, and α is a scaling parameter (Hanski 1994). This measure assigns the greatest weight to patches in close proximity. For the parameter α, we used a value based on the capture-mark-recapture of Apollo done in the study area (0.27; Brommer and Fred 1999). A higher value indicates greater isolation from nectar sources. The second measure, \(-\sum (exp\left(-\alpha {D}_{ij}\right)*patch\;size \left(j\right))\), evaluates the interpatch isolation, incorporating the size of each neighbouring outcrop j. This measure accounts for both spatial proximity and size disparities between outcrops, with larger values indicating increased isolation among outcrops. Both Din and Dij were measured from the centre to the centre of a patch in units of 100 m, as described by Hanski et al. (1994). These measures collectively provide a robust evaluation of spatial isolation in relation to both nectar sources and neighbouring rocky outcrops within the study landscape.
Geographical information systems (GIS) data
The landscape features used in the analysis are the raster layer (16 m × 16 m) of the growing stock volume for all tree species (1 m3/ha) (Luke 2021) and the raster layer (2 m × 2 m) of the digital elevation model, DEM (NLS 2023). The patch size area and distances between rocky outcrop centroids and nectar patches were calculated, and the values were extracted from the raster layers via QGIS (version 3.22, 2022). Because the slope and aspect of a rocky outcrop affect its potential exposure to sunlight, we used the digital elevation model (NLS 2023) to calculate the insolation (WH/m2) of each rocky outcrop to describe its microclimate. Insolation was computed using ArcGIS Pro (version 3.1.0, ESRI 2023) with the analysis tool “area solar radiation”. The time period used for calculating the insolation was May 2022.
Statistical analyses
The presence/absence of Apollo was recorded in several rocky outcrops in two seasons, and we, therefore, applied a generalised linear mixed-effects model (GLMM) in which the patch ID was used as a random intercept to account for the dependency among observations made on the same rocky outcrop. The binomial GLMM model was implemented with a logit function in the R package glmmTMB (Brooks et al. 2017). We used a rocky outcrop's occupancy (unoccupied 0 or occupied 1) as the response variable. The fixed effects included the number of host plants (continuous), the size of the patch (continuous), the mean value of the tree volume of the growing stock in a patch (continuous), the nectar isolation (continuous), the mean value of the elevation of a patch (continuous), the interpatch isolation (continuous), and the insolation of a patch (continuous). Temporal variation was captured by including the survey date (continuous), the survey year (categorical with two levels), and spatial variation by including the survey block (categorical with four levels). We considered meaningful interactions survey year × block number to capture spatial–temporal interactions at the above-patch level. We chose not to utilise stepwise model selection methods due to well-documented concerns about their reliability and interpretability (Mundry and Nunn 2009). Instead, we present the full model with all predictor variables included simultaneously. This approach allows for a comprehensive evaluation of each variable's effect while minimising the risk of erroneous conclusions associated with stepwise techniques. All estimates were standardised to facilitate interpretation, ensuring that effect sizes were comparable across predictors. We used the restricted maximum likelihood (REML) approach and the Wald test for hypothesis testing. All analyses were performed with the R program (R Core Team 2023), version 4.3.1.
Results
The data comprised 327 rocky outcrops surveyed over two years (Table 1). The size of the outcrops averaged 16.36 ± 1.30 SE ha (Table 2). Apollo larvae were detected on 46% (150/327) of these rocky outcrops (Table 1), and this proportion did not differ between years (Table 1; X21 = 0.07, P = 0.79). Of the 327 patches surveyed in two years, 50 patches (15%) had fewer than five host plants at the time of the survey. However, in 3 of these patches, in one patch in 2022 and two in 2023, an Apollo larva was detected. In most blocks, especially in 2023, approximately 40 rocky outcrops were surveyed (Table 1). The number of host plants in the surveyed outcrops over two years varied, averaging 42 host plants per outcrop (Table 2). The growing stock volume for all tree species in the surveyed outcrops also showed variation, as did the isolation measure of nectar patches and interpatch (i.e. between rocky outcrops) (Table 2). The elevation of the surveyed outcrops ranged from low to moderate levels above sea level. Insolation values were relatively consistent but displayed some variation, and the distance to the nearest neighbouring rocky outcrop also varied (Table 2). The correlation coefficients among habitat characteristics (number of host plants, patch size, tree volume, insolation, nectar isolation, and interpatch isolation) ranged from − 0.44 to 0.46, where the highest correlation (0.46) was between host plant number and patch size. These findings indicate no strong correlations among the variables examined in our study, and we, therefore, included all as explanatory variables when modelling patch occupancy.
Of the 104 rocky outcrops surveyed in both years, 36 (35%) were occupied, and no larvae were detected in 39 (38%). Occupancy changed between years in 29 (28%) rocky outcrops, indicating occupancy dynamics. Apollo was more likely to occur on rocky outcrops with more abundant host plants and closer to nectar plant patches but was less likely to occur on rocky outcrops with a large growing stock volume of trees (Table 3, Fig. 2). In addition, we found (P < 0.05) that outcrops with higher elevations were more likely to be occupied (Table 3, Fig. 2). Finally, there was an indication of spatial variation in occupancy across the study area (“Block” in Table 3; P < 0.05), with occupancy being exceptionally high in one block (Table 3, Fig. 2).
Marginal effects for four significant model terms of a GLMM on the predicted probability of Apollo occupancy (Table 3). The marginal effects of a the number of host plants/rocky outcrop, b the average tree (growing stock) volume of an outcrop, c within-study-area spatial differences (between survey blocks) and d elevation of a rocky outcrop are plotted here
Discussion
We examine characteristics of the rocky outcrops and other landscape elements supporting the occupancy of the nationally endangered Apollo butterfly in the coastal population of Southwest Finland. We find that rocky outcrops with a higher abundance of host plants and nearby nectar plants are significant for Apollo. Moreover, we observed that elevated rocky outcrops also play a significant role in the butterfly's habitat. We also find that the encroachment of rocky outcrops, in terms of a greater tree volume of growing stock, lowers the probability of the rocky outcrop being used for reproduction by Apollo. Finally, we find spatial variation (included here by surveying four “blocks” of 20–40 rocky outcrops in this population) in Apollo occupancy.
Our finding that the abundance of larval resources (host plant) and proximity of adult resources (nectar plants) are essential for Apollo is consistent with results obtained some 20 years ago in one part of this population (Brommer and Fred 1999; Fred and Brommer 2003, 2009, 2010; Fred et al. 2006), roughly coinciding with block 2. Our finding that these two resources are crucial for Apollo when considering a larger area two decades later underlines their importance in this Apollo population. This is consistent with broader butterfly ecology, where the availability of these resources is crucial for many species (Boggs et al. 2003; Dennis et al. 2003, 2006; Hardy et al. 2007; Wallisdevries et al. 2012; Curtis et al. 2015). Previous research in other Apollo populations (Nakonieczny et al. 2007; Adamski and Ćmiel 2022; Sbaraglia et al. 2023) highlights similar dependencies, demonstrating that conservation strategies focused on these resources are widely applicable. For example, Baz (2002) emphasised that successful conservation management requires habitat management and restoration focused on host and nectar plants. This principle applies broadly across butterfly species (Smallidge and Leopold 1997; Dennis et al. 2006; Hardy et al. 2007; Wallisdevries et al. 2012; Curtis et al. 2015), making our findings relevant to general butterfly ecology and conservation.
We found that tree encroachment on rocky outcrops significantly lowers the probability of Apollo occupancy. This phenomenon is not unique to the Apollo butterfly; many butterfly species are sensitive to habitat structure changes caused by encroachment and succession. Encroachment reduces the open habitats required by many specialised butterflies, as seen with the Clouded Apollo butterfly (Parnassius mnemosyne) (Konvička and Kuras 1999; Välimäki and Itämies 2005). Encroachment is a significant threat to European butterflies in general, particularly those dependent on grasslands that become forests due to land abandonment or the cessation of grazing (Kuussaari et al. 2007; Warren et al. 2021; Sunde et al. 2023). Our findings contribute to the broader understanding that managing open habitats is crucial for conserving many specialised butterfly species.
The elevation of the outcrop, despite its seemingly unremarkable range from 7 to 56 m a.s.l., was found to be a significant factor influencing Apollo occupancy. This unexpected finding could be attributed to the mate-locating behaviour of male butterflies, who use hilltops as landmarks for finding potential mates (Rutowski 1991). This hill-topping behaviour, exhibited by various patrolling butterflies and species of Papilionidae (Rutowski et al. 1989; Takeuchi 2019), including Apollo (Baz 2002; Adamski and Witkowski 2006), could explain the significance of the elevation. However, our findings suggest that the openness of the outcrop is a more significant characteristic for the Apollo larvae than its elevation in this lowland population.
We find strong evidence for spatial differences in Apollo occupancy within our relatively small study area. As this spatial heterogeneity is apparent in a model that also considers the effect of all the above-discussed landscape elements, this finding implies that—in addition to these landscape elements—there are other factors affecting Apollo occupancy that we did not consider here. Within our study area, the most favourable part for Apollo is located in the northern part of the central island (Fig. 1; block 3). In this part of the study area, biodiversity is promoted by managing the land. For instance, unpaved roadsides with nectar flowers are mowed once relatively late in the summer (Valtonen and Saarinen 2005), and sheep graze on some outcrops after the larval season, which could benefit Apollo in terms of critical resources. Furthermore, the above-described small-scale restoration work has been performed for the Apollo in the area. It seems likely that the details of land management related to aspects other than larval and adult resources and encroachment benefit Apollo. Another aspect is that the fields are cultivated biologically and organically in block three. There is evidence that organic farms offer higher-quality habitat for butterflies than conventional farms (Goded et al. 2019; Van Deynze et al. 2024). More research is thus needed to identify which aspects of land management favour Apollo.
We did not observe any significant impact of connectivity on Apollo occupancy in our study area, likely due to the relatively high density of rocky outcrops within the survey blocks (the longest distance to the nearest neighbour was < 800 m). A recent study by Graser et al. (2023) also concluded that habitat quality is a more influential factor than patch connectivity for two light-demanding butterfly species. Although our research did not identify habitat connectivity and patch size as significant factors, we acknowledge that these elements are well-established determinants influencing butterfly populations globally (e.g., Haddad and Tewksbury 2005; Binzenhöfer et al. 2008; Brückmann et al. 2010; Jangjoo et al. 2016; Paterson et al. 2019; Stilley and Gabler 2021; Popović and Nowicki 2023).
Implications for conservation
The Apollo butterfly is a species listed in Annex IV of the EU Habitats Directive that requires strict protection (Council Directive 92/43/EEC). The Habitats Directive mandates that all Member States establish a strict protection regime for the species listed in Annex IV within and outside protected areas (European Commission 2024). In particular, Member States must prohibit the deterioration or destruction of these species' breeding or resting sites (European Commission 2024). Despite strict protection, a major threat to Apollo in this and other populations is the deterioration of its breeding habitats. In our study population, one threat is that both large and smaller construction projects are carried out on the rocky outcrops (breeding habitat) of Apollo (The Supreme Administrative Court 1999, Nieminen and Ahola 2017). This Apollo population is scattered across various rocky outcrops. In this patchy population (Brommer and Fred 1999), Apollo adults move across several rocky outcrops in the landscape, and arguably, the importance of a single outcrop for the entire population is relatively small. However, habitat quality at a specific location in a given year is determined by its inherent spatial attributes and ever-changing environmental conditions (Hanski 2005). Rocky outcrops not used in one year may be crucial in another year and vice versa. In particular, our finding that rocky outcrops near nectar plants are more likely to produce the next generation of Apollo implies that the system is very dynamic, as nectar plants grow on ephemeral sites and are likely to change location from year to year. Hence, a network of intact outcrops with host plants near nectar resources is needed for Apollo to persist in this area. Chipping away rocky outcrops from this network for infrastructure development likely will, at some point, make the network unsuitable for Apollo, and more research is needed to understand better whether and where a critical threshold exists.
Our findings suggest strategies for restoring or offsetting Apollo's habitat loss. Offsets are a way to achieve additional or equivalent biodiversity benefits to compensate for the losses caused by development. In general, offsetting is the last resort in the mitigation hierarchy. First, developers must try to avoid, minimise, and reverse the predicted impacts of biodiversity. Our findings imply that it may be possible to offset Apollo by opening previously suitable habitats and ensuring high host plant abundance and the presence of nearby nectar resources (cf. Nieminen and Ahola 2017).
Rocky outcrops form potential breeding habitats for Apollo in SW Finland, and conveniently, these can be readily delineated. Importantly, however, we find that the area of the rocky outcrop itself has little importance, but it is the number of host plants that affect Apollo occupancy. Thus, not only large rocky outcrops are important for Apollo reproduction since even small rocky outcrops can contain a relatively high (to their area) abundance of host plants. The ramification of this finding is that responsible infrastructure development in this Apollo population requires knowledge of host plant numbers on rocky outcrops and taking this information into consideration. However, as far as we know, this detailed information is not easily obtained through remote sensing, but field surveys are needed. The Apollo butterfly is, in that sense, one of many butterfly species that Dennis et al. (2006) describe as relying on resources found in small or even tiny pockets that are widely dispersed. Surveying for these small resources can be challenging due to limited access, search time, and the number of surveyors compared to the area being covered. Nevertheless, orpine is a perennial herb that is easy to census in early spring, as it is one of the first to grow after snow melts. Thus, orpine will likely persist in the same area if conditions remain favourable.
The Apollo butterfly is facing a concerning future, as its populations show declining trends at all levels—global, European, and national (Swaay et al. 2010; Hyvärinen et al. 2019; Nadler et al. 2021). Furthermore, the cold-adapted, sedentary nature of Apollo, along with its specialisation in habitat and host plants, all predict that this declining trend will continue (Pöyry et al. 2009; Eskildsen et al. 2015; Sugimoto et al. 1971; Shirey et al. 2024). Translocation of Apollo in Finland to other suitable sites has proven challenging (Fred and Brommer 2015), and the few Apollo populations remaining thus warrant conservation actions.
Our study's findings provide insights into the general principles of butterfly ecology and conservation. Resource availability, habitat structure, and spatial configuration are relevant to many specialised and threatened butterfly species. Conservation actions should focus on preserving and enhancing these critical habitat features. This includes ensuring the availability of host and nectar plants, managing habitats to prevent encroachment, and maintaining habitat connectivity.
Furthermore, our research underscores the complex interplay of various factors in determining the occupancy dynamics of the Apollo butterfly, with broader implications for the conservation of other specialised butterflies. The importance of specific resources and habitat characteristics identified in our study can inform general conservation strategies, making this study relevant for a wider audience interested in butterfly ecology and conservation. Efficient conservation efforts require a multifaceted approach, considering the specific needs of butterflies throughout their life cycles and addressing the challenges posed by habitat loss and fragmentation.
Data availability
No datasets were generated or analysed during the current study.
References
Adamski P, Witkowski Z (2006) Male patrolling modes in Apollo butterfly Parnassius apollo [L.]: simulation of optimal choice [Lepidoptera: Papilionidae]. Nature and Conservation
Adamski P, Ćmiel AM (2022) The long-term effect of over-supplementation on recovered populations: why restraint is a virtue. Oryx 56(4):564–571. https://doi.org/10.1017/S0030605321000296
Baz A (2002) Nectar plant sources for the threatened apollo butterfly (Parnassius apollo L 1758) in populations of central Spain. Biological Conservation 103(3):277–282. https://doi.org/10.1016/S0006-3207(01)00138-0
Binzenhöfer B, Biedermann R, Settele J, Schröder B (2008) Connectivity compensates for low habitat quality and small patch size in the butterfly Cupido minimus. Ecol Res 23(2):259–269. https://doi.org/10.1007/s11284-007-0376-x
Boggs CL, Watt WB, Ehrlich PR (2003) Butterflies : ecology and evolution taking flight. University of Chicago Press, Chicago, p 736
Börschig C, Klein AM, Von Wehrden H, Krauss J (2013) Traits of butterfly communities change from specialist to generalist characteristics with increasing land-use intensity. Basic Appl Ecol 14(7):547–554. https://doi.org/10.1016/j.baae.2013.09.002
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Mächler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. The R Journal 9(2):378. https://doi.org/10.32614/RJ-2017-066
Brommer JE, Fred MS (1999) Movement of the apollo butterfly Parnassius apollo related to host plant and nectar plant patches: population structure and movement of Parnassius apollo. Ecological Entomology 24(2):125–131. https://doi.org/10.1046/j.1365-2311.1999.00190.x
Brückmann SV, Krauss J, Steffan-Dewenter I (2010) Butterfly and plant specialists suffer from reduced connectivity in fragmented landscapes. J Appl Ecol 47(4):799–809. https://doi.org/10.1111/j.1365-2664.2010.01828.x
Cardinale BJ, Duffy JE, Gonzalez A, Hooper DU, Perrings C, Venail P, Narwani A, Mace GM, Tilman D, Wardle DA, Kinzig AP, Daily GC, Loreau M, Grace JB, Larigauderie A, Srivastava DS, Naeem S (2012) Biodiversity loss and its impact on humanity. Nature 486(7401):59–67. https://doi.org/10.1038/nature11148
Cardoso P (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv 242:108426. https://doi.org/10.1016/j.biocon.2020.108426
Ceballos G, Ehrlich PR, Raven PH (2020) Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proceedings of the National Academy of Sciences of the United States of America 117(24):13596–13602. https://doi.org/10.1073/pnas.1922686117
Council Directive (2013) 92/43/EEC of 21 May 1992 on the Conservation of Natural Habitats and of Wild Fauna and Flora (Annex IV, Article 12). http://data.europa.eu/eli/dir/1992/43/2013-07-01/eng Accessed 18 March 2024
Crone EE, Schultz CB (2003) Movement behavior and minimum patch size for butterfly population persistence. butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago, pp 561–576
Curtis RJ, Brereton TM, Dennis RLH, Carbone C, Isaac NJB (2015) Butterfly abundance is determined by food availability and is mediated by species traits. J Appl Ecol. https://doi.org/10.1111/1365-2664.12523
Dennis RLH, Shreeve TG, Van Dyck H (2003) Towards a functional resource-based concept for habitat: a butterfly biology viewpoint. Oikos 102(2):417–426. https://doi.org/10.1034/j.1600-0579.2003.12492.x
Dennis RLH, Shreeve TG, Van Dyck H (2006) Habitats and resources: the need for a resource-based definition to conserve butterflies. Biodivers Conserv 15(6):1943–1966. https://doi.org/10.1007/s10531-005-4314-3
European Commission The Habitats Directive—European Commission (2024), https://environment.ec.europa.eu/topics/nature-and-biodiversity/habitats-directive_en Accessed 18 March 2024
Eskildsen A, Carvalheiro LG, Kissling WD, Biesmeijer JC, Schweiger O, Høye TT (2015) Ecological specialization matters: long-term trends in butterfly species richness and assemblage composition depend on multiple functional traits. Divers Distrib 21(7):792–802. https://doi.org/10.1111/ddi.12340
ESRI (2023) ArcGIS Pro ESRI. Redlands, California
FinBIF Parnassius Apollo (2023). Finnish Biodiversity Info Facility (FinBIF), https://laji.fi/en/taxon/MX.60724/occurrence Accessed 5 October 2023
Fischer J, Manning AD, Steffen W, Rose DB, Daniell K, Felton A, Garnett S, Gilna B, Heinsohn R, Lindenmayer DB, MacDonald B, Mills F, Newell B, Reid J, Robin L, Sherren K, Wade A (2007) Mind the sustainability gap. Trends Ecol Evol 22(12):621–624. https://doi.org/10.1016/j.tree.2007.08.016
Fred MS (1998) A case study of a patchy population of the Apollo butterfly (Parnassius apollo); the importance of nectar sources. University of Helsinki, Helsinki
Fred MS, Brommer JE (2003) Influence of habitat quality and patch size on occupancy and persistence in two populations of the Apollo butterfly (Parnassius apollo). J Insect Conserv 7(2):85–98. https://doi.org/10.1023/A:1025522603446
Fred MS, Brommer JE (2009) Resources influence dispersal and population structure in an endangered butterfly. Insect Conservation and Diversity 2(3):176–182. https://doi.org/10.1111/j.1752-4598.2009.00059.x
Fred MS, Brommer JE (2010) Olfaction and vision in host plant location by Parnassius apollo larvae: consequences for survival and dynamics. Animal Behaviour 79(2):313–320. https://doi.org/10.1016/j.anbehav.2009.11.001
Fred MS, Brommer JE (2015) Translocation of the endangered apollo butterfly Parnassius apollo in southern Finland. Conservation Evidence 12:8–13
Fred MS, O’Hara RB, Brommer JE (2006) Consequences of the spatial configuration of resources for the distribution and dynamics of the endangered Parnassius apollo butterfly. Biol Cons 130(2):183–192. https://doi.org/10.1016/j.biocon.2005.12.012
Fred M (2004) Influence of resource distribution and abundance on the population structure and dynamics of Parnassius apollo. University of Helsinki, Helsinki, 1–32 pp
Goded S, Ekroos J, Azcárate JG, Guitián JA, Smith HG (2019) Effects of organic farming on plant and butterfly functional diversity in mosaic landscapes. Agr Ecosyst Environ 284:106600. https://doi.org/10.1016/j.agee.2019.106600
Graser A, Kelling M, Pabst R, Schulz M, Hölzel N, Kamp J (2023) Habitat quality, not patch isolation, drives distribution and abundance of two light-demanding butterflies in fragmented coppice landscapes. J Insect Conserv 27(5):743–758. https://doi.org/10.1007/s10841-023-00494-8
Haddad NM, Tewksbury JJ (2005) Low-quality habitat corridors as movement conduits for two butterfly species. Ecol Appl 15(1):250–257. https://doi.org/10.1890/03-5327
Häkkinen SL (1976) Apolloperhosen (Parnassius apollo L.) levinneisyyden ja runsauden muutokset suomessa 1900-luvulla. University of Helsinki, Helsinki
Hanski I (1994) Patch-occupancy dynamics in fragmented landscapes. Trends Ecol Evol 9(4):131–135. https://doi.org/10.1016/0169-5347(94)90177-5
Hanski I (2011) Habitat Loss, the Dynamics of Biodiversity, and a Perspective on Conservation. Ambio 40(3):248–255. https://doi.org/10.1007/s13280-011-0147-3
Hanski I (2005) Habitat Loss the shrinking world: ecological consequences of habitat loss excellence in ecology book 14. International Ecology Institute, Oldendorf
Hanski I, Kuussaari M, Nieminen M (1994) Metapopulation structure and migration in the butterfly Melitaea cinxia. Ecol 75(3):747–762. https://doi.org/10.2307/1941732
Hardy PB, Sparks TH, Isaac NJB, Dennis RLH (2007) Specialism for larval and adult consumer resources among British butterflies: Implications for conservation. Biol Cons 138(3–4):440–452. https://doi.org/10.1016/j.biocon.2007.05.014
Hogue AS, Breon K (2022) The greatest threats to species. Conservation Science and Practice 4(5):e12670. https://doi.org/10.1111/csp2.12670
Horváth Z, Ptacnik R, Vad CF, Chase JM (2019) Habitat loss over six decades accelerates regional and local biodiversity loss via changing landscape connectance. Ecol Lett 22(6):1019–1027. https://doi.org/10.1111/ele.13260
Hyvärinen E, Juslén A, Kemppainen E, Uddström A, Liukko UM (eds.) (2019) The 2019 Red List of Finnish Species. Ministry of the Environment & Finnish Environment Institute, Helsinki, 704 pp
Jangjoo M, Matter SF, Roland J, Keyghobadi N (2016) Connectivity rescues genetic diversity after a demographic bottleneck in a butterfly population network. Proc Natl Acad Sci 113(39):10914–10919. https://doi.org/10.1073/pnas.1600865113
Janz N (2005) The relationship between habitat selection and preference for adult and larval food resources in the Polyphagous Butterfly Vanessa cardui (Lepidoptera: Nymphalidae). Journal of Insect Behavior 18(6):767–780. https://doi.org/10.1007/s10905-005-8739-z
Kontula T, Raunio A (2019) Threatened habitat types in Finland 2018 red list of habitats—results and basis for assessment. The Finnish Environment Institute and Ministry of the Environment, Helsinki
Kontula T, Raunio A (2018) Threatened habitat types in Finland 2018. Red list of habitats—part 2: descriptions of habitat types. The Finnish environment 5/2018. Ministry of the Environment & Finnish Environment Institute, Helsinki, p 925
Konvička M, Kuras T (1999) Population structure, behaviour and selection of oviposition sites of an endangered butterfly, Parnassius mnemosyne, in Litovelské Pomoravíl. Czech Republic. J Insect Conserv 3(3):211–223. https://doi.org/10.1023/A:1009641618795
Kukkonen JM, Mussaari M, Fred MS, Brommer JE (2022) A strong decline of the endangered apollo butterfly over 20 years in the archipelago of Southern Finland. J Insect Conserv 26(4):673–681. https://doi.org/10.1007/s10841-022-00413-3
Kuussaari M, Heliölä J, Pöyry J, Saarinen K (2007) Contrasting trends of butterfly species preferring semi-natural grasslands, field margins and forest edges in Northern Europe. J Insect Conserv 11(4):351–366. https://doi.org/10.1007/s10841-006-9052-7
Laaksonlaita J (2023) Exploring the impacts of microclimatic temperature on occupancy of the apollo butterfly. University of Turku, Turku
Luke (2021) The multi-source national forest inventory raster maps of 2021. The Natural Resources Institute Finland, Helsinki
Macias-Fauria M, Johnson EA (2013) Warming-induced upslope advance of subalpine forest is severely limited by geomorphic processes. Proc Natl Acad Sci 110(20):8117–8122. https://doi.org/10.1073/pnas.1221278110
Marttila O, Ojalainen P, Marttila M, Heiskanen H (1991) Suomen päiväperhoset, 2nd ed. Kirjayhtymä, Helsinki, 370 pp
McWilliams C, Lurgi M, Montoya JM, Sauve A, Montoya D (2019) The stability of multitrophic communities under habitat loss. Nat Commun 10(1):2322. https://doi.org/10.1038/s41467-019-10370-2
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: wetlands and water synthesis: a report of the Millennium Ecosystem Assessment. World Resources Institute, Washington
Ministry of the Environment (2017) Action plan for protection of threatened species. The Finnish Environment, Helsinki
Mundry R, Nunn CL (2009) Stepwise model fitting and statistical inference: turning noise into signal pollution. Am Nat 173(1):119–123. https://doi.org/10.1086/593303
Nadler J, Bonelli S, Dapporto L, Karaçetin E, Lukhtanov V, López Munguira M, Micevski N, Settele J, Tzortzakaki O, Verovnik R, Warren M, Wiemers M, Wynhoff I, van Swaay C (2021) Parnassius apollo. The IUCN red list of threatened species 2021:e.T16249A122600528. https://doi.org/10.2305/IUCN.UK.2021-1.RLTS.T16249A122600528.en
NAFI (2023). The National Butterfly Survey of Finland (NAFI). https://laji.fi/en/project/MHL.6/stats?time=all&taxonId=MX.60724&page=1 (Accessed 24 October 2023)
Nakonieczny M, Kedziorski A, Michalczyk K (2007) Apollo butterfly (Parnassius apollo L) in Europe—its history, decline and perspectives of conservation. Functional Ecosystems and Communities 1(1):56–79
Newbold T, Hudson LN, Hill SLL, Contu S, Lysenko I, Senior RA, Börger L, Bennett DJ, Choimes A, Collen B, Day J, De Palma A, Díaz S, Echeverria-Londoño S, Edgar MJ, Feldman A, Garon M, Harrison MLK, Alhusseini T, Ingram DJ, Itescu Y, Kattge J, Kemp V, Kirkpatrick L, Kleyer M, Correia DLP, Martin CD, Meiri S, Novosolov M, Pan Y, Phillips HRP, Purves DW, Robinson A, Simpson J, Tuck SL, Weiher E, White HJ, Ewers RM, Mace GM, Scharlemann JPW, Purvis A (2015) Global effects of land use on local terrestrial biodiversity. Nature 520(7545):45–50. https://doi.org/10.1038/nature14324
Nieminen M, Ahola A (2017) Presentation of the species (except for bats). In: Nieminen M, Ahola A (eds) Annex IV of the European union’s habitats directive. The Finnish Environment, Helsinki
NLS (2023) Elevation model 2 m (CC BY 4.0). National land survey of Finland topographic database 08/2023, National land survey of Finland, Helsinki. https://asiointi.maanmittauslaitos.fi/karttapaikka/tiedostopalvelu/korkeusmalli
Paterson GB, Smart G, McKenzie P, Cook S (2019) Prioritising sites for pollinators in a fragmented coastal nectar habitat network in Western Europe. Landscape Ecol 34(12):2791–2805. https://doi.org/10.1007/s10980-019-00884-x
Popović M, Nowicki P (2023) Movements of a Specialist Butterfly in Relation to Mowing Management of Its Habitat Patches. Biology 12(3):344. https://doi.org/10.3390/biology12030344
Potts SG, Imperatriz-Fonseca V, Ngo HT, Aizen MA, Biesmeijer JC, Breeze TD, Dicks LV, Garibaldi LA, Hill R, Settele J, Vanbergen AJ (2016) Safeguarding pollinators and their values to human well-being. Nature 540(7632):220–229. https://doi.org/10.1038/nature20588
Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K (2009) Species traits explain recent range shifts of Finnish butterflies. Glob Change Biol 15(3):732–743. https://doi.org/10.1111/j.1365-2486.2008.01789.x
QGIS (2022) QGIS geographic information system. QGIS Association, London
R Core Team (2023) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Raven PH, Wagner DL (2021) Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc Natl Acad Sci 118(2):e2002548117. https://doi.org/10.1073/pnas.2002548117
Rutowski RL (1991) The evolution of male mate-locating behavior in butterflies. The American Naturalist 138(5):1121–1139
Rutowski RL, Alcock J, Carey M (1989) Hilltopping in the pipevine swallowtail butterfly (Battus philenor). Ethology 82(3):244–254. https://doi.org/10.1111/j.1439-0310.1989.tb00505.x
Ruuska R, Helenius J (1996) GIS analysis of change in an agriculture landscape in Central Finland. Agricultural and Food Science 5(6):567–576. https://doi.org/10.23986/afsci.72770
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: a review of its drivers. Biological Conservation. https://doi.org/10.1016/j.biocon.2019.01.020
Sbaraglia C, Samraoui KR, Massolo A, Bartoňová AS, Konvička M, Fric ZF (2023) Back to the future: climate change effects on habitat suitability of Parnassius apollo throughout the quaternary glacial cycles. Insect Conservation and Diversity 16(2):231–242. https://doi.org/10.1111/icad.12615
Shirey V, Neupane N, Guralnick R, Ries L (2024) Rising minimum temperatures contribute to 50 years of occupancy decline among cold-adapted Arctic and boreal butterflies in North America. Glob Change Biol 30(2):e17205. https://doi.org/10.1111/gcb.17205
Smallidge PJ, Leopold DJ (1997) Vegetation management for the maintenance and conservation of butterfly habitats in temperate human-dominated landscapes. Landsc Urban Plan 38(3):259–280. https://doi.org/10.1016/S0169-2046(97)00038-8
Sollai G, Solari P (2022) An overview of “insect biodiversity.” Diversity. https://doi.org/10.3390/d14020134
Stilley JA, Gabler CA (2021) Effects of patch size, fragmentation, and invasive species on plant and Lepidoptera communities in Southern Texas. Insects 12(9):777. https://doi.org/10.3390/insects12090777
Sugimoto N, Fukasawa K, Asahara A, Kasada M, Matsuba M (1971) Miyashita T (2022) Positive and negative effects of land abandonment on butterfly communities revealed by a hierarchical sampling design across climatic regions. Proceedings of the Royal Society b: Biological Sciences 289:20212222. https://doi.org/10.1098/rspb.2021.2222
Sunde J, Franzén M, Betzholtz PE, Francioli Y, Pettersson LB, Pöyry J, Ryrholm N, Forsman A (2023) Century-long butterfly range expansions in northern Europe depend on climate, land use and species traits. Communications Biology 6(1):1–14. https://doi.org/10.1038/s42003-023-04967-z
Takeuchi T (2019) Mating behavior of the old world swallowtail. Papilio Machaon Lepidoptera Science 70(1):17–24. https://doi.org/10.18984/lepid.70.1_17
The Supreme Administrative Court (1999) FINLEX ®—Korkeimman hallinto-oikeuden ratkaisuja: 25.10.1999/2853. Finlex. https://finlex.fi/fi/oikeus/kho/lyhyet/1999/199902853 Accessed 6 Mar 2024
Välimäki P, Itämies J (2005) Effects of canopy coverage on the immature stages of the clouded apollo butterfly Parnassius mnemosyne L. with observations on larval behaviour. Entomologica Fennica 16(2):117–123
Valtonen A, Saarinen K (2005) A highway intersection as an alternative habitat for a meadow butterfly: effect of mowing, habitat geometry and roads on the ringlet (Aphantopus hyperantus). Ann Zool Fenn 42(5):545–556
Van Swaay C, Warren M (1999) Red data book of european butterflies (Rhopalocera). Council of Europe, Strasbourg
Van Deynze B, Swinton SM, Hennessy DA, Haddad NM, Ries L (2024) Insecticides, more than herbicides, land use, and climate, are associated with declines in butterfly species richness and abundance in the American Midwest. PLoS ONE 19(6):e0304319. https://doi.org/10.1371/journal.pone.0304319
Van Swaay C, Cuttelod A, Collins S, Maes D, López Munguira M, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhoff I (2010) European red list of butterflies. Publications Office of the European Union, Luxembourg
Wagner DL (2020) Insect declines in the anthropocene. Annual Review of Entomology. https://doi.org/10.1146/ANNUREV-ENTO-011019-025151
Wagner DL, Grames EM, Forister ML, Berenbaum MR, Stopak D (2021) Insect decline in the anthropocene: death by a thousand cuts. Proc Natl Acad Sci 118(2):e2023989118. https://doi.org/10.1073/pnas.2023989118
Wallisdevries MF, Van Swaay CAM, Plate CL (2012) Changes in nectar supply: a possible cause of widespread butterfly decline. Current Zoology 58(3):384–391. https://doi.org/10.1093/czoolo/58.3.384
Warren MS, Maes D, van Swaay CAM, Goffart P, Dyck HV, Bourn NAD, Wynhoff I, Hoare D, Ellis S (2021) The decline of butterflies in Europe: problems, significance, and possible solutions. Proceedings of the National Academy of Sciences. https://doi.org/10.1073/PNAS.2002551117
Wiens JJ, Graham CH (2005) Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology Evolution and Systematics. https://doi.org/10.1146/annurev.ecolsys.36.102803.095431
Acknowledgements
We express our gratitude to the following funding providers: Societas pro Fauna et Flora Fennica (to JMK), Otto A. Malm Stiftelse (to JMK), The Finnish Foundation for Nature Conservation: Wärtsilä Oyj Fund (to JMK) and Oskar Öflunds Stiftelse (to JMK). We also thank Jussi Laaksonlaita and Arkipelagia volunteers (Jouko Lehtonen, Heikki Wendelin, Oili Pyysalo & Juha Varrela) for their support with the field surveys. We are grateful to Miguel Baltazar Soares for providing comments on the draft. The comments of two reviewers considerably improved the manuscript.
Funding
Open Access funding provided by University of Turku (including Turku University Central Hospital).
Author information
Authors and Affiliations
Contributions
JMK: Data collection (lead); methodology (supporting); GIS analyses (lead); formal analysis (lead); investigation (equal); visualisation (lead); writing—original draft (lead); writing—review and editing (supporting). MN: Conceptualisation (supporting); methodology (supporting); supervision (supporting); visualisation (supporting); GIS analyses—review (lead) and writing—review and editing (supporting). JEB: Conceptualisation (lead); methodology (lead); supervision (lead); formal analysis (supporting); investigation (equal); visualisation (supporting); writing—original draft (supporting); writing—review and editing (lead).
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kukkonen, J.M., von Numers, M. & Brommer, J.E. Conserving apollo butterflies: habitat characteristics and conservation implications in Southwest Finland. J Insect Conserv (2024). https://doi.org/10.1007/s10841-024-00617-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10841-024-00617-9