Abstract
Habitat size, habitat isolation and habitat quality are regarded as the main determinants of butterfly occurrence in fragmented landscapes. To analyze the relationship between the occurrence of the butterfly Cupido minimus and these factors, patch occupancy of the immature stages in patches of its host plant Anthyllis vulneraria was studied in the nature reserve Hohe Wann in Bavaria (Germany). In 2001 and 2002, 82 A. vulneraria patches were surveyed for the presence of C. minimus larvae. The occurrence was largely affected by the size of the food plant patches. In a habitat model that uses multiple logistic regression, the type of management and habitat connectivity are further determinants of species distribution. Internal and temporal validation demonstrate the stability and robustness of the developed habitat models. Additionally, it was proved that the colonization rate of C. minimus was significantly influenced by the distance to the next occupied Anthyllis patch. Concerning long-term survival of (meta-) populations in fragmented landscapes, the results show that lower habitat quality may be compensated by higher connectivity between host plant patches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In metapopulation biology, habitat size and isolation are assumed to be the most important factors for the occurrence and long-time survival of butterflies in fragmented habitats (Hanski and Gilpin 1991; Thomas et al. 1992; Hanski 1994a). Theoretical (Hanski 1994a; Hanski and Thomas 1994) and empirical studies (Harrison 1991; Hanski et al. 1994; Hill et al. 1996) among Lepidoptera have demonstrated that the greater the patch size and the connectivity to other occupied patches, the higher the colonization probability (Wilcox 1980; Hovestadt 1990; Poethke et al. 1996). Extinction rates of local populations are higher in small and isolated patches.
However, recent studies have demonstrated that habitat quality might also be a good determinant of lepidopteran occurrence and may improve the comprehension of metapopulation dynamics (Kuussaari et al. 1996; Dennis and Eales 1997; Thomas et al. 2001; Wahlberg et al. 2002). For example, Dennis and Eales (1997) asserted that patch occupancy of Coenonympha tullia was as successfully explained by habitat quality as by habitat size and isolation together. In the study of Thomas et al. (2001), habitat quality was the best predictor for the occurrence of three butterfly species in comparison to isolation and patch size. Further, Thomas et al. (1998) showed that within the genus Maculinea, increasing extinction rates due to habitat loss and isolation could be much reduced—in a preconditioned minimum area—if the habitat quality was optimized. According to Thomas et al. (2001), variation in habitat quality is the missing third parameter in metapopulation dynamics beside the conventional spatial parameters of isolation and area.
Habitat models are widely used to specify functional relationships between the occurrence of a species and its environment (Guisan and Zimmermann 2000; Austin 2002) and to quantify habitat requirements (Morrison et al. 1998). In this study, we developed habitat models based on presence–absence data using logistic regression (Trexler and Travis 1993; Guisan and Zimmermann 2000) to test: (1) which of three factors—patch size, patch quality and isolation—best explains species distribution, and (2) to what extent they are responsible for species persistence. The study species, the Small Blue Cupido minimus (Fuessli 1775) (Lepidoptera: Lycaenidae), is widely distributed in Europe, but has declined sharply in many countries during the last decades (Asher et al. 2001). Therefore, the results of our study may enhance our understanding of the (meta-) population biology of C. minimus and help in choosing effective conservation strategies.
To estimate the predictive performance of habitat models, they should be transferred to independent data (Manel et al. 1999; Guisan and Zimmermann 2000; Pearce and Ferrier 2000a, b; Reineking and Schröder 2003). In addition to using internal validation techniques, we tested our habitat models also under different landscape conditions by externally validating the models in space and time.
Methods
Study species
The Small Blue C. minimus colonizes poor, arid and often calcareous grasslands with kidney vetch (Anthyllis vulneraria) as feedstock (Ebert and Rennwald 1991; Weidemann 1995). The larvae feed only on Anthyllis vulneraria in the study area. C. minimus is a xerothermophilic species (Blab and Kudrna 1982) and is univoltine. The flight period in the study area lasts from the beginning of May until the beginning of July.
C. minimus occurs in Europe from Spain to Scandinavia, and also across Asia and Mongolia. While the distribution is assumed to be stable in many European countries, there is a particularly serious decline in north-western Europe (>50% decrease in distribution in 25 years; Asher et al. 2001, pp. 145–146). In Germany, Weidemann (1995) described the decline of this species in many regions during the last decades, and C. minimus is regarded as ‘near threatened’ (Pretscher 1998). Agricultural intensification as well as abandonment are regarded as the main causes for the decline (e.g. Kudrna 1986; Feldmann et al. 2000).
Field work and identification of relevant habitat factors
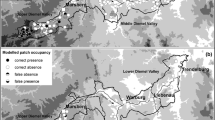
The main study area, about 21 km2, is the nature reserve ‘Hohe Wann’ in Northern Bavaria, Germany (50°03′N, 10°35′E). The mean annual temperature is 8.8°C, and the mean annual precipitation 650 mm (Deutscher Wetterdienst 2002). The region is highly structured through the geological (Trias: Middle Keuper), geomorphological and microclimatic heterogeneity of the landscape. While the leveled areas (plateaus, valleys) are in intensive agricultural use, the slopes are used extensively or are fallow land. Thus, on the one hand, the landscape is characterized by a small-scale mosaic of crop fields, fallow land and intensively managed meadows and on the other hand by poor grassland, thermophilic fringes, hedges and forests.
As the recording of C. minimus is most reliable accredit out by searching for the eggs and larvae in the flower heads of A. vulneraria (Hermann 2000), in 2000 all patches of kidney vetch (n = 82) were mapped in the study area and subsequently plotted in aerial photographs (scale 1:3,500). Anthyllis patches were considered as separate if they were at least 10 m apart. The size of small patches was measured in the field, for larger patches a Geographic Information System (GIS, ESRI ArcView 3.2) was used. During the main flight periods in 2001 and 2002 the incidence of the immature stages of C. minimus was recorded in all Anthyllis patches. If the species was not detected at the first sampling occasion, the patch was searched for a second time at the end of the flight period. Each patch was scanned for eggs and larvae in time periods proportional to its area, with a maximum of 15 min per patch. The patch sizes range from 1m2 to 6,300 m2. Additionally, further parameters of the habitat quality of the Anthyllis patches were recorded in the field: vegetation structure (e.g. plant cover, vegetation height) of the different vegetation strata and of the Anthyllis plants, succession parameters (degree of bush encroachment) and the management regime (four different categories). Insolation parameters are exposition, inclination and scale of shading. The habitat type (three different categories) was detected from a vegetation map (see Table 1 for more details). The complete survey of the Anthyllis sites in the main study area enabled us to calculate patch isolation and connectivity. The distance to the next occupied Anthyllis-patch was used as a simple measure of isolation. Moreover, the connectivity S i according to Hanski (Hanski 1994b; Moilanen and Nieminen 2002) was calculated:
S i = patch connectivity, p j = occupancy of patch i [0 or 1], α = parameter scaling the effect of distance on dispersal success, d ij = distance between patch i and j measured from center to center [km], A j = size of patch j [m2], b = scaling of immigration
Based on the results of Leon-Cortes et al. (2003) and Krauss et al. (2004) on C. minimus in Britain and Germany (Lower Saxony) respectively, as well as on the result of a colonization experiment with two artificial Anthyllis patches in the main study area (Binzenhöfer; unpubl. data) an average dispersal distance of 200 m was estimated. Consequently, we used α = 5 and according to Krauss et al. (2004), b = 1, assuming a proportional rise of emigration rate with increasing patch area.
To test the transferability of the habitat models under different geographical conditions (Dennis and Eales 1999; Schröder and Richter 1999; Schröder 2000; Fleishman et al. 2003), an additional study area with C. minimus occurrence was chosen in 2002: the nature reserve Leutratal near Jena in Thuringia (50°52’N, 11°34’E). This study area (0.5 km2) is characterized by shell-limestone slopes (lower Triassic limestone) of the river Saale valley covered with mesoxerophytic grassland in combination with semi-arid grasslands and thermophile fringes at different successional stages (Heinrich et al. 1998; Hirsch et al. 1998). Normally, the grassland is mown in late summer (not before the end of July) or autumn. The climate is warmer (mean annual temperature is 9.3°C) and dryer (mean annual precipitation 587 mm) compared to our main study in Northern Bavaria (Heinrich et al. 1998).
Using an identical sampling protocol to that used in the main study area, 39 Anthyllis patches were found here, with patch sizes ranging from 1 m2 to 1,500 m2.
Statistical analyses
The relationships between the occurrence of C. minimus and the parameters patch size, connectivity (or isolation) and habitat quality of the patches were analyzed using logistic regression. All statistical analyses were performed with R 2.2.0 (R Core Development Team, http://www.r-project.org) using the packages Hmisc and Design provided by F. Harrell.
Model selection
First, univariate analyses were conducted, in order to examine the importance and relevance of each explanatory variable (Hosmer and Lemeshow 2000) before entering these parameters into a multiple model. Parameters with P-values > 0.2 were excluded from the analysis (Hosmer and Lemeshow 2000). The influence of the remaining variables was quantified by their odds ratios, which is a measure to estimate the effect of a predictor by specifying the ratio of odds between the presence or absence of a species when the value of the explanatory variable is altered by one unit. Further, the number of habitat parameters was reduced to avoid strong multicollinearity between the predictor variables. If there was a strong correlation between two explanatory variables (Spearman rank correlation r s > 0.5, see also Fielding and Haworth 1995; Binzenhöfer et al. 2005), only the parameter that correlated most strongly with the incidence was selected for further modeling. For variable selection prior to estimating multiple models, we applied stepwise backward selection (Hosmer and Lemeshow 2000) with the Akaike Information Criterion (AIC) as a selection criterion (Burnham and Anderson 2002, Reineking and Schröder 2006). Since the biases and shortcomings of stepwise variable selection are known (e.g. Whittingham et al. 2006) we acknowledged model uncertainty by comparing our “final” model with a set of almost equally good models resulting from AIC-based best subset regression (cf. Tables 5 and 6, electronic appendix).
Model evaluation
Different kinds of performance criteria can be used to evaluate a habitat model. Nagelkerke’s (1991) R 2N quantifies the proportion of variance explained by the model. Values exceeding 0.4 indicate a good calibration (Backhaus et al 2000; Steyerberg et al. 2001). Model discrimination describes the ability to correctly separate occupied from unoccupied habitats. Due to the failure to use all information of the classifier (Fielding and Bell 1997), a threshold-independent measure for discrimination was applied, namely the area under the receiver operating characteristic curve (AUC). AUC-values above 0.7 describe an acceptable discrimination, values between 0.8 and 0.9 indicate a good discrimination, and values above 0.9 an excellent discrimination (Hosmer and Lemeshow 2000). For the comparison of different alternative models we used AIC. The model with the lowest AIC represents the best compromise between goodness of fit and the lowest number of predictors (Burnham and Anderson 2002).
Model validation
To test the accuracy and transferability of the final habitat models, we applied internal as well as external validation methods to get an unbiased estimate of model performance (Verbyla and Litvaitis 1989; Guisan and Zimmermann 2000). The models were internally validated by means of bootstrapping with 1,000 bootstrap replicates (Reineking and Schröder 2003, Peppler-Lisbach and Schröder 2004). To judge the quality of the model predictions, independent, external data were used. We collected data in a 2nd year in the main study area and in a second study area. Following Schröder (2000), we applied the significance test according to Beck and Shultz (1986) to verify the transferability in space and time, whereby the evaluation is deemed successful if the AUC-values of the model transferred significantly exceed a critical AUC-value (here: AUCcrit = 0.7; cf. Bonn and Schröder 2001). Because of 100% patch occupancy in the second test area, we were not able to calculate AUC-values and to execute this transferability test for spatial validation. Therefore, we checked the plausibility of estimated occurrence probabilities compared to the prevalence.
Effects of geographical parameters on population dynamics
Based on the 2-year survey of the immature stages of C. minimus, the influence of geographical parameters on the extinction rate and the colonization rate was tested. The effects of the geographical parameters on the colonization and the extinction events were analyzed by logistic regression.
Results
Prevalence and spatial patch characteristics
In the nature reserve Hohe Wann, the prevalence of the pre-imago stages of C. minimus increased from 55% in 2001 to 82% in 2002 (see Table 2), whereas the number of the Anthyllis patches decreased in the same period by about 21% (2001: n = 82, 2002: n = 65). Eleven and four Anthyllis patches were found in 2001 and 2002 respectively with only one or two plants. In both years three of them were occupied with eggs or larvae from C. minimus. Their distances to the next occupied patch were less than 100 m (2001: 11–73 m, 2002: 62–78 m). We detected C. minimus larvae in each of thirteen patches larger than 1000 m2. The mean distance to the next occupied Anthyllis patch was 182 m in 2001 and 203 m in 2002. In 2001 and 2002 the most isolated habitat was 1,025 m away from the next C. minimus population with sizes of 500 m2 and 588 m2 respectively.
In the study area Leutratal, eggs or larvae occupied all 39 patches in 2002. The distance between the patches ranged from 20 to 115 m. The two largest Anthyllis patches measured 1,500 m2, the smallest 1 m2.
Effects of environmental factors on the occurrence of Cupido minimus
Selection and relevance of single parameters
After parameter reduction on the basis of univariate regression analyses and Spearman rank correlations, only six variables remained for further modeling (see Table 3 for significance levels, R 2N and odd ratios). Patch size yielded the highest explanatory power (R 2N = 0.39). The odds ratio of C. minimus nearly doubled for each 100-m2 area of larval food plants. Management regime had also a great influence on species occurrence. The most adequately managed sites are those with extensive shepherding, followed by mown grasslands, cattle-grazed meadows and fallow land. The parameter ‘date of first management’ showed a unimodal response (Fig. 1), whereas the period between middle of July and middle of August resulted in the highest predicted probabilities. Very early farmed sites (before 15th June) and particularly fallow land featured the lowest predicted probabilities. Within the parameter habitat type, the odds ratio was six fold higher for the extensively managed grassland and doubled for the mesoxerophytic meadow in comparison with the thermophilic fringes. Connectivity had a positive effect on the occurrence of C. minimus. Cover of shrub layer showed a negative, but slight influence on occurrence.
In a univariate model, the parameter ‘date of first management’ can be considered either as a numeric or as a categorical predictor. The values of the predictor (x-axis) represent: 1 = until 15th June; 2 = until 15th July; 3 = until 15th August; 4 = after 15th August; 5 = fallow. Black Predicted occurrence probabilities of C. minimus for this variable taken as a categorical predictor, grey unimodal response curve considering the predictor as numeric
Multivariate effects of environmental factors on habitat suitability for Cupido minimus
As habitat suitability is not specified by one parameter alone, a multiple logistic regression analysis was performed to investigate the influence of different combinations of predictors on C. minimus occurrence.
Logistic regression with backward selection resulted in a model that considers the predictors ‘Anthyllis patch size’, ‘connectivity’ and ‘date of first management’ (Table 4; Fig. 2). The performance criteria indicate a good calibration and discrimination of the model after bootstrapping. Patches exceeding sizes of 50 m2 (log-value 1.699) enhance the predicted probability independently of habitat connectivity and date of first management. At sites greater than 1,800 m2 (log-value 3.255), the occurrence probability is 100% (Fig. 2a, c for lowest connectivity). For the highest connectivity value in the main study area (1.04) but for unfavorable date of first management an occurrence probability between 41% and 45% is already predicted for very small sites (10 m2). For optimal date of first management and the highest connectivity value, an occurrence probability of 85% is estimated independently of Anthyllis patch size (Fig. 2b).
Therefore, and as already demonstrated in the univariate models, the factor patch size plays the most important role for explaining C. minimus occurrence. In the multiple model the variance is mainly explained by patch size. Including the other two predictor variables and the interaction term, R²N increased from 0.39 to 0.46 and the AUC-value from 0.784 to 0.853 (Table 4).
Finally, we compared our ‘final’ model to a set of alternative models having a similarly good fit in terms of AIC, which were derived from an all-possible subset procedure. Each of those considered ‘Anthyllis patch size’ and ‘connectivity, but also ‘habitat type’ or ‘management type’ (cf. Tables 5 and 6, electronic appendix). Estimated regression coefficients (and standard errors) resemble the ones presented in Table 4.
Transferability of model results
The generality of the habitat models was tested by transferring them in time. The transferability in time (from 2001 to 2002) of both habitat models was significant (AUC = 0.891 significantly exceeding AUCcrit = 0.7, with P < 0.0001). Validation by spatial model transfer was not possible applying this transferability test, since C. minimus was recorded in all Anthyllis patches in the Leutratal; which corresponds with the high occurrence probabilities (mean: 0.749 ± 0.237 SD, min: 0.307, max: 1) predicted for this area.
Effects of spatial landscape structure on population dynamic processes
The total extinction rate of C. minimus from 2001 to 2002 was 10% (eight extinction events), of which six patches went extinct due to patch eradication and two local C. minimus populations went extinct in persistent patches. The abandoned Anthyllis sites were 263 m and 1,025 m from the next occupied patch and 64 m2 and 500 m2 in size. Due to the small sampling size (only two ‘real’ extinction events), statistical analysis was not feasible.
All in all, 16 (25%) of the persistent patches were (re)colonized for the first time in 2002. Within univariate logistic regression the distance to the next occupied patch was found to affect colonization (P < 0.04, R 2N = 0.21). The most distant patch, which was newly colonized, was 534 m apart from the next occupied patch and 180 m2 in size. Though the influence of patch size on colonization rate was positive, it was not significant (P < 0.09, R 2N = 0.14). No relationship was found between colonization and connectivity (P < 0.76, R 2N = 0.005).
Discussion
Effect of single environmental factors on habitat suitability for Cupido minimus
The influence of habitat quality factors
The target species was restricted to three habitat types: extensively managed meadows, mesoxerophytic grasslands, and thermophilic fringes. In contrast to Ebert and Rennwald (1991), C. minimus was predominantly recorded in extensively managed meadows and not in mesoxerophytic grasslands. This may be explained by the fact that many Anthyllis plants in the study area grew at locations which were cleared of bushes no more than a few years ago. Therefore, many patches are currently just in a transitional stage between extensively managed meadows and mesoxerophytic grasslands. This is because a higher number and cover of typical species of mesoxerophytic grassland still have to immigrate at first, contrary to Anthyllis vulneraria, which is a pioneer on immature soils. Thermophilic fringes are the habitat type with the lowest prevalence in the nature reserve Hohe Wann. This effect could be traced back again to the fact that the cover of bush encroachment due to the extensive management is normally very high in fringes, and this habitat type therefore is suboptimal as larval food plant and probably for the xerothermophilic butterfly, too. The weak, but negative relationship between cover of shrub layer and C. minimus occurrence in the present study also corroborates this effect. Further, population size of C. minimus is negatively correlated with cover of shrub layer as demonstrated by Krauss et al. (2004) in a study near Göttingen in southern Lower Saxony (Germany). These results correspond with the studies of Ebert and Rennwald (1991) and Weidemann (1995), who regard early or initial successional stages as habitats for C. minimus.
The date of first management also influenced the occurrence of C. minimus. The highest predicted probabilities were found at sites first managed between middle of July and middle of August, after the majority of the larvae have already hatched out. Sites farmed very early (until 15th June) and fallow land are most inappropriate, because the former, as a rule, have fertile soils and will be managed several times a year. Thus, the immature stages of C. minimus will be damaged on the larval food plant. Furthermore, as pioneers are weak competitors, Anthyllis vulneraria will be swamped out as a result of strong bush encroachment (see above).
In addition, the type of management explains the species occurrence. C. minimus most strongly benefited from extensive shepherding. Conspicuously less suitable were mown grasslands, followed by cattle-grazed meadows. In agreement with management time the lowest occurrence probabilities were found on fallow grounds. To sum up, pasturing—if possible with avoidance during the development phase of the immature stages—seems to be the most adequate management method for C. minimus, if management does not occur too frequently or too intensively (e.g. with cattle). Under these conditions, on the one hand there still remain sufficient flowering Anthyllis plants for egg deposition or nectar, and on the other hand bare ground will be generated, which again promotes this pioneer plant. Consequently, abandoned farmland negatively affects C. minimus occurrence. The study results correspond greatly with recommendations from other authors. According to Feldmann et al. (2000), C. minimus habitats should not be mown or intensively grazed before the beginning of July. Also, according to Ebert and Rennwald (1991), overgrazing and wrong time-phased (at the development-phase of the immature stages) or repeated mowing per year should be avoided. Asher et al. (2001) advocated an adopted, extensive grazing regime in summer time and recommended pasturing in autumn or winter. From their point of view, periodic ground disturbance may be essential on sites that cannot be grazed, as seeds of Anthyllis vulneraria can only germinate on bare ground. Ebert and Rennwald (1991) as well as Weidemann (1995) also highlight the potential importance of sites without natural cover for Anthyllis settlement and for C. minimus survival (like slopes with sparse vegetation or rural roads). Likewise, in the present study areas of such locations are occupied by C. minimus.
The influence of patch size and habitat connectivity
In the univariate as well as in the multiple models, patch size and habitat connectivity were shown to be important factors for explaining C. minimus occurrence. Patch size explained a large amount of variance in the distribution of C. minimus in the host plant patches of different sizes. Such a positive area-incidence relationship has been found in a number of studies on butterflies (e.g. Wahlberg et al. 1996, Dennis and Eales 1999) and other insects (e.g. Hanski 2001, Biedermann 2003) in fragmented landscapes. In addition, habitat connectivity is influencing the presence of C. minimus. This interconnection is indirectly affirmed by Asher et al. (2001). In their study, the extinction risk of C. minimus populations was strongly increased by isolation of small habitat patches. In our study, the variable with the highest explanatory power was Anthyllis patch size, which is confirmed by the investigations of Krauss et al. (2004). The high importance of food-plant availability, due to the strong dependency of immature stages on the host plants, might be the limiting factor for butterfly distribution (Thomas et al. 2001). According to Krauss et al. (2004), connectivity nevertheless did not play a significant role in determining the population size of C. minimus. The authors found Anthyllis patches isolated up to 4.4 km, but they were occupied by C. minimus. Presumably connectivity plays an important role for the persistence of C. minimus populations whenever the conditions are suboptimal. While our study mainly took place in Keuper soil with only a small proportion of lime, the study of Krauss et al. (2004) was performed in soils of shell-limestone with a high proportion of lime, where Anthyllis vulneraria has its main distribution (Oberdorfer 2001). Thus, the density of food plants and of C. minimus adults is much higher in shell-limestone regions such as the second study area Leutratal. There, but also near Göttingen, all Anthyllis patches were occupied by C. minimus without exception. Our results show that classical metapopulation dynamics (extinction and/or (re)colonization events: 25% of patches re-colonized in 2002) occur when habitats are too fragmented, i.e. when connectivity falls below a certain threshold—a threshold which due to smaller distances wasn’t reached in the test area. These smaller distances were at least partly due to rather good edaphic conditions.
Furthermore, logistic regression analyses conducted by Leon-Cortes et al. (2003, p. 473) confirmed that C. minimus in North Wales usually went extinct when host plants were at low densities. In a mark–release–recapture study of C. minimus in chalk grassland in southern Belgium, Baguette et al. (2000) did not observe butterfly movements between habitat patches which were more than 762 m apart, although within their study exchange could theoretically have been observed with distances between 1,334 m and 2,568 m. Maybe the management between the Belgium habitats is too intensive (mainly fertilized grassland), and consequently dispersal corridors are not available. In general, according to Dennis and Eales (1997), habitat quality and patch size may be more influential factors for butterfly occurrence than isolation. But in areas where site eradication and fragmentation have progressed further, connectivity falls below a certain threshold, and isolation is likely to be a more prominent factor (Dennis and Eales 1999), as is the case in our main research area.
Multivariate effects of environmental factors on habitat suitability for Cupido minimus
Multiple regression analyses resulted in a final habitat model including three significant habitat factors (see Table 4). In addition to the two landscape parameters patch size and connectivity, date of first management was the only factor among all habitat quality predictors which remained in the multiple model presented here. Alternative models with similar performance considered habitat type or management type instead of date of first management, but do not change our interpretation. The preferred land-use type, shepherding, in the study area mostly occurs on poor grasslands (extensively managed meadows or mesoxerophytic grasslands) during an optimal timeframe, and prevents a high shrub cover. Obviously, the date of first management is a good surrogate for the real factors driving habitat quality of C. minimus.
In the final model, Anthyllis patch size, connectivity and date of first management determine the patch occupancy of the study species. Model calibration and discrimination of the model are evaluated as “good”. However, in the multiple model of Krauss et al. (2004) only patch size remained as a predictor. This may be explained by the fact that (1) the habitat factors investigated did not consider the management methods, and (2) the habitat conditions are probably optimal, and hence the isolation effect did not significantly affect occurrence (see above). The relationship between incidence, patch size, connectivity (or isolation) and habitat quality is also known for other butterflies (Thomas and Harrison 1992; Hanski 1994b; Hill et al. 1996; Thomas and Hanski 1997; Dennis and Eales 1999; Gutierrez et al. 2001; Thomas et al. 2001) and other insects (beetles: Roslin and Koivunen 2001; grasshoppers: Kindvall and Ahlen 1992; Appelt and Poethke 1997; Kuhn and Kleyer 1999; leafhoppers: Biedermann 2000, 2004). Patch size affects species presence because the habitat size is correlated with population size, and large populations will become extinct less frequently (Wilcox 1980; Hovestadt 1990; Poethke et al. 1996). Connectivity is important for patch occupancy, as the colonization probability of an unoccupied patch decreases with increasing isolation (Hanski 1994b). According to Leon-Cortes et al. (2003), the number of eggs and larvae of C. minimus is significantly correlated with the number of inflorescences per Anthyllis plant, which is a result of habitat quality. Thomas et al. (2001) have demonstrated that—in addition to isolation and patch size—habitat quality is a major determinant of species survival. The persistence of three different butterfly species was two to three times better explained by variations in habitat characteristics than by site isolation. According to these authors, the three factors operate at different hierarchical levels. While habitat quality contributes more to species persistence, patch size and isolation more strongly influence the recolonization of empty habitats. For instance, patch occupancy and extinction of the butterfly Speyreira nokomis apacheana were best modeled by measures of habitat quality, rather than by patch size and isolation (Fleishman et al. 2002).
If habitat quality is high, small and isolated habitats are also suitable for butterflies (Thomas et al. 2001). This conclusion is affirmed by the investigations of Krauss et al. (2004), who detected 100% patch occupancy of C. minimus in spite of great variances in patch size and isolation. In the two investigation years of the present study, the prevalence of this species constitutes 55% and 82% in the Hohe Wann, probably due to the comparatively unfavorable habitat conditions. In contrast, in the nature reserve Leutratal, with optimal geological formation and soil type for host plant settlement, all potential habitats were occupied. This comparison supports in turn the statement of Thomas et al. (2001) that habitat quality is the third parameter affecting (meta-)population dynamics.
The final habitat model shows a very good transferability in time. In contrast, the high patch occupancy was the reason why spatial validation did not work. However, this fact in particular highlights the general validity of the presented model. Anthyllis vulneraria grows on extensively managed shell-limestone slopes in such high densities that the maximum distance to the next Anthyllis patch is only 115 m, and thus lower than the assumed mean dispersal distance of 200 m. The ubiquity of C. minimus in the nature reserve Leutratal could therefore be ascribed to high habitat quality, large Anthyllis sites and high connectivity.
Population structure and population dynamics of Cupido minimus
The results of our 2-year survey of C. minimus in the main study area indicate a metapopulation structure (Hanski and Gilpin 1991; Hanski and Gilpin 1997; Reich and Grimm 1996). The species occupied discrete host-plant patches separated by non-habitat and showed turnover in its incidence. Furthermore, population dynamics are assumed to be asynchronous, as indicated by the simultaneous occurrence of local extinction and (re)colonization events. In their study of C. minimus in southern Belgium, Baguette et al. (2000) also classified the network of colonies as a metapopulation. Only two of three habitat patches were colonized due to low dispersal ability and high habitat isolation.
In our study, the patch occupancy of C. minimus increased about 25% from 2001 to 2002, although the host-plant distribution decreased by about 21%. Asher et al. (2001) also report large fluctuations of C. minimus populations from year to year, possibly in relation to flowering cycles in the host plant. Leon-Cortes et al. (2003) suggest that food plant dynamics strongly affect C. minimus persistence. The high colonization rate, in spite of the host-plant decline, in our study presumably results from the management history, rather than from recording bias in the previous year. The newly emerging foodplant patches, due to the clearing of bushes a few years ago, were colonized with a delay, since A. vulneraria have to be 1 to 5 years old before they flower (Sterk et al. 1982). As a result of the newly emerged Anthyllis patches, the extinction events of C. minimus are low (3%) in comparison to colonization events. This again supports the statement of Baguette et al. (2000) that conservation of this species implies the creation of more proximate suitable patches.
Contrary to the occurrence probability of C. minimus, no relationship was found between colonization rate and connectivity. However, there was a significant correlation between colonization rate and distance to the next occupied patch. This result may be traced back to the fact that the two attributes integrate different time frames and operate at different levels of population dynamic processes. Colonization is influenced by the regional process of immigration and occurs from one year to the next. On the other hand, patch occupancy is more affected by processes at the local level (like birth and death), or by events dating back some time ago (e.g. management history, climatic disasters).
Connectivity and the distance to occupied patches are crucial factors, which must be considered together with dispersal capabilities of a species in order to build up an adequate habitat network for the persistence of a metapopulation (Baguette et al. 2000). In the main study area, the maximum distance between one C. minimus colony and the next one was 1,025 m. Immigration into habitat patches was observed over distances of several hundred meters (534 m maximum) from occupied patches. In mark–recapture studies in Great Britain some movements over 1 km were detected, and vagrants 17 km from known colonies were recorded (Asher et al. 2001). However, the great majority of C. minimus individuals in a population is very sedentary. Based on a colonization experiment with two artificial Anthyllis patches in the present main study area (Binzenhöfer; unpubl. data) an average dispersal distance of 200 m was estimated. In Southern Belgium, 91% of the marked individuals remained in the same habitat during the flight period (Baguette et al. 2000), and the adults of the mark–recapture experiment in the UK rarely moved more than 40 m (Asher et al. 2001).
Conclusions
Our study demonstrates that Anthyllis patch size had the strongest effect on the occurrence of C. minimus, also when occasionally very small food plant patches were occupied. A further important factor for the occurrence was habitat quality, in which date of first management was especially important. The results show that lower habitat quality may be compensated by higher connectivity between the host plant patches to ensure long-term survival of (meta-)populations in fragmented landscapes.
References
Appelt M, Poethke HJ (1997) Metapopulation dynamics in a regional population of the Blue-Winged Grasshopper (Oedipoda caerulescens). J Insect Conserv 1:205–214
Asher J, Warren M, Fox R, Harding P, Jeffcoate C, Jeffcoate S (2001) The millennium atlas of butterflies in Britain and Ireland. Oxford University Press, Oxford
Austin MP (2002) Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol Model 157:101–118
Backhaus K, Erichson B, Plinke W, Weiber R (2000) Multivariate Analysemethoden - Eine anwendungsorientierte Einführung. Springer, Berlin
Baguette M, Petit S, Queva F (2000) Population spatial structure and migration of three butterfly species within the same habitat network: consequence for conservation. J Appl Ecol 37:100–108
Beck JR, Shultz EK (1986) The use of ROC curves in test performance evaluation. Arch Pathol Lab Med 110:13–20
Biedermann R (2000) Metapopulation dynamics of the froghopper Neophilaenus albipennis (F., 1798) (Homoptera, Cercopidae)—what is the minimum viable metapopulation size? J Insect Conserv 4:99–107
Biedermann R (2003) Body size and area-incidence relationships: is there a general pattern? Global Ecol Biogeogr 12:381–387
Biedermann R (2004) Patch occupancy of two hemipterans sharing a common host plant. J Biogeogr 31:1179–1184
Binzenhöfer B, Schröder B, Biedermann R, Strauß B, Settele J (2005) Habitat models and habitat connectivity analysis for butterflies and burnet moths—the example of Zygaena carniolica and Coenonympha arcania. Biol Conserv 126:247–259
Blab J, Kudrna O (1982) Hilfsprogramm für Schmetterlinge. Kilda, Greven
Bonn A, Schröder B (2001) Habitat model and their transfer for single and multi species groups: a case study of carabids in an alluvial forest. Ecography 24:483–496
Burnham KP, Anderson DR (2002) Model selection and multi-model inference. Springer, Heidelberg
Dennis RLH, Eales HT (1997) Patch occupancy in Coenonympha tullia (Müller, 1764) (Lepidoptera: Satyrinae): habitat quality matters as much as patch size and isolation. J Insect Conserv 1:167–176
Dennis RLH, Eales HT (1999) Probability of site occupancy in the large heath butterfly Coenonympha tullia determined from geographical and ecological data. Biol Conserv 87:295–301
Deutscher Wetterdienst (2002) http://www.klimadiagramme.de/Deutschland/Bamberg2.html
Ebert G, Rennwald E (1991) Die Schmetterlinge Baden-Württembergs - Band 2 Tagfalter II. Ulmer, Stuttgart
Feldmann R, Reinhardt R, Settele J (2000) Bestimmung und Kurzcharakteristik der außeralpinen Tagfalter Deutschlands. In: Settele J, Feldmann R, Reinhardt R (eds) Die Tagfalter Deutschlands - Ein Handbuch für Freilandökologen, Umweltplaner und Naturschützer. Ulmer, Stuttgart, pp 247–369
Fielding AH, Haworth PF (1995) Testing the generality of bird-habitat models. Conserv Biol 9:1466–1481
Fielding AH, Bell J (1997) A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ Conserv 24:38–49
Fleishman E, Ray C, Sjögren-Gulve P, Boggs CL, Murphy DD (2002) Assessing the roles of patch quality, area and isolation in predicting metapopulation dynamics. Conserv Biol 16:706–716
Fleishman E, Mac Nally R, Fay JP (2003) Validation tests of predictive models of butterfly occurrence based on environmental variables. Conserv Biol 17:806–817
Guisan A, Zimmermann NE (2000) Predictive habitat distribution models in ecology. Ecol Model 135:147–186
Gutierrez D, Leon-Cortes JL, Menendez R, Wilson RJ, Cowley MJR, Thomas CD (2001) Metapopulations of four lepidopteran herbivores on a single host plant, Lotus corniculatus. Ecology 82:1371–1386
Hanski I (1994a) Patch occupancy dynamics in fragmented landscapes. TREE 9:131–135
Hanski I (1994b) A practical model of metapopulation dynamics. J Anim Ecol 63:151–162
Hanski I (2001) Spatially realistic theory of metapopulation ecology. Naturwissenschaften 88:372–381
Hanski I, Gilpin ME (1991) Metapopulation dynamics: brief history and conceptual domain. Biol J Linn Soc 42:3–16
Hanski I, Thomas CD (1994) Metapopulation dynamics and conservation: a spatially explicit model applied to butterflies. Biol Conserv 68:167–180
Hanski I, Kuussaari M, Nieminen M (1994) Metapopulation structure and migration in the butterfly Melitaea cinxia. Ecology 75:747–762
Hanski I, Gilpin ME (1997) Metapopulation biology: ecology, genetics and evolution. Academic Press, Toronto
Harrison S (1991) Local extinction in a metapopulation context: an empirical evaluation. Biol J Linn Soc 42:73–78
Heinrich W, Marstaller R, Bährmann R, Perner J, Schäller G (1998) Das Naturschutzgebiet “Leutratal” bei Jena - Struktur- und Sukzessionsforschung in Grasland-Ökosystemen. Naturschutzreport 14, Jena
Hermann G (2000) Methoden der qualitativen Erfassung von Tagfalter. In: Settele J, Feldmann R, Reinhardt R (eds) Die Tagfalter Deutschlands - Ein Handbuch für Freilandökologen, Umweltplaner und Naturschützer. Ulmer, Stuttgart, pp 124–143
Hill JK, Thomas CD, Lewis OT (1996) Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. J Anim Ecol 63:151–162
Hirsch G, Mann M, Müller O (1998) Naturschutzgroßprojekt Orchideenregion Jena - Muschelkalkhänge im Mittleren Saaletal, Thüringen. Natur und Landschaft 73:334–349
Hosmer DW, Lemeshow S (2000) Applied logistic regression. Wiley, New York
Hovestadt T (1990) Die Bedeutung zufälligen Aussterbens für die Naturschutzplanung. Natur und Landschaft 65:3–8
Kindvall O, Ahlen I (1992) Geometrical factors and metapopulation dynamics of the bush cricket, Metrioptera bicolor Philippi (Orthoptera, Tettigoniidae). Conserv Biol 6:520–529
Krauss J, Steffan-Dewenter I, Tscharntke T (2004) Landscape occupancy and local population size depends on host plant distribution in the butterfly Cupido minimus. Biol Conserv 120:359–365
Kudrna O (1986) Aspects of the conservation of butterflies in Europe. Aula, Wiesbaden
Kuhn W, Kleyer M (1999) A statistical habitat model for the blue winged grasshopper (Oedipoda caerulescens) considering the habitat connectivity. J Nature Conserv 8:207–218
Kuussaari M, Nieminen M, Hanski I (1996) An experimental study of migration in the glanville fritillary Melitaea cinxia. J Anim Ecol 65:791–801
Leon-Cortes JL, Lennon JJ, Thomas CD (2003) Ecological dynamics of extinct species in empty habitat networks. 2. The role of host plant dynamics. Oikos 102:465–477
Londo G (1976) The decimal scala for releves of permanent quadrats. Vegetatio 33:1–61
Manel S, Dias JM, Ormerod SJ (1999) Comparing discriminant analysis, neural networks and logistic regression for predicted species distributions: a case study with a Himalayan river bird. Ecol Model 120:337–347
Moilanen A, Nieminen M (2002) Simple connectivity measures in spatial ecology. Ecology 83:1131–1145
Morrison ML, Marcot BG, Mannan RW (1998) Wild-life habitat relationship—concepts and applications. University of Wisconsin Press, Madison
Nagelkerke NJD (1991) A note on general definition of the coefficient of determination. Biometrika 78: 691–692
Oberdorfer E (2001) Pflanzensoziologische Exkursionsflora für Deutschland und angrenzende Gebiete. Ulmer, Stuttgart
Pearce J, Ferrier S (2000a) Evaluating the predictive performance of habitat models developed using logistic regression. Ecol Model 133:224–245
Pearce J, Ferrier S (2000b) An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol Model 128:127–147
Peppler-Lisbach C, Schröder B (2004) Predicting the species composition of mat-grass communities (Nardetalia) by logistic regression modelling. J Veg Sci 15:623–634
Poethke HJ, Gottschalk E, Seitz A (1996) Gefährdungsanalyse einer räumlich strukturierten Population der Westlichen Beißschrecke (Patycleis albopunctata): Ein Beispiel für den Einsatz des Metapopulationskonzeptes im Artenschutz. J Nature Conserv 5:229–242
Pretscher P (1998) Rote Liste der Großschmetterlinge (Macrolepidoptera). In: Binot M, Bless R, Boye P, Gruttke H, Pretscher P (eds) Rote Liste gefährdeter Tiere Deutschlands. Schriftenreihe für Landschaftspflege und Naturschutz 55:87–111
Reich M, Grimm V (1996) Das Metapopulationskonzept in Ökologie und Naturschutz: Eine kritische Bestandsaufnahme. J Nature Conserv 5:123–139
Reineking B, Schröder B (2003) Computer-intensive methods in the analysis of species-habitat relationships. In: Reuter H, Breckling B, Mittwollen A (eds) Gene, Bits und Ökosysteme. GfÖ Arbeitskreis Theorie in der Ökologie 2003, pp 165–182
Reineking B, Schröder B (2006) Constrain to perform: regularization of habitat models. Ecol Model 193:675–690
Roslin T, Koivunen A (2001) Distribution and abundance of dung beetles in fragmented landscapes. Oecologia 127:69–77
Schröder B, Richter O (1999) Are habitat models transferable in space and time? J Nature Conserv 8:195–207
Schröder B (2000) Zwischen Naturschutz und theoretischer Ökologie: Modelle zur Habitateignung und räumlicher Populationsdynamik für Heuschrecken im Niedermoor. Landschaftsökologie und Umweltforschung 35. PhD thesis, TU Braunschweig
Sterk A, von Duykeren A, Hogervorts J, Verbeek EDM (1982) Demographic studies of Anthyllis vulneraria L. in the Netherlands. II. Population density fluctuations, seed populations, seedling mortality and influence of the biocenosis on demographic features. Acta Botanica Neerlandica 24:315–337
Steyerberg EW, Harrell FEJ, Borsboom GJJM, Eijkemans MJC, Vergouwe Y, Habbema JDF (2001) Internal validation of predictive models—efficiency of some procedures for logistic regression analysis. J Clinical Epidemiol 54:774–781
Thomas CD, Harrison S (1992) Spatial dynamics of a patchily distributed butterfly species. J Anim Ecol 61:437–446
Thomas CD, Thomas JA, Warren MS (1992) Distribution of occupied and vacant butterfly habitats in fragmented landscapes. Oecologia 92:563–567
Thomas CD, Hanski I (1997) Butterfly metapopulations. In: Hanski I, Gilpin ME (eds) Metapopulation biology: ecology, genetics and evolution. Academic Press, San Diego, pp 359–386
Thomas JA, Clarke RT, Elmes GW, Hochberg ME (1998) Population dynamics in the genus Maculinea. In: Dempster JP, McLean IFG (eds) Insect population dynamics: in theory and practise. Chapman & Hall, London, pp 261–290
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc Royal Soc Lond B 268:1791–1796
Trexler JC, Travis J (1993) Nontraditional regression analyses. Ecology 74:1629–1637
Verbyla DL, Litvaitis JA (1989) Resampling methods for evaluating classification accuracy of wildlife habitat models. Environ Manage 13:783–787
Wahlberg N, Moilanen A, Hanski I (1996) Predicting the occurrence of endangered species in fragmented landscapes. Science 273:1536–1538
Wahlberg N, Klemetti T, Hanski I (2002) Dynamic populations in a dynamic landscape: the metapopulation structure of the marsh fritillary butterfly. Ecography 25:224–232
Weidemann HJ (1995) Tagfalter: beobachten, bestimmen. Naturbuch, Augsburg
Whittingham MJ, Stephens PA, Bradbury BR, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour? J Anim Ecol 75:1182–1189
Wilcox BA (1980) Insular ecology and conservation. In: Soule ME, Wilcox BA (eds) Conservation biology: an evolutionary-ecological perspective. Sinauer Associates Inc., Sunderland, pp 95–117
Acknowledgments
We thank Zdenek Fric, Ferenc Kassai and Alban Pfeifer for field assistance. Many thanks to two anonymous referees and the editor Yoh Iwasa for their constructive comments on the manuscript. This study is part of the MOSAIK-project, and is financially supported by the German Federal Ministry of Education and Research (BMBF, grant 01LN0007).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
About this article
Cite this article
Binzenhöfer, B., Biedermann, R., Settele, J. et al. Connectivity compensates for low habitat quality and small patch size in the butterfly Cupido minimus . Ecol Res 23, 259–269 (2008). https://doi.org/10.1007/s11284-007-0376-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11284-007-0376-x