Abstract
Insects are under-represented in current assessments of biodiversity loss at global and national scales. Butterflies, and a few other insect taxa, have been used as indicators of biodiversity change and as flagships for conservation, especially in temperate zones, but these groups are typically species-poor and may not be representative of insects as a whole. Macro-moths, on the other hand, are an important component of UK biodiversity, in terms of both species richness and ecosystem function. Moth abundance has decreased significantly in the UK and there is rapidly growing interest in the surveillance and monitoring of macro-moths among amateur and professional scientists. The Moths Count project was instigated to raise awareness of moths, increase moth recording and set up a long-term National Moth Recording Scheme for c.900 species of macro-moth in the UK. Since 2006, Moths Count has promoted moths to millions of people, trained and engaged thousands in recording and collated over eight million species distribution records into the National Moth Recording Scheme. These data, though incomplete at present, are already contributing to assessments of biodiversity loss and to conservation initiatives. We present initial results which show that substantial changes are already evident for macro-moths in the UK. The northern range margins of a sample of 12 species have shifted northwards by 194.8 km on average between 1982 and 2009 (7.8 km year−1 over the 25 years between survey mid-points). Provisional distribution trends (not corrected for variation in recording effort) were calculated for 43 species and the results corroborated both positive and negative population-level changes assessed previously.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Biodiversity loss in the form of species extinction and habitat destruction is now a major global issue. Modern extinction rates among well-documented taxa are several orders of magnitude greater than the long-term average (Pimm et al. 1995; May 2010) and, in conjunction with regional and national biodiversity declines, have led to widespread concern about the sustainability of our planet’s ecosystems and the essential ‘services’ they provide to humankind (Balmford and Bond 2005; Millennium Ecosystem Assessment 2005; Mooney 2010). Political recognition of the threats led to various international governmental targets to halt, or reduce the rate of, biodiversity loss by 2010.
In this context, insects occupy an important but problematic position. Insects form the most diverse animal taxon on earth and provide numerous, critical functions within ecosystems (Kremen and Chaplin-Kramer 2007). Knowledge of extinction rates and changing species richness among insects is, therefore, paramount to an understanding of global biodiversity loss and its implications. However, basic information from which to derive such knowledge is inadequate. The number of insect species is unknown and as many as 90% are yet to be discovered (Thomas 2005). Among described species, baseline information about extent of distribution, abundance and ecological requirements is scant and biased heavily towards temperate biomes. Only a tiny proportion of known insect species have been evaluated to assess their risk of extinction (Baillie et al. 2004) and few insect extinctions have been documented, although many are suspected (Dunn 2005). Global assessments of the biodiversity impacts of climate change have been undertaken with little reference to insects (Parmesan and Yohe 2003; Thomas et al. 2004a).
In spite of these substantial impediments, considerable efforts have been made in many countries, particularly in Europe, to develop surveillance and monitoring of insects (de Heer et al. 2005; van Swaay et al. 2008). In a few cases, notably in the United Kingdom, longer time-series of data are available (Thomas 2005; Conrad et al. 2007). The UK Butterfly Monitoring Scheme (Pollard and Yates 1993; Roy et al. 2007; Oliver et al. 2010), Rothamsted Insect Survey light-trap network (Woiwod and Harrington 1994; Conrad et al. 2004) and the Rothamsted Insect Survey suction-trap network (Benton et al. 2002; Shortall et al. 2009) have provided important results from intensive, fixed-point monitoring of insect populations. In Britain and Ireland, species surveillance, or biological recording, is a long-standing popular pastime with many thousands of members of the public engaged in a wide variety of local and national schemes run mainly by non-governmental organisations. Thus, considerable UK datasets exist for certain ‘charismatic’ insect taxa, such as butterflies, dragonflies, hoverflies and ladybirds. In the best case scenario, that of butterflies, these data have been utilised to calculate temporal trends in range size (Warren et al. 2001; Fox et al. 2006a), population size (Pollard and Yates 1993), phenology (Roy and Sparks 2000), shifts in range margins (Hill et al. 2002) and patterns of species richness (Menéndez et al. 2006) and community composition (González-Megías et al. 2008). Perhaps most significantly, it has proved possible to directly compare biodiversity loss of an insect group (butterflies) with vertebrates (birds) and vascular plants at a national scale (Thomas et al. 2004b). This study revealed significantly greater losses (population extinctions) and national extinctions among butterflies than in the other taxa, adding to concern that, without including insects, current assessments severely underestimate biodiversity loss.
Almost all insect taxa that have been monitored effectively have relatively low species richness. It is clearly advantageous to have trends on a few insect taxa rather than none, but are the declines of butterflies (Warren et al. 2001) or the northward spread of dragonflies (Hickling et al. 2005) in Britain representative of other insects, at the national or global scale? A considerable recent literature exists on the use of butterflies and other taxa as indicators, but little consensus has emerged (e.g. Ricketts et al. 2002; Kremen et al. 2003; Maes and Van Dyck 2005; Thomas 2005; Fleishman and Murphy 2009). A preferable approach would be to directly assess larger numbers of insect species.
There is one species-rich insect taxon, the moths, with potential for comprehensive surveillance and monitoring in the UK and elsewhere, in order to assess biodiversity change beyond estimates based on small ‘charismatic’ insect groups and vertebrates. Here we report on the development of national surveillance for the macro-moths in the UK, undertaken as part of the Moths Count project run by Butterfly Conservation. We provide some initial findings relating to increases and decreases in species occurrence and the first quantitative assessments of northward range margin shifts for macro-moths.
Macro-moths are uniquely placed for surveillance to better understand biodiversity change. The macro-moths comprise a relatively large and ecologically diverse group of insects in the UK (some 900 species in 19 lepidopteran families: Hepialidae, Cossidae, Zygaenidae, Limacodidae, Sesiidae, Lasiocampidae, Saturniidae, Endromidae, Drepanidae, Thyatiridae, Geometridae, Sphingidae, Notodontidae, Thaumetopoeidae, Lymantriidae, Arctiidae, Ctenuchidae, Nolidae and Noctuidae), an order of magnitude increase in species richness over the butterflies or dragonflies. Macro-moths are representative of almost all terrestrial biotopes, have a relatively stable taxonomy, and most are readily identified. There is a large UK workforce of skilled amateur moth recorders, at least 2,000 strong, and evidence that this number is increasing rapidly (Fox et al. 2005). A questionnaire of 1,032 moth recorders, carried out in 2003/04, found that 36% of respondents had been recording moths for less than 4 years (Spalding et al. 2005). The UK’s moth recorders and their predecessors have generated a substantial dataset of historical information that, if collated and computerised, could provide a baseline against which to assess distribution change for each species.
There is already a long-standing national monitoring network for macro-moths, in the form of the Rothamsted Insect Survey light-trap network (Woiwod and Harrington 1994). Recent analysis of 35 year (1968–2002) population trends from the Rothamsted Insect Survey highlighted significant declines in the overall abundance of nocturnal moths in Britain and found that 66% of 337 widespread and common macro-moth species studied had declined (Conrad et al. 2006). Declines have also been reported from The Netherlands (Groenendijk and Ellis in press). Evidence of such widespread declines, as well as national-level extinctions (Parsons 2010) and highly threatened species (Parsons 2004), demands a conservation response. A national distribution recording scheme for macro-moths will provide essential knowledge of species occurrence in the landscape, underpinning almost all initiatives to reverse biodiversity loss or conserve species. It will also enable assessment of hundreds of less common macro-moth species not included in the Rothamsted population trend analysis. This paper describes the development of such a scheme and some initial results.
Methods
The Moths Count project
The use of volunteers to collect biodiversity surveillance and monitoring data has a long history in the UK. This approach has been validated by the numerous research and policy outputs emanating from such schemes, but also by recent research (e.g. Newman et al. 2003; Schmeller et al. 2009). Butterfly Conservation devised a four-year project, Moths Count, to develop a national surveillance system for macro-moths in the UK, Isle of Man and Channel Islands (the National Moth Recording Scheme, NMRS) that would harness the skills and effort of existing volunteer moth recorders, collate historical data sources, and recruit and train new volunteers. The willingness of the existing moth recorders to support the project was determined in advance by use of questionnaires, focus group meetings and promotional articles. The Moths Count project was led by Butterfly Conservation, but was an active partnership of many national and local organisations (including government agencies), businesses and individuals. Funding to run the project was secured from a variety of public and charitable sector sources, with the majority coming from the Heritage Lottery Fund, as well as private and business sector donations. The total project budget was over £1 million, with a team of five full-time staff over 4 years.
Promotion, feedback and training of volunteer scientists
The experience of organisations and professionals co-ordinating volunteer-based surveillance and monitoring programmes is that feedback to participants is vital to maintain motivation. This has also been shown in research, which demonstrated the high value placed by volunteers on the social aspects of such work (Bell et al. 2008; Kühn et al. 2008). The Moths Count project invested a considerable portion of staff time and budget expenditure in outreach work, designed to promote awareness of and interest in moths and moth recording, training and feedback.
Specific targets of the outreach work included holding 90 ‘public moth events’, informal opportunities for the public to experience moth recording in the company of skilled volunteers, improving the profile of moths in the media, producing accessible education materials (leaflets and websites) and developing simple ‘citizen science’ surveys that could be undertaken by the public with no training or equipment. As volunteer involvement in the study and recording of moths prior to the Moths Count project appeared to be heavily biased toward older men, there was also an intention to engage more women and young people.
The project objective for training was to provide a framework of learning opportunities to help members of the public progress from complete beginners through to knowledgeable moth recorders, able to contribute high quality data to the NMRS in order to inform biodiversity conservation policy and practice. A target programme of 100 training courses was set, each to be led by a professional tutor and with continual monitoring of the quality of training provision (via feedback questionnaires completed by course participants). Increasing the number of active moth recorders and their level of expertise provides a direct benefit to the NMRS by increasing surveillance coverage at both national and local levels, but also leads to the establishment of sustainable, locally-based volunteer networks to continue recording in the future.
Substantial project resources were allocated to feedback for actual or potential participants in the NMRS. Volunteers study moths for a variety of personal reasons including enjoyment, relaxation and intellectual stimulation. However, they are generally willing to participate in surveillance and monitoring schemes, such as the NMRS, if the rationale behind the activity is made clear. Indeed, many gain additional motivation and satisfaction from helping to produce utilitarian benefits such as biodiversity conservation (Bell et al. 2008). The Moths Count project held regular moth recorders’ conferences in each of the four UK nations, providing opportunities for two-way, face-to-face feedback between project staff, partner organisations and volunteer recorders, as well as informal social networking between individuals. A project website, email newsletter and magazine-style annual project newsletter were also used to provide feedback, in addition to direct contact between project staff and volunteers. This wide variety of media and approaches ensured that volunteers were involved in the project, received accurate and timely information about progress and understood the linkages between the NMRS and wider policy and conservation initiatives.
The National Moth Recording Scheme
The main aim of the Moths Count project was to develop a National Moth Recording Scheme through which spatially and temporally referenced macro-moth distribution records, both past and future, could be collated into a comprehensive, accurate and accessible database. The NMRS was developed to cover the c.900 macro-moth species present within the geographical area of the UK, Isle of Man and Channel Islands. The NMRS contributes to the long-term conservation of moths and their habitats, by making available the data needed to implement effective conservation policies and action at the local, regional, national and international levels by members of the public, communities, and public, private and charitable sector organisations (e.g. by shaping policy, informing decisions and contributing to public awareness and scientific knowledge).
The project planned to mobilize the experience and expertise of the existing moth recording community to create a successful and highly cost-effective recording network that could be sustained in the long-term. The NMRS was developed, therefore, using a hierarchical approach, which has worked successfully for other national surveillance and monitoring schemes: members of the public and volunteer recorders pass their moth observations to volunteer county moth recorders and, in due course, collated, computerised and verified county datasets of moth records are passed to the NMRS database. Feedback was disseminated effectively in the opposite direction, from the Moths Count project team to county moth recorders and then to volunteer recorders, local communities and wider audiences.
Commencing in 2007, a network of county moth recorders was established to form the backbone of the NMRS. Some of these highly-skilled volunteers were already in place at the outset of the Moths Count project, others had to be recruited. A national database was established by Butterfly Conservation and guidance provided to county moth recorders. The first county dataset of macro-moth records was incorporated into the NMRS database at the end of 2007 and it has grown rapidly since then (see Results). It is not possible at this early stage in the NMRS to undertake full analysis of every macro-moth species. However, here we present some preliminary analyses utilising the NMRS database, and of relevance to issues of biodiversity loss and climate change.
Northward shifts in range margins of British macro-moths
With much recent evidence of poleward range margin shifts among terrestrial organisms (e.g. Parmesan et al. 1999; Hickling et al. 2006; Hitch and Leberg 2007; Huntley et al. 2008), we examined the distribution data in the NMRS for 12 species of macro-moth, each with a southern distribution in Great Britain. These species were not chosen at random or systematically. Anecdotal evidence from the ‘grey’ literature, suggested that each of these species had moved northwards, but change in the northern range margins of these moths had not been assessed quantitatively. Eight of the macro-moths selected were long-term resident species in Great Britain, with populations supported by native host plants. Four of the species, in contrast, colonised Britain during the twentieth century and are wholly or largely reliant on alien host plant species. Blair’s shoulder-knot Lithophane leautieri and cypress pug Eupithecia phoeniceata use non-native cypress trees and shrubs in the family Cupressaceae as larval host plants, varied coronet Hadena compta primarily utilises sweet William Dianthus barbatus and toadflax brocade Calophasia lunula uses the naturalised garden-escape purple toadflax Linaria purpurea (as well as native plants, including common toadflax L. vulgaris and pale toadflax L. repens).
We calculated the position of the species’ northern range margin in Great Britain (i.e. excluding Northern Ireland, the Isle of Man and Channel Islands) in two separate time periods (1960–1982 and 1983–2009), corresponding to previous survey activity. In each time period, the range margin was determined as the average latitude of the 10 most northerly occupied 10 km × 10 km grid squares of the Ordnance Survey National Grid of Great Britain (following the method developed by Hill et al. 2002). The shift in range margin for each species was calculated as the difference (in km) between the locations of the range margin in the two time periods.
The NMRS database was used to determine range margins in the 1983–2009 period. For the earlier period the maps published in Heath and Emmet (1983a, b) were used, where possible. However, for the three Geometridae species selected (Eupithecia phoeniceata, dwarf cream wave Idaea fuscovenosa and least carpet I. rusticata) no such maps have been published, so recently-computerised, historical data from the Biological Records Centre were used via www.nbn.org.uk.
Distribution change of UK macro-moths
A key aim of the NMRS is to determine distribution trends over time for all macro-moth species. It is premature to undertake this large-scale analysis at present as the spatial and temporal coverage of the NMRS database is insufficient. Datasets of this kind are also subject to a variety of biases stemming from the opportunistic nature of recording and substantial variation in observer effort over time and space (see Discussion). These need to be accounted for in a full analysis of macro-moth distribution trends over time. However, with these caveats, the data gathered to date can be used to examine preliminary trends.
We selected 40 macro-moth species to examine distribution change from the provisional NMRS data. These were chosen from the species showing the greatest increases and decreases in population index based on the analysis of Rothamsted Insect Survey data in Conrad et al. (2006). Strong positive correlation has been shown for UK butterflies between population trends and distribution trends (Warren et al. 2001), and we hypothesise that the same relationship will hold for macro-moths. We cannot yet test this hypothesis, but examining patterns of distribution change at the extremes of recorded population trends may be indicative. We also assessed distribution change for three species that were considered stable. Two of these, scorched wing Plagodis dolabraria and flame shoulder Ochropleura plecta, had extremely low annual change rates in the 35 year population trends calculated by Conrad et al. (2006). The third ‘stable’ species, large yellow underwing Noctua pronuba, is a ubiquitous species found commonly throughout Britain and Ireland. The actual distribution of this species cannot increase substantially (as it already occurs almost everywhere), so any change in the recorded distribution will give information about bias in the NMRS database.
Distribution change was examined from the NMRS database. Extent of distribution for each of the 43 species was determined as the number of 10 km × 10 km grid squares in which the species was recorded at least once in a given time period. Two time periods were used, pre-2000 and 2000 onwards. These periods were chosen with reference to over-representation of recent moth records in the NMRS (see Results). The proportional change in distribution was calculated as the number of occupied grid squares 2000 onwards minus the number of pre-2000 occupied squares, divided by the number of pre-2000 occupied squares.
Results
Promotion, feedback and training of volunteer scientists
These aspects of the project were successful. The UK media, which had previously reflected the generally negative attitude of the public towards moths (especially in contrast to butterflies, New 2004), took interest in Moths Count from the outset. The project achieved seven appearances on national television and 18 on national radio, as well as 32 articles in national newspapers and over 60 articles in popular magazines. In one memorable newspaper headline, moths were described as the “Glory of our gardens” (Daily Mail 21 June 2008) to over two million readers. Journalists and producers now actively seek moth-related news and feature items, a sea change in attitude for which the Moths Count project can take some credit.
The public was also actively engaged. Ninety-five public moth events were held across the UK (2007–2009) attended by a total of 2,010 people and involving many partner organisations. At least 30% of participants were women and over a quarter were under 16. Over 100,000 leaflets were distributed and the project website received 17,500 hits in its first 8 months of operation (July 2009–March 2010). Simple online surveys of widespread and easily identified moths (e.g. humming-bird hawk-moth Macroglossum stellatarum) also proved effective. In 2009, over 2,300 people contributed to these surveys (www.butterfly-conservation.org).
Training courses were run throughout the UK and exceeded the project target. By the end of 2009, 110 training events had been held as part of the Moths Count project, attended by 1,481 people. The quality and appropriateness of the training provided was highly rated by participants. Feedback questionnaires revealed a mean annual satisfaction score of 95%. Many training courses focused on UK Biodiversity Action Plan priority species, including slender scotch burnet Zygaena loti, chalk carpet Scotopteryx bipunctaria, straw belle Aspitates gilvaria, narrow-bordered bee hawk-moth Hemaris tityus, marsh moth Athetis pallustris and four-spotted Tyta luctuosa among others, thus contributing towards conservation targets for these threatened species.
The National Moth Recording Scheme
The first local dataset of approximately 161,000 records (unique combinations of species occurrence, spatial location and date) was imported into the NMRS database in December 2007. Just over 2 years later, by March 2010, the NMRS comprised 8.2 million distribution records of macro-moths, making it the largest dataset for any animal taxon on the UK’s National Biodiversity Network Gateway (www.nbn.org.uk). Ninety-three percent of counties had contributed datasets to the NMRS and the project aims to achieve complete county-level coverage during the first half of 2010.
At this stage the NMRS database shows a strong bias towards recent records (Fig. 1). The records spanned the period 1769 (a record of death’s head hawk-moth Acherontia atropos) to 2009, but 61% of records were from the period 2000–2009 and only 9% were pre-1980. The relative paucity of data for 2008 and 2009 in the NMRS reflects time lags in the flow of records from field recorder, via county moth recorder, to the national scheme.
The bias towards modern records is in part a reflection of increasing recording effort over time, but is also an artefact of NMRS policy. Guidance given to county moth recorders prioritised recent records, recommending that they worked through moth records progressively backwards in time. The representation of historical records in the NMRS will increase (although the relative proportion may not), as county recorders alleviate backlogs of recent data and move onto older records. Furthermore, substantial centralised datasets of historical records were repatriated to county recorders by the Moths Count project during 2009 and 2010, and these will enter the NMRS database in due course.
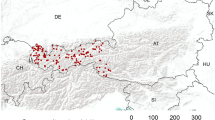
Overall geographical coverage in the NMRS database was already comprehensive by March 2010 (Fig. 2). The aim of any such scheme is to determine the actual distribution of species in the landscape rather than simply mapping the location of active recorders. The widespread coverage of data collated into the NMRS in the short period of time since the recording scheme commenced, gives confidence that robust assessments of species’ status and trend will be possible in the future.
Map of Britain and Ireland showing coverage of all macro-moth records (1769–2009), at 10 km grid square resolution, in the National Moth Recording Scheme database at March 2010. Major gaps represent local datasets that have yet to be incorporated into the database. The Republic of Ireland is not included in the NMRS
Northward shifts in range margins of British macro-moths
Each of the 12 macro-moth species selected for assessment of range margin change showed a substantial northward shift (mean = 194.8 km, n = 12) between the two date periods (25 years between mid-points) (Table 1). This equates to a mean annual shift of 7.8 km year−1 over the 25 years between survey mid-points. The mean range margin shift for macro-moths utilising native host plants (198.8 km, 8.0 km year−1, n = 8) was not significantly different from that for those using alien host plants (mean = 187.0 km, 7.5 km year−1, n = 4) (T-test, t = 0.28, P = 0.78). Maps showing the northward spread of some of these species are given in (Fig. 3).
Maps of selected macro-moth species that show substantial northward shifts of range margin (Table 1). Black dots indicate all 10 km grid squares with records of the species 1960–1982; grey dots indicate 10 km grid squares with only 1983–2009 records of the species in the NMRS database. Species are as follows: a red-necked footman Atolmis rubricollis, b shuttle-shaped dart Agrotis puta, c vine’s rustic Hoplodrina ambigua, d buff footman Eilema depressa, e red underwing Catocala nupta, f dingy footman Eilema griseola, g Blair’s shoulder-knot Lithophane leautieri, h toadflax brocade Calophasia lunula, i varied coronet Hadena compta. Species a–f utilise native larval host plants, while species g–i wholly or partially utilise alien host plant species. It should be noted that the data shown on these maps, drawn from the NMRS database, are not identical to those used to assess range margin shift, taken from Heath and Emmet (1983a, b)
Distribution change of UK macro-moths
A simple assessment of species distribution trend from the NMRS database revealed large-scale changes between the pre-2000 and 2000 onwards recording periods (Table 2). Each of the 20 macro-moth species selected because of large population index increases in Conrad et al. (2006) showed a positive distribution trend (mean = 64%, n = 20). The three species expected to indicate stability also showed substantial distribution increases (mean = 46%, n = 3). In contrast, the 20 species selected as examples of macro-moths with the greatest population declines in Conrad et al. (2006), had an overall decrease in distribution (mean = −14%, n = 20). Maps illustrating the distribution decreases for double dart Graphiphora augur and v-moth Macaria wauaria are shown in Fig. 4. Nevertheless, five species in this group had increased in distribution (but see discussion).
Maps showing distribution decreases of two macro-moth species in the UK: a V-moth Macaria wauaria, 77% decrease in occupied squares, b double dart Graphiphora augur, 48% decrease. Solid dots indicate all 10 km grid squares with 2000–2009 records of the species in the NMRS database; open dots indicate 10 km grid squares with only pre-2000 occurrence records of the species
Discussion
Moths are often regarded as the poor cousins of butterflies. This attitude is especially prevalent among the public and in the media, but is also encountered among scientists and policy makers, where butterflies are firmly established as model organisms for research (Watt and Boggs 2003) and as flagships and indicators for biodiversity conservation (Samways 2005; van Swaay et al. 2008). Yet moths, because of their diversity and abundance, are of much greater functional significance in ecosystems (Proctor et al. 1996; Vaughan 1997; Wilson et al. 1999; Wickramasinghe et al. 2004; Fox et al. 2006b).
We have shown, through the Moths Count project, the potential to challenge negative attitudes successfully in the media and engage large numbers of the public in the study of macro-moths. We have also, in a short space of time, collated a substantial time-series of species occurrence data at the national scale in the UK. Despite the existence of a population monitoring scheme (the Rothamsted Insect Survey) covering macro-moths, these new data, collated from thousands of volunteers through the NMRS, are of great importance for biodiversity conservation. Robust quantitative trends in distribution over time have not been published for any species-rich terrestrial invertebrate taxon in the UK (although they have for marine zooplankton e.g. Beaugrand and Reid 2003; Beaugrand et al. 2009), but the NMRS will enable this to be done in the near future for c.900 species of macro-moth. Furthermore, the detailed spatial information in the NMRS can be applied in numerous ways to target and implement measures to reduce and reverse biodiversity loss.
Each of the 12 macro-moth species we examined for range margin change showed a strong northward shift. This was to be expected since we had selected them, from a pool of some 900 species, specifically because northward colonisation had been reported in personal communication, the ‘grey’ literature and recent field guides (e.g. Waring et al. 2009). Nevertheless, these range margin shifts have now been measured quantitatively and they add to and support the growing body of evidence for poleward shifts in species’ distributions during a period of climatic warming (Parmesan and Yohe 2003). Butterflies have been particularly well-studied in this context both in Britain (Hill et al. 1999; Warren et al. 2001; Hill et al. 2002; Willis et al. 2009) and elsewhere (Parmesan et al. 1999; Crozier 2003, 2004; Mitikka et al. 2008; Pöyry et al. 2009). However, the total numbers of species assessed remain relatively low. The most inclusive of these analyses identified northward shifts in the range margins (either northern or southern limits of range) for only 36 species of butterflies (Parmesan et al. 1999). In Britain, Hill et al. (2002) found that 11 of 46 southerly distributed butterflies had extended their range northwards. Thus, in our provisional, selective and incomplete assessment of macro-moth range margins, we have identified more northward shifts than have been previously identified for butterflies in Britain. Hickling et al. (2006) conducted a range margin assessment for 329 species in 16 taxonomic groups in Britain, including 177 insect species in eight taxa (not including moths). The northern range margins of a total of 275 species moved northwards, including 151 insects (CD Thomas pers. comm.). When, in due course, we analyse range margin shifts for all southerly distributed macro-moths in Britain, the results may be expected to add significantly to the total number of insect examples currently in the literature.
The range margin shifts recorded for macro-moths in this study are towards the upper end of measurements from British butterfly studies. The 170 km northward shift (8.1 km year−1) of the comma butterfly Polygonia c-album (between the periods 1970–1982 and 1995–1999) was the only species to exceed 100 km in the study by Hill et al. (2002). The common darter dragonfly Sympetrum striolatum, on the other hand, had shifted 346 km (13.8 km year−1) northwards in Britain (between 1960–1970 and 1985–1995), and two other Odonata species by almost 200 km, rivalling the range extensions we recorded for Atolmis rubricollis (15.7 km year−1) and Lithophane leautieri (14.6 km year−1) (Hickling et al. 2005).
Distribution change over the 25 year period between the mid-points of sampling periods 1960–1982 and 1983–2009, was assessed for 40 macro-moths likely to have shown substantial change and three further species expected to have changed little. Measuring distribution change over time from recording scheme datasets is problematic. In most such schemes, including the butterfly recording scheme for Britain and Ireland (Fox et al. 2006a), species occurrence records are collected opportunistically by volunteer recorders, with little direction and no random or systematic sampling of the landscape. It has been established that such data may contain bias emanating from uneven geographical recording effort (e.g. as a result of access and favoured ‘hot spot’ areas) (Dennis and Thomas 2000), differences in the apparency of species due to size, colouration and behaviour (Dennis et al. 2006), uneven recording over time (Dennis et al. 1999; Dennis 2001) and issues of scale (Thomas and Abery 1995). These causes of bias have not been investigated in detail in the NMRS database, although the issue of uneven recording over time is clear (Fig. 1).
In light of this, the distribution change analyses reported in this paper must be viewed tentatively, as early indications of patterns of change from a dataset that is still being developed (particularly with regard to the historical baseline). We would expect species to show increases in distribution over time simply as a result of the strong bias of records towards the modern period, which has not been accounted for in our analysis. The positive distribution trends (mean = 46% increase) measured for the ‘stable’ species Plagodis dolabraria, Ochropleura plecta and Noctua pronuba support this hypothesis and are indicative of what might be expected for species whose distributions have not actually changed over time. This, therefore, gives greater confidence that the distribution declines presented here are qualitatively correct and likely to be conservative estimates of change. All but one of the 20 species with large population declines (as well as eight of the species with large population increases) had distribution changes below this 46% threshold and are likely to have declined in real terms.
Despite the provisional nature of this analysis, it is clear that some macro-moth species have undergone substantial changes in distribution, both positive and negative, within the UK. Caution must be exercised in interpreting the results, but the distribution increases and decreases of macro-moths assessed here are in keeping with trends found in other studies in the UK and elsewhere. All of the moths for which distribution change was calculated are considered widespread and common species (a prerequisite of inclusion in the Rothamsted population trend study, Conrad et al. 2004). The greatest decreases measured here are comparable with the most rapid declines recorded among highly threatened butterflies species in Britain (Fox et al. 2006a). Macaria wauaria (77% decrease) compares to the 79% decline of high brown fritillary Argynnis adippe over a similar period, while Graphiphora augur (48% decrease) and Dasypolia templi (46% decrease) compare to the 46% decline of marsh fritillary Euphydryas aurinia. Both these butterflies are listed as priority species in the UK Biodiversity Action Plan and assessed as Critically Endangered and Vulnerable, respectively, in the Red List of British butterflies (Fox et al. 2010).
Conrad et al. (2006) reported population declines in 66% of a sample of 337 widespread macro-moth species in Britain, with 21% (71 species) decreasing rapidly (>30% 10 year−1). Overall decreases in moth biodiversity have also been reported from The Netherlands (Groenendijk and van der Meulen 2004; Groenendijk and Ellis in press) and Finland (Mattila et al. 2006, 2008). Warren et al. (2001) found that the distributions of 74% of 46 butterfly species had declined in Britain since the 1970 s, while Fox et al. (2006a) reported decreases for 76% of 54 resident British butterflies, 14 of which had lost >40% of their distribution since the 1970 s. The butterfly faunas of Flanders (northern Belgium) and The Netherlands appear to have fared even worse (Maes and Van Dyck 2001; Van Dyck et al. 2009), while van Swaay et al. (2006, 2010) reported overall decreases of butterfly distributions at the European scale.
Simultaneously, other species have increased their distributions, often, but not exclusively, including a shift in range margin. The buff footman Eilema depressa, least carpet Idaea rusticata and dingy footman E. griseola, for example, have each doubled their recorded distribution and spread northwards by approximately 100 km. Whilst the distribution trends presented in this paper are by no means an exhaustive treatment, they do provide tentative support for a correlation between changes at the population level and distribution level, as has been shown previously for British butterflies (Warren et al. 2001).
The development of analytical methods for assessing change from biased datasets of species occurrence records is an area of intense research activity at present. Approaches dealing with static ‘snap-shot’ distribution data (e.g. Wilson et al. 2004) and time-series data (e.g. Maes and van Swaay 1997; Telfer et al. 2002; Dennis and Shreeve 2003; Thomas et al. 2004b; Fox et al. 2006a; Menéndez et al. 2006) provide a rich resource for full analysis of UK macro-moths once gross geographical and temporal coverage issues have been resolved.
Little research has been undertaken as yet into the causes of change in the extent and range margins of macro-moths in Britain. Climate has been implicated in the distribution decline of the garden tiger Arctia caja in Britain (Conrad et al. 2002, 2003), in the pattern of immigration to Britain (Sparks et al. 2005) and in altitudinal shifts of tropical moths in Borneo (Chen et al. 2009). Both the range margin shifts of the eight macro-moths that use native host plants and some of the distribution increases examined here are consistent with responses to recent climate change. However, there may be other causal factors. Four of the species examined for range margin shift utilise alien host plants exclusively or primarily. Lithophane leautieri, Calophasia lunula, Eupithecia phoeniceata and Hadena compta have colonised Great Britain and extended their range margins as a result of the widespread cultivation of their host plants, principally in domestic gardens. The potential influence of human planting of alien host plants can also be seen among the group of moths showing substantial increases in distribution size. For example, Thera britannica, Panolis flammea, Deileptenia ribeata and T. juniperata each make use of alien conifer species, which have been widely planted in commercial forests and domestic gardens.
Habitat loss or degradation is a likely causal factor in distribution decreases, as has been found for biodiversity worldwide. For example, Merckx et al. (2009) found positive effects of landscape features including hedgerow trees and wide, uncultivated field margins on macro-moth abundance and diversity in British farmland. Macro-moth population data from the Rothamsted Insect Survey also provide evidence of declines in response to agricultural improvement (Woiwod and Harrington 1994).
Furthermore, the trends we present here hint at causal factors relating to pollution. Several species expanding their distributions and range margins (e.g. Eilema depressa, E. griseola and Atolmis rubricollis) use lichens and algae as larval hosts, raising the potential of air quality as a driver of recent increases. In contrast, decreasing species such as Macaria wauaria and Eulithis mellinata utilise currants Ribes spp. and may be impacted by increased use of pesticides. Finally, light pollution should not be ruled out as a potential cause of biodiversity loss (Eisenbeis 2006; Frank 2006), although research in this area is urgently required (Sutherland et al. 2006).
Instigated by the Moths Count project, the National Moth Recording Scheme has, in just 27 months, collated one of the UK’s largest datasets of species’ occurrence records and one that will continue to grow rapidly. These data will be critical to understand the rapid changes taking place in populations, ranges and ecological communities, and to halt and reverse the biodiversity losses that increasingly threaten human welfare. Political, as well as scientific, commitment is needed and, therefore, public concern and support is important. The Moths Count project has engaged successfully with both politicians and the public, raising awareness of the issues among millions of people via the media and involving thousands directly in moth recording for conservation in the UK.
References
Baillie JEM, Hilton-Taylor C, Stuart SN (2004) 2004 IUCN Red List of threatened species. A global species assessment. IUCN, Gland and Cambridge
Balmford A, Bond W (2005) Trends in the state of nature and their implications for human well being. Ecol Lett 8:1218–1234
Beaugrand G, Reid PC (2003) Long-term changes in phytoplankton, zooplankton and salmon related to climate. Glob Change Biol 9:801–817
Beaugrand G, Luczak C, Edwards M (2009) Rapid biogeographical plankton shifts in the North Atlantic Ocean. Glob Change Biol 15:1790–1803
Bell S, Marzano M, Cent J, Kobierska H, Podjed D, Vandzinskaite D, Reinert H, Armaitiene A, Grodzińska-Jurczak M, Muršič R (2008) What counts? Volunteers and their organisations in the recording and monitoring of biodiversity. Biodivers Conserv 17:3443–3454
Benton TG, Bryant DM, Cole L, Crick HQP (2002) Linking agricultural practice to insect and bird populations: a historical study over three decades. J Appl Ecol 39:673–687
Chen I-C, Shiu H-J, Benedick S, Holloway JD, Khen Chey V, Barlow HS, Hill JK, Thomas CD (2009) Elevation increases in moth assemblages over 42 years on a tropical mountain. Proc Nat Acad Sci 106:1479–1483
Conrad KF, Woiwod IP, Perry JN (2002) Long-term decline in abundance and distribution of the garden tiger moth (Arctia caja) in Great Britain. Biol Conserv 106:329–337
Conrad KF, Woiwod IP, Perry JN (2003) East Atlantic teleconnection pattern and the decline of a common arctiid moth. Glob Change Biol 9:13–125
Conrad KF, Woiwod IP, Parsons M, Fox R, Warren M (2004) Long-term population trends in widespread British moths. J Insect Conserv 8:119–136
Conrad KF, Warren M, Fox R, Parsons M, Woiwod IP (2006) Rapid declines of common, widespread British moths provide evidence of an insect biodiversity crisis. Biol Conserv 132:279–291
Conrad KF, Fox R, Woiwod IP (2007) Monitoring biodiversity: measuring long-term changes in insect abundance. In: Stewart AJA, New TR, Lewis OT (eds) Insect conservation biology. Royal Entomological Society, London, pp 203–225
Crozier L (2003) Winter warming facilitates range expansion: cold tolerance of the butterfly Atalopedes campestris. Oecologia 135:648–656
Crozier LG (2004) Field transplants reveal summer constraints on a butterfly range expansion. Oecologia 14:148–157
De Heer M, Kapos V, Ten Brink BJE (2005) Biodiversity trends in Europe: development and testing of a species trend indicator for evaluating progress towards the 2010 target. Phil Trans R Soc B 360:297–308
Dennis RLH (2001) Progressive bias in species status is symptomatic of fine-grained mapping units subject to repeated sampling. Biodivers Conserv 10:483–494
Dennis RLH, Shreeve TG (2003) Gains and losses of French butterflies: tests of predictions, under-recording and regional extinction from data in a new atlas. Biol Conserv 110:131–139
Dennis RLH, Thomas CD (2000) Bias in butterfly distribution maps: the influence of hot spots and access. J Insect Conserv 4:73–77
Dennis RLH, Sparks TH, Hardy PB (1999) Bias in butterfly distribution maps: the effects of sampling effort. J Insect Conserv 3:33–42
Dennis RLH, Shreeve TG, Isaac NJB, Roy DB, Hardy PB, Fox R, Asher J (2006) The effects of visual appearance on bias in butterfly recording and monitoring. Biol Conserv 128:486–492
Dunn RR (2005) Modern insect extinctions, the neglected majority. Conserv Biol 19:1030–1036
Eisenbeis G (2006) Artifical night lighting and insects: attraction of insects to streetlamps in a rural setting in Germany. In: Rich C, Longcore T (eds) Ecological consequences of artificial night lighting. Island Press, Washington, pp 281–304
Fleishman E, Murphy DD (2009) A realistic assessment of the indicator potential of butterflies and other charismatic taxonomic groups. Conserv Biol 23:1109–1116
Fox R, Spalding A, Tunmore M, Parsons M (2005) Planning a new national macro-moth recording scheme. Br J Ent Nat Hist 18:26–36
Fox R, Asher J, Brereton T, Roy D, Warren M (2006a) The state of butterflies in Britain and Ireland. Pisces Publications, Newbury
Fox R, Conrad KF, Parsons MS, Warren MS, Woiwod IP (2006b) The state of Britain’s larger moths. Butterfly Conservation and Rothamsted Research, Wareham
Fox R, Warren MS, Brereton TM (2010) A new red list of British butterflies. Species status no. 12. Joint Nature Conservation Committee, Peterborough
Frank KD (2006) Effects of artificial night lighting on moths. In: Rich C, Longcore T (eds) Ecological consequences of artificial night lighting. Island Press, Washington, pp 305–344
González-Megías A, Menéndez R, Roy D, Brereton T, Thomas CD (2008) Changes in the composition of British butterfly assemblages over two decades. Glob Change Biol 14:1464–1474
Groenendijk D, Ellis WN (in press) The state of the Dutch larger moth fauna. J Insect Conserv
Groenendijk D, van der Meulen J (2004) Conservation of moths in The Netherlands: population trends, distribution patterns and monitoring techniques of day-flying moths. J Insect Conserv 8:109–118
Heath J, Emmet AM (1983a) The moths and butterflies of Great Britain and Ireland volume 9 Sphingidae—Noctuidae (part I). Harley Books, Colchester
Heath J, Emmet AM (1983b) The moths and butterflies of Great Britain and Ireland volume 10 Noctuidae (part II) and Agaristidae. Harley Books, Colchester
Hickling R, Roy DB, Hill JK, Thomas CD (2005) A northward shift of range margins in British Odonata. Glob Change Biol 11:502–506
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD (2006) The distributions of a wide range of taxonomic groups are expanding polewards. Glob Change Biol 12:450–455
Hill JK, Thomas CD, Huntley B (1999) Climate and habitat availability determine 20th century changes in a butterfly’s range margins. Proc R Soc B 266:1197–1206
Hill JK, Thomas CD, Fox R, Telfer MG, Willis SG, Asher J, Huntley B (2002) Responses of butterflies to 20th century climate warming: implications for future ranges. Proc R Soc B 269:2163–2171
Hitch AT, Leberg PL (2007) Breeding distributions of North American bird species moving north as a result of climate change. Conserv Biol 21:534–539
Huntley B, Collingham YC, Willis SG, Green RE (2008) Potential impacts of climatic change on European breeding birds. PLoS One. doi:10.1371/journal.pone.0001439
Kremen C, Chaplin-Kramer R (2007) Insects as providers of ecosystem services: crop pollination and pest control. In: Stewart AJA, New TR, Lewis OT (eds) Insect conservation biology. Royal Entomological Society, London, pp 349–382
Kremen C, Lees DC, Fay JP (2003) Butterflies and conservation planning in Madagascar: from pattern to practice. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies - ecology and evolution taking flight. University of Chicago Press, Chicago, pp 517–540
Kühn E, Feldmann R, Harpke A, Hirneisen N, Musche M, Leopold P, Settele J (2008) Getting the public involved in butterfly conservation: Lessons learned from a new monitoring scheme in Germany. Israel J Ecol Evol 54:89–103
Maes D, Van Dyck H (2001) Butterfly diversity loss in Flanders (North Belgium): Europe’s worst case scenario? Biol Conserv 99:263–276
Maes D, Van Dyck H (2005) Habitat quality and biodiversity indicator performances of a threatened butterfly versus a multispecies group for wet heathland in Belgium. Biol Conserv 123:177–187
Maes D, Van Swaay CAM (1997) A new methodology for compiling national Red Lists applied to butterflies (Lepidoptera, Rhopalocera) in Flanders (N-Belgium) and the Netherlands. J Insect Conserv 1:113–124
Mattila N, Kaitala V, Komonen A, Kotiaho JS, Päivinen J (2006) Ecological determinants of distribution decline and risk of extinction in moths. Conserv Biol 20:1161–1168
Mattila N, Kotiaho JS, Kaitala V, Komonen A (2008) The use of ecological traits in extinction risk assessments: a case study on geometrid moths. Biol Conserv 141:2322–2328
May RM (2010) Ecological science and tomorrow’s world. Phil Trans R Soc B 365:41–47
Menéndez R, González-Megías A, Hill JK, Braschler B, Willis SG, Collingham Y, Fox R, Roy DB, Thomas CD (2006) Species richness changes lag behind climate change. Proc R Soc B 273:1465–1470
Merckx T, Feber RE, Riordan P, Townsend MC, Bourn NAD, Parsons MS, Macdonald DW (2009) Optimizing the biodiversity gain from agri-environment schemes. Agric Ecosyst Environ 130:177–182
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: synthesis. Island Press, Washington DC
Mitikka V, Heikkinen RK, Luoto M, Araújo MB, Saarinen K, Pöyry J, Fronzek S (2008) Predicting range expansion of the map butterfly in Northern Europe using bioclimatic models. Biodivers Conserv 17:623–641
Mooney HA (2010) The ecosystem-service chain and the biological diversity crisis. Phil Trans R Soc B 365:31–39
New TR (2004) Moths (Insecta: Lepidoptera) and conservation: background and perspective. J Insect Conserv 8:79–94
Newman C, Buesching CD, Macdonald DW (2003) Validating mammal monitoring methods and assessing the performance of volunteers in wildlife conservation—“Sed quis custodiet ipsos custodies?”. Biol Conserv 113:189–197
Oliver TH, Roy DB, Hill JK, Brereton T, Thomas CD (2010) Heterogeneous landscapes promote population stability. Ecol Lett 13:473–484
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across. Natural systems. Nature 421:37–42
Parmesan C, Ryrholm N, Stefanescu C, Hill JK, Thomas CD, Descimon H, Huntley B, Kaila L, Kullberg J, Tammaru T, Tennant J, Thomas JA, Warren M (1999) Polewards shifts in geographical ranges of butterfly species associated with regional warming. Nature 399:579–583
Parsons MS (2004) The United Kingdom biodiversity action plan moths—selection, status and progress on conservation. J Insect Conserv 8:95–107
Parsons M (2010) The changing moth and butterfly fauna of Britain–the first decade of the twenty-first century (2000–2009). Entomologist’s Rec J Var 122:13–22
Pimm SL, Russell GJ, Gittleman JL, Brooks TM (1995) The future of biodiversity. Science 269:347–350
Pollard E, Yates TJ (1993) Monitoring butterflies for ecology and conservation. Chapman & Hall, London
Pöyry J, Luoto M, Heikkinen RK, Kuussaari M, Saarinen K (2009) Species traits explain recent range shifts of Finnish butterflies. Glob Change Biol 15:732–743
Proctor M, Yeo P, Lack A (1996) The natural history of pollination. Harper Collins, London
Ricketts TH, Daily GC, Ehrlich PR (2002) Does butterfly diversity predict moth diversity? Testing a popular indicator taxon at local scales. Biol Conserv 103:361–370
Roy DB, Sparks TH (2000) Phenology of British butterflies and climate change. Glob Change Biol 6:407–416
Roy DB, Rothery P, Brereton T (2007) Reduced-effort schemes for monitoring butterfly populations. J Appl Ecol 44:993–1000
Samways MJ (2005) Insect diversity conservation. Cambridge University Press, Cambridge
Schmeller DS, Henry P-Y, Julliard R, Gruber B, Clobert J, Dziock F, Lengyel S, Nowicki P, Déri E, Budrys E, Kull T, Tali K, Bauch B, Settele J, van Swaay C, Kobler A, Babij V, Papastergiadou E, Henle K (2009) Advantages of volunteer-based biodiversity monitoring in Europe. Conserv Biol 23:307–316
Shortall CR, Moore A, Smith E, Hall MJ, Woiwod IP, Harrington R (2009) Long-term changes in the abundance of flying insects. Insect Conserv Divers 2:251–260
Spalding A, Tunmore M, Parsons M, Fox R (2005) The state of moth recording in Britain: the results of the national macro-moth recording scheme consultation questionnaire. Atropos 24:9–19
Sparks TH, Roy DB, Dennis RLH (2005) The influence of temperature on migration of Lepidoptera into Britain. Glob Change Biol 11:507–514
Sutherland WJ, Armstrong-Brown S, Armsworth PR, Brereton T, Brickland J, Campbell CD, Chamberlain DE, Cooke AI, Dulvy NK, Dusic NR et al (2006) The identification of 100 ecological questions of high policy relevance in the UK. J Appl Ecol 43:617–627
Telfer MG, Preston CD, Rothery P (2002) A general method for measuring relative change in range size from biological atlas data. Biol Conserv 107:99–109
Thomas JA (2005) Monitoring change in the abundance and distribution of insects using butterflies and other indicator groups. Phil Trans R Soc B 360:339–357
Thomas CD, Abery JCG (1995) Estimating rates of butterfly decline from distribution maps: the effect of scale. Biol Conserv 73:59–65
Thomas CD, Cameron A, Green RE, Bakkenes M, Beamont LJ, Collingham YC, Erasmus BFN, Ferreira de Siqueira M, Grainger A, Hannah L, Hughes L, Huntley B, van Jaarsveld AS, Midgley GF, Miles L, Ortega-Huerta MA, Peterson AT, Phillips OL, Williams SE (2004a) Extinction risk from climate change. Nature 427:145–148
Thomas JA, Telfer MG, Roy DB, Preston CD, Greenwood JJD, Asher J, Fox R, Clarke RT, Lawton JH (2004b) Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science 303:1879–1881
Van Dyck H, van Strien AJ, Maes D, van Swaay CAM (2009) Declines in common, widespread butterflies in a landscape under intense human use. Conserv Biol 23:957–965
Van Swaay C, Warren MS, Loïs G (2006) Biotope use and trends of European butterflies. J Insect Conserv 10:189–209
Van Swaay CAM, Nowicki P, Settele J, van Strien AJ (2008) Butterfly monitoring in Europe: methods, applications and perspectives. Biodivers Conserv 17:3455–3469
Van Swaay C, Cuttelod A, Collins S, Maes D, Lopez Munguira M, Šašić M, Settele J, Verovnik R, Verstrael T, Warren M, Wiemers M, Wynhof I (2010) European red list of butterflies. Publications Office of the European Union, Luxembourg
Vaughan N (1997) The diets of British bats (Chiroptera). Mamm Rev 27:77–94
Waring P, Townsend M, Lewington R (2009) Field guide to the moths of Great Britain and Ireland, 2nd edn. British Wildlife Publishing, Gillingham
Warren MS, Hill JK, Thomas JA, Asher J, Fox R, Huntley B, Roy DB, Telfer MG, Jeffcoate S, Harding P, Jeffcoate G, Willis SG, Greatorex-Davies JN, Moss D, Thomas CD (2001) Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 414:65–69
Watt WB, Boggs CL (2003) Synthesis: butterflies as model systems in ecology and evolution—present and future. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies—ecology and evolution taking flight. University of Chicago Press, Chicago, pp 603–613
Wickramasinghe LP, Harris S, Jones G, Jennings N (2004) Abundance and species richness of nocturnal insects on organic and conventional farms: effects of agricultural intensification on bat foraging. Conserv Biol 18:1283–1292
Willis SG, Thomas CD, Hill JK, Collingham YC, Telfer MG, Fox R, Huntley B (2009) Dynamic distribution modelling: predicting the present from the past. Ecography 32:5–12
Wilson JD, Morris AJ, Arroyo BE, Clark SC, Bradbury RB (1999) A review of the abundance and diversity of invertebrate and plant foods of granivorous birds in northern Europe in relation to agricultural change. Agric Ecosyst Environ 75:13–30
Wilson RJ, Thomas CD, Fox R, Roy DB, Kunin WE (2004) Spatial patterns in species distributions reveal biodiversity change. Nature 432:393–396
Woiwod IP, Harrington R (1994) Flying in the face of change: the rothamsted insect survey. In: Leigh R, Johnston A (eds) Long-term experiments in agricultural and ecological sciences. CAB International, Wallingford, pp 321–342
Acknowledgments
We are very grateful to the thousands of volunteers past and present, especially the county moth recorders, for collecting the distribution records now collated into the NMRS. We would also like to thank the many partner organisations and individuals for their support of the Moths Count project. Principal funders included the Heritage Lottery Fund, Butterfly Conservation, Environment Agency, Natural England, Countryside Council for Wales, City Bridge Trust, Northern Ireland Environment Agency, Royal Entomological Society, Scottish Natural Heritage, Royal Society for the Protection of Birds, D’Oyly Carte Charitable Trust, The 1989 Willan Charitable Trust, John Spedan Lewis Foundation and British Entomological and Natural History Society. Many other organisations are involved, providing support and helping to host events. A full list of partners is provided at www.mothscount.org. We thank Martin Warren for guidance, Roger Dennis for statistical advice and two anonymous referees for constructive comments. RF, ZR, LH, SA and LW were funded by Heritage Lottery Fund project HG-05-00610.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fox, R., Randle, Z., Hill, L. et al. Moths count: recording moths for conservation in the UK. J Insect Conserv 15, 55–68 (2011). https://doi.org/10.1007/s10841-010-9309-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-010-9309-z