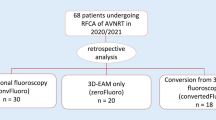
Abstract
Background
Radiofrequency catheter ablation (RFCA) of the slow pathway in atrioventricular nodal reentry tachycardia (AVNRT) is associated with high efficacy and low risk of total perioperative or late atrioventricular block. This study aimed to evaluate the efficacy, safety, and feasibility of slow-pathway RFCA for AVNRT using a zero-fluoroscopy approach.
Methods
Data were obtained from a prospective multicenter registry of catheter ablation from January 2012 to February 2018. Consecutive unselected patients with the final diagnosis of AVNRT were recruited. Electrophysiological and 3-dimensional (3D) electroanatomical mapping systems were used to create 3D maps and to navigate only 2 catheters from the femoral access. Acute procedural efficacy was evaluated using the isoproterenol and/or atropine test, with 15-min observation after ablation. Each case of recurrence or complication was consulted at an outpatient clinic during long-term follow-up.
Results
Of the 1032 procedures, 1007 (97.5%) were completed without fluoroscopy. Conversion to fluoroscopy was required in 25 patients (2.5%), mainly due to an atypical location of the coronary sinus (n = 7) and catheter instability (n = 7). The mean radiation exposure time was 1.95 ± 1.3 min for these cases. The mean fluoroscopy time for the entire study cohort was 0.05 ± 0.4 min. The mean total procedure time was 44.8 ± 18.6 min. There were no significant in-hospital complications. The total success rate was 96.1% (n = 992), and the recurrence rate was 3.9% (n = 40).
Conclusion
Slow-pathway RFCA can be safely performed without fluoroscopy, with a minimal risk of complications and a high success rate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Background
Atrioventricular nodal reentry tachycardia (AVNRT) is the most common type of reentrant supraventricular tachycardia [1, 2]. The substrate for AVNRT is the presence of dual atrioventricular (AV) nodal pathways. In most patients, percutaneous radiofrequency catheter ablation (RFCA) remains the gold standard and most effective treatment of symptomatic AVNRT. It is also considered to be the first-line therapy [1]. Patients subjected to this procedure usually do not require chronic pharmacological treatment [1, 2].
Slow-pathway RFCA or cryoablation is the preferred target during AVNRT ablation. Moreover, it is curative in approximately 95 to 98% of cases and is associated with a low risk of complications [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. The major complication of RFCA, reported in less than 1% of cases, is accidental damage to the fast pathway in the AV node, leading to permanent AV block [2,3,4, 11, 12]. Conventional catheter ablation methods are based on the analysis of intracardiac signals to introduce and guide catheter movement using fluoroscopy. On the other hand, novel techniques employ precise 3-dimensional (3D) electroanatomic mapping of the cardiac structures. This allows clinicians to reduce radiation exposure during treatment, or in some cases even completely eliminate it [2,3,4,5,6,7,8,9,10, 12,13,14,15,16, 18,19,20,21,22]. Zero fluoroscopy is defined as the achievement of ablation without the use of any fluoroscopy [10, 21]. A systematic review and meta-analysis revealed that zero or near-zero fluoroscopic guidance significantly reduced fluoroscopic time, radiation dose, ablation time, and lifetime risk of malignancy, as compared with conventional fluoroscopic guidance [10]. At the same time, it was associated with a similar procedure duration, success rate, and the rate of recurrences and complications [10].
The use of the zero-fluoroscopy approach is especially important in children, pregnant women, young adults, and patients with immune dysfunction or repeated exposure to radiation [8, 9, 12, 14, 16, 17, 19,20,21,22]. The reduction of exposure to ionizing radiation is not only beneficial for patients but also for operators during their long interventional career [8, 12, 21, 23, 24]. Apart from lowering carcinogenic risk associated with X-rays, the zero-fluoroscopy approach significantly improves operator comfort by making lead aprons and special eye protection unnecessary [12, 15, 23].
The main objective of our study was to assess the safety, feasibility, efficacy, and limitations of the zero-fluoroscopy approach for catheter ablation of AVNRT guided by the Ensite NavX system. Moreover, the effect of selected demographic and clinical characteristics on procedure duration and recurrence of AVNRT was assessed.
2 Methods
Data were obtained from a prospective standardized multicenter ELEKTRA and RARE-A CARE Registry of patients who had undergone a catheter ablation and an electrophysiological study (EPS) between January 2012 and February 2018. Unselected consecutive patients with the final diagnosis of AVNRT were recruited. The electrophysiological system (EP-Tracer, CardioTek, Maastricht, the Netherlands) and the 3D electroanatomical mapping system (EnsiteVelocityNavX, St. Jude Medical, St. Paul, MN, USA) were used to create 3D maps and to navigate only 2 catheters from the femoral access. The procedures were performed by 3 experts and 7 middle-advanced electrophysiology fellows-in-training. The acute efficacy of the procedure was evaluated using the isoproterenol and/or atropine test, with 15 min of observation postablation. The long-term follow-up of patients involved electrophysiologists, physicians, and a research nurse. Each case of recurrence or complication was consulted at an outpatient clinic. Antiarrhythmic drugs were discontinued for a minimum of 5 half-lives prior to the study. The only contraindication to the zero-fluoroscopy approach was the presence of cardiac implantable electronic devices. Clinical and procedural data were obtained from a computer database.
A rare disease was defined as any disease that affects fewer than 1 in 2000 people, according to the definition established by the European Commission [25].
The following procedural parameters were assessed: the total procedure duration, time of consecutive procedural steps (time to first and last radiofrequency energy application, observation time), application time, radiation exposure (conversion to fluoroscopy, fluoroscopic time), ablation complication rate, as well as acute and long-term (at least 1 month after ablation) success and recurrence rates. Selected demographic and clinical characteristics associated with a longer procedure duration and arrhythmia recurrence were identified. First, the effect of these characteristics on the duration of the procedure was assessed using the Mann–Whitney test. Subsequently, a multivariate linear regression analysis was performed.
Patients were followed from January 2016 to August 2018. Moreover, we assessed a subgroup of patients with AVNRT with easily inducible persistent arrhythmia (spontaneous, mechanical, or after S1–S2 stimulation protocol without drug facilitation) for a differential diagnosis and endpoint analysis.
All patients provided their written informed consent prior to the procedure, and the study was performed with the consent of the Bioethics Committee in accordance with the Declaration of Helsinki. The RARE-A-CARE-REGISTRY of rare cardiac arrhythmias and their current or unusual treatment was approved by the Bioethics Committee of the University of Rzeszów (Act No 5/4/2017).
2.1 Electrophysiological study and ablation
For the procedure with conversion to fluoroscopy, we used settings that involved minimum fluoroscopy exposure (4–8 frames/s) as well as nonfluoroscopic navigation and imaging. All centers used a similar 2-catheter approach with the femoral access. The EP-Tracer recording system (CardioTek) was used for all procedures to simultaneously record the input from all 12 electrocardiographic (ECG) leads and intracardiac signals. Under fluoroscopic or nonfluoroscopic guidance, one decapolar (APT Medical Inc., Shenzen, China; St. Jude Medical; or Biotronik, Berlin, Germany) nonsteerable catheter was placed in the coronary sinus (CS), and one steerable 7-French quadripolar catheter with an integrated thermocouple (4-mm, 8-mm, or irrigated gold-tip catheters, Biotronik, St. Jude Medical) was used for mapping and ablation. The decapolar catheter was positioned in the CS, with a distal pair of electrodes (CS 9–10) located in the proximal CS and with a prominent arteriovenous ratio. The other catheter was positioned using a dynamic approach in the His bundle region, proximal CS, and right atrium. The standard protocol included 5-min CS cannulation, which is obligatory in minimally invasive and zero-fluoroscopy approach and in all fluoroscopy-guided procedures. Subsequently, an EPS was performed in each patient [12, 13].
During the EPS, fast programmable atrial and ventricular stimulation was used. If induction of tachycardia failed, 2 to 3 extra stimuli were applied during the sinus rhythm. Subsequent attempts to induce arrhythmia as well as other diagnostic maneuvers were performed after a bolus administration of isoproterenol and/or atropine. Once the indication for ablation was confirmed, the procedure was initiated. The ablation site was chosen based on the electroanatomical mapping of the mid-septum with low-voltage atrial fractionated potentials with an A/V ratio of 1/4 to 1/6. Overdrive right ventricular pacing during tachycardia, along with the evaluation of the transition zone as well as SA and AA fix intervals, was performed to exclude accessory pathways [12, 13]. Standard or modified criteria for AVNRT were used [12, 13].
After at least 3 ablation applications, an EPS was repeated with (obligatorily) and without isoproterenol infusion. In patients with a difficult or atypical induction (e.g., thermal mapping), several attempts were made. In all cases, including children, the final EPS was performed during conscious sedation.
The total procedure time was defined as an interval between catheter insertion and catheter removal. The fluoroscopy time was defined as the total duration of exposure during the procedure. Finally, the observation time was measured from the last radiofrequency application to catheter removal.
2.2 Zero-fluoroscopy approach
EnSite NavX™ is one of the most modern mapping and navigation systems. It allows a visualization and navigation of the intracardiac catheter arrangement in each cavity of the heart for diagnostic and therapeutic purposes. The system was introduced at our institution in 2012, and the zero-fluoroscopy approach was implemented within 3 months. The EnSite Precision™ Cardiac Mapping System has been used since 2018.
With the NavX system, clinicians can quickly create a detailed model of heart anatomy. Each catheter can be used to collect data, create static and potential maps, and perform ablation. Catheters were inserted into the heart through vessels in the right and left anterior oblique views. One of the catheters in the heart was used as a reference for reconstruction of the cardiac geometry. It was marked by applying a shadow to control the change of its position. The characteristic anatomic reference points were marked, and the location of the veins was determined by comparing multiple markers. Subsequently, virtual 3D intracardiac geometry was obtained (Fig. 1). During the procedure, the optimization and compensation breathing were performed. The nonfluoroscopic cardiac mapping system enables the evaluation of catheter–tissue contact and catheter stability in real time. It also allows clinicians to assess the arrangement of cardiac anatomy as well a correlation between the ablation catheter and other intracardiac catheters. Thanks to these capabilities, catheter displacement and insufficient contact with the wall can be easily recognized even without fluoroscopy (Fig. 2).
2.3 Radiofrequency ablation
Radiofrequency energy was delivered in the temperature control mode to the target area with a power output of 50 to 60 W and a temperature of 50 to 60 °C. For this purpose, an EP-Shuttle generator (Stockert, Bar Diamond, CA, USA) was used. After ablation, pacing and infusion of isoproterenol and/or atropine were performed during a 15-min observation to confirm ablation success. High-output energy was applied for a short period of time. Radiofrequency applications were stopped when AVNRT was induced or after up to 8 consecutive junctional ectopic beats. Moreover, radiofrequency energy delivery was stopped, and the catheter was repositioned if no junctional ectopic beats were recorded or no retrograde or anterograde AV block appeared within 15 to 20 s (Fig. 3) [12, 13].
2.4 Long-term follow-up
Patients underwent regular checkups with a Holter monitor test and a consultation at 1, 3, 6, and 12 months. The final follow-up visits (at least 1 month after ablation) were scheduled between March and December 2018. The mean and median follow-up duration was 26 and 24 months, respectively.
Complications were defined either as those directly related to the procedure or those requiring additional interventions or a prolonged hospital stay. Long-term procedural success was confirmed if no clinical arrhythmia was present on repeated ECGs and Holter monitoring and if no pre-excitation was documented on ECG. In cases of short periods of unclear palpitations, transmission by a tele-ECG monitoring system or EPS was advised. Recurrence was defined as reappearance of the same signs and symptoms as documented by an ECG recording of recurrent tachycardia.
2.5 Statistical analysis
The results were presented as means ± standard deviation, median, and quartiles. Statistical significance was established at a p value of less than 0.05. The characteristics of patients were compared using the Mann–Whitney U test and the χ2 test. Significant variables were identified using the stepwise regression model. The multivariate analysis of factors associated with procedure duration was performed using linear regression, while the analysis of factors associated with AVNRT recurrence was performed using the logistic regression model.
The logistic regression model formula for estimating the probability of AVNRT recurrence after ablation for coexisting congenital defect and total procedure time is as follows:
The odds ratios and 95% confidence intervals were calculated. Data were collected using a Microsoft Excel spreadsheet and analyzed using Statistica 13.1 (StatSoft, Inc., Tulsa, OK, USA).
3 Results
3.1 Characteristics of the study group and ablation procedures
Between January 2012 and February 2018, 1032 consecutive patients were referred for electrophysiological procedures. After an EPS, patients with the final diagnosis of AVNRT were included in the analysis. Descriptive characteristics of the study sample are shown in Table 1.
Of the 1032 procedures, 1007 (97.6%) were completed without fluoroscopy. The reasons for conversion to fluoroscopy in 25 patients were as follows: atypical location of the CS (n = 7), catheter instability (n = 7), need to control the passage of the catheter (n = 5), high skin impedance (n = 2), system failure (n = 2), another coexisting arrhythmia (n = 1), and difficulty in vascular access (n = 1). The mean radiation exposure time for these cases was 1.95 ± 1.3 min, while the mean fluoroscopy time for the entire study cohort was 0.05 ± 0.4 min. Only patients with implanted devices (pacemakers or implantable cardioverter-defibrillators) were not included in the registry.
The mean total procedure time was 44.8 ± 18.6 min. The median time was 40 min (lower quartile, 32 min; upper quartile, 53 min). The distribution of the total procedure time in the study group is shown in Fig. 4. The results for consecutive procedural steps were as follows: time to first application, 21 ± 10.3 min; time to last application, 32.5 ± 25 min; and observation time, 13 ± 6.5 min. The mean application time was 5.48 ± 4.18 min.
The total success and recurrence rates are reported in Table 1. None of the patients experienced permanent AV block (the most common complication of RFCA). No significant in-hospital complications occurred. Minor vascular complications such as hematomas and femoral artery pseudoaneurysm at the injection site were detected in less than 1% of patients (hematoma, n = 9; pseudoaneurysm, n = 1) (Table 1).
Of all the procedures, 125 (12%) were performed in patients under the age of 19. Only 1 procedure required conversion to fluoroscopy during ablation, but it lasted only 0.07 min due to several vascular access failures with ultrasound-guided puncture in a patient with spina bifida (single catheter approach from the jugular vein).
3.2 Association between selected factors and procedure duration
We assessed the effect of selected demographic and clinical characteristics on the duration of the procedure. The total procedure time was significantly longer in patients with coexisting arrhythmias, rare diseases, atypical type of AVNRT, and heart valve disease. On the other hand, the presence of hypertension and age older than 60 years were associated with a shorter procedure duration. The results are presented in Table 2.
The stepwise forward regression analysis identified 5 independent variables associated with a prolonged procedure time. Ablation of the second substrate during the same procedure was associated with an increase in the procedure time by an average of 14.7%, followed by the presence of heart valve disease (12.6%), atypical type of AVNRT (7.8%), and coexisting arrhythmia (4.9%). On the other hand, older age was shown to reduce the procedure time by approximately 0.3% per year (e.g., in a 10-year-older patient, the procedure time is shorter by about 3.2%). The results are presented in Table 3.
3.3 Association between selected factors and recurrence of atrioventricular nodal reentry tachycardia
Recurrence of AVNRT was reported in 40 patients (3.9% of all procedures). These patients were compared with the remaining group. The analysis showed that patients with recurrence had a higher incidence of congenital defects and a longer procedure time (Table 4). Therefore, the presence of a congenital defect and a longer procedure time can be considered as risk factors for AVNRT recurrence.
The results of the univariate analysis were in line with the findings from the multivariate analysis using logistic regression (Table 5). The presence of a congenital defect was shown to increase the risk of recurrence after ablation by up to 7.6-fold, and each additional minute of the procedure increased the risk by 1.4%. The most frequent congenital heart defect was atrial septal defect in 11 patients (1%), ventricular septal defect in 6 patients (0.6%), coarctation of the aorta in 3 patients (0.3%), persistent left superior vena cava in 2 patients (0.2%), and tetralogy of Fallot in 1 patient (0.1%).
4 Discussion
Over the last years, various strategies have been used to reduce radiation exposure during arrhythmia ablation. This study presents the feasibility and safety of the minimally invasive and zero-fluoroscopy approach for catheter ablation of AVNRT that almost eliminates radiation and the need to use protective lead aprons by medical staff. In the current study, this method was shown to be effective in most patients with AVNRT, and almost all procedures (97.6%) were successfully completed without fluoroscopy. Moreover, none of the patients experienced permanent AV block or any other significant complications. Our data are in line with the 2018 Spanish Registry of Catheter Ablation, in which 3525 AVNRT ablation procedures were performed in 96 hospitals, with a success rate of 96%. Three-dimensional electroanatomical systems were used in 10.9% of cases, and most of these procedures were performed without fluoroscopy [2].
4.1 Application of the EnSite system
The EPS study and ablation did not differ between fluoroscopy and EnSite systems: in both cases, 2 electrodes were used. The quadripolar catheter was placed near the His bundle, as shown on the map. The steerable decapolar catheter was used to perform the mapping of the superior vena cava and right atrium, as well as to establish the outline of the tricuspid valve annulus. Subsequently, it was placed in the CS. The precise electrophysiological mapping of the Koch’s triangle and the search of the slow pathway’s electrical potential were performed before radiofrequency application [12, 13].
It is generally believed that catheters longer than 4 mm are less precise for ablation. However, in our study, we used 8-mm catheters in 87 cases (8.43%) without compromising the efficacy of the procedure. Moreover, no differences in the safety profile were observed between procedures done with 8-mm catheters versus those employing 4-mm catheters. Most patients had comorbid arrhythmia that was treated during the same ablation procedure. In the Spanish registry, most procedures (96%) were performed with conventional 4-mm-tip catheters, while 8-mm-tip catheters were used only in 1.1% of cases [2].
The main objective of RFCA with the electroanatomical system is an attempt to limit fluoroscopy time and radiation dose. There is growing awareness of the long-term effect of radiation exposure on the human body. Recently, several studies assessing the risks of such exposure have been published [16, 21,22,23,24], focusing primarily on long-term cancer risk. The introduction of the 3D mapping ablation systems has significantly reduced the radiation exposure during the procedure both for medical personnel and patients [2,3,4, 12,13,14,15,16, 19,20,21,22,23].
In a recent study, Matevž et al. [9] assessed the feasibility, safety, and efficacy of radiofrequency ablation and cryoablation of AVNRT guided by the 3D electroanatomical mapping system without fluoroscopy. They concluded that fluoroless radiofrequency ablation or cryoablation can be routinely performed in all patients with AVNRT without compromising the safety, effectiveness, or duration of the procedure [9].
Li et al. [19] investigated the feasibility of RFCA without fluoroscopy in pregnant patients. The authors demonstrated that the zero-fluoroscopy approach has an acceptable safety and efficacy profile and constitutes a viable therapeutic option for pregnant women with a highly prevalent drug-resistant arrhythmia. In our registry, 4 pregnant women were referred for nonfluoroscopic AVNRT procedures, achieving full therapeutic success with no recurrence and no perioperative or late complications.
The use of the completely zero-fluoroscopy approach is also of great importance in children.
Krauze et al. [17] reported that the catheter ablation of various tachycardia substrates was overall effective and safe in pediatric patients, with a low complication rate and a high success rate at 1-year follow-up. In our study, we assessed 125 procedures (12%) performed in patients under the age of 19 years, and 96% of them were successful without recurrence.
The NO-PARTY multicenter randomized controlled trial compared conventional fluoroscopy-guided procedures with procedures performed using the Ensite NavX system in patients undergoing an EPS for supraventricular tachycardia. The results showed that a zero-fluoroscopy approach in the ablation of supraventricular tachycardia significantly reduced patient exposure to radiation while maintaining acceptable efficacy and safety profiles [20].
Another study assessing the safety, feasibility, and efficacy of a completely zero-fluoroscopy approach for catheter ablation of supraventricular tachycardia using the EnSite NavX navigation system versus conventional fluoroscopy approach was performed by Chen et al. [14]. They confirmed that the EnSite NavX navigation system eliminated the need to use fluoroscopy, while achieving comparable success and complication rates to those observed for a conventional fluoroscopy approach.
The zero-fluoroscopy approach for mapping and navigation during EPS and catheter ablation requires expertise in the management of patients with normal and structural heart disease. Świętoniowska-Mścisz et al. [26] reported successful right-sided zero-fluoroscopy ablation of 3 types of supraventricular tachycardia in a single patient with atypical vein connections such as persistent left superior vena cava. The zero-fluoroscopy approach can be used by experienced clinicians as a standard approach, and even cases with rare and complex cardiac anatomies, heart disease, or atypical blood vessels can be effectively treated. Morka et al. [27] reported that the minimally invasive zero-fluoroscopy approach was successfully implemented in most ablation procedures of regular supraventricular tachycardia in patients with structural heart diseases and should become a standard approach.
In our study, conversion to fluoroscopy in the zero-fluoroscopy approach was necessary only in 25 procedures, and the mean radiation exposure time for those procedures was less than 2 min. The main reason for the use of fluoroscopy was to verify the position of the diagnostic electrode and to assess differences in cardiac anatomical structure.
In our study, only patients with implanted devices (pacemakers or implantable cardioverter-defibrillators) were considered ineligible for the zero-fluoroscopy approach, as per the internal standards at our center. From the clinical perspective, our near-zero protocol for ablation has always included preoperative and postoperative fluoroscopy to assess the condition of endocardial leads. There are other imaging modalities that enable the zero-fluoroscopy approach in these patients; however, neither intracardiac echocardiography nor early or late cardiac electronic implantable device implantation was used as eligibility criterion for zero-fluoroscopy approach. These patients were primarily accepted for the near-zero fluoroscopy approach with an additional reduction of fluoroscopy time (data not shown). Recently, the first study was published showing that zero-fluoroscopy ablation for supraventricular arrhythmia using 3D mapping and intracardiac echocardiography in patients with cardiac electronic implantable device leads was feasible with careful catheter manipulation [28].
The introduction of the 3D electroanatomic mapping systems has created new possibilities for improving the efficiency and safety of ablation procedures. EnSite NavX is currently one of the most modern mapping and navigation systems [12,13,14, 18, 21, 22, 29, 30]. It offers a simultaneous visualization of all catheters and is compatible with almost every available catheter, including a pacemaker electrode.
In clinical practice, it is increasingly common to create computed tomography or magnetic resonance imaging datasets before the study. This method enables to precisely reconstruct unusual anatomical features or difficult areas, particularly in the left atrium. It also facilitates catheter navigation and tissue contact visualization during the proper ablation procedure. [31].
The learning curve analysis is also an important component in evaluating the effectiveness of ablation procedures [14, 18, 21, 22, 32]. Difficulty in catheter insertion into the CS was the primary factor affecting the duration of the time to first application. A growing experience with this radiation-free approach should lead to complete elimination of fluoroscopy during AVNRT ablations and during the training of a new generation of invasive electrophysiologists.
5 Conclusions
With the advent of the 3D mapping systems, radiation exposure during ablation procedures has been reduced, both for the medical personnel and for patients. Our results show that slow-pathway RFCA can be successfully performed without fluoroscopy, offering a minimal risk of complications and a high success rate.
References
Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: executive summary: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2016;13:e92-135.
Ibáñez Criado JL, Quesada A, Cózar R; Spanish Catheter Ablation Registry collaborators; Appendix 1. REGISTRY COLLABORATORS. Spanish Catheter Ablation Registry. 18th Official Report of the Spanish Society of Cardiology Working Group on Electrophysiology and Arrhythmias (2018). Rev Esp Cardiol (Engl Ed). 2019;72:1031–42.
Álvarez M, Bertomeu-González V, Arcocha MF, Moriña P, Tercedor L, Ferrero de Loma Á, et al; on behalf of investigators of the Spanish Multicenter Registry of Fluoroscopy-free Ablation. Nonfluoroscopic catheter ablation. Results from a prospective multicenter registry. Rev Esp Cardiol (Engl Ed). 2017;70:699–705.
Jan M, Yazici M, Kalinšek TP, Žižek D, Kuhelj D, Pernat A, Lakič N. Fluoroless radiofrequency and cryo-ablation of atrioventricular nodal reentry tachycardia in adults and children: a single-center experience. J Interv Card Electrophysiol. 2020. https://doi.org/10.1007/s10840-020-00791-1.
Hanninen M, Yeung-Lai-Wah N, Massel D, Gula LJ, Skanes AC, Yee R, et al. Cryoablation versus RF ablation for AVNRT: a meta-analysis and systematic review. J Cardiovasc Electrophysiol. 2013;24:1354–60.
Schwagten B, Knops P, Janse P, Kimman G, Van Belle Y, Szili-Torok T, et al. Long-term follow-up after catheter ablation for atrioventricular nodal reentrant tachycardia: a comparison of cryothermal and radiofrequency energy in a large series of patients. J Interv Card Electrophysiol. 2011;30:55–61.
Balli S, Kucuk M, Orhan Bulut M, Kemal Yucel I, Celebi A. Transcatheter cryoablation procedures without fluoroscopy in pediatric patients with atrioventricular nodal reentrant tachycardia: a single-center experience. Acta Cardiol Sin. 2018;34:337–43.
Alvarez M, Tercedor L, Almansa I, Ros N, Galdeano RS, Burillo F, et al. Safety and feasibility of catheter ablation for atrioventricular nodal re-entrant tachycardia without fluoroscopic guidance. Heart Rhythm. 2009;6:1714–20.
Jan M, Žižek D, Rupar K, Mazić U, Kuhelj D, Lakič N, et al. Fluoroless catheter ablation of various right and left sided supraventricular tachycardias in children and adolescents. Int J Card Imaging. 2016;32:1609–16.
Yang L, Sun G, Chen X, Chen G, Yang S, Guo P, et al. Meta-Analysis of zero or near-zero fluoroscopy use during ablation of cardiac arrhythmias. Am J Cardiol. 2016;118:1511–8.
Katritsis DG, Zografos T, Siontis KC, Giannopoulos G, Muthalaly RG, Liu Q, et al. Endpoints for successful slow pathway catheter ablation in typical and atypical atrioventricular nodal re-entrant tachycardia: a contemporary, multicenter study. JACC Clin Electrophysiol. 2019;5:113–9.
Stec S, Sledz J, Mazij M, Ras M, Ludwik B, Chrabąszcz M, et al. Feasibility of implementation of a “simplified, No-X-Ray, no-lead apron, two-catheter approach” for ablation of supraventricular arrhythmias in children and adults. J Cardiovasc Electrophysiol. 2014;25:866–74.
Stec S, Sledź J, Mazij M, Ludwik B, Labus M, Spikowski J, et al. Simplified automated right ventricular overdrive pacing for rapid diagnosis of supraventricular tachycardia. Cardiology. 2014;129:93–102.
Chen G, Wang Y, Proietti R, Wang X, Ouyang F, Ma CS, et al. Zero-fluoroscopy approach for ablation of supraventricular tachycardia using the Ensite NavX system: a multicenter experience. BMC Cardiovasc Disord. 2020;20:48.
Fernández-Gómez JM, Moriña-Vázquez P, Morales Edel R, Venegas-Gamero J, Barba-Pichardo R, Carranza MH. Exclusion of fluoroscopy use in catheter ablation procedures: six years of experience at a single center. J Cardiovasc Electrophysiol. 2014;25:638–44.
Wan G, Shannon KM, Moore JP. Factors associated with fluoroscopy exposure during pediatric catheter ablation utilizing electroanatomical mapping. J Interv Card Electrophysiol. 2012;35:235–42.
Krause U, Paul T, Bella PD, Gulletta S, Gebauer RA, Paech C, Kubus P, Janousek J, Ferrari P, De Filippo P. Pediatric catheter ablation at the beginning of the 21st century: results from the European Multicenter Pediatric Catheter Ablation Registry “EUROPA.” Europace. 2021;23:431–40.
Santoro A, Di Clemente F, Baiocchi C, Zacà V, Bianchi C, Bellini C, et al. From near-zero to zero fluoroscopy catheter ablation procedures. J Cardiovasc Electrophysiol. 2019;30:2397–404.
Li MM, Sang CH, Jiang CX, Guo XY, Li SN, Wang W, et al. Maternal arrhythmia in structurally normal heart: prevalence and feasibility of catheter ablation without fluoroscopy. Pacing Clin Electrophysiol. 2019;42:1566–72.
Casella M, Dello Russo A, Pelargonio G, Del Greco M, Zingarini G, Piacenti M, et al. Near zerO fluoroscopic exPosure during catheter ablAtion of supRavenTricular arrhYthmias: the NOPARTY multicentre randomized trial. Europace. 2016;18:1565–72.
Anselmino M, Sillano D, Casolati D, Ferraris F, Scaglione M, Gaita F. A new electrophysiology era: zero fluoroscopy. J Cardiovasc Med (Hagerstown). 2013;14:221–7.
Chen G, Sun G, Xu R, Chen X, Yang L, Bai Y, et al. Zero-fluoroscopy catheter ablation of severe drug-resistant arrhythmia guided by Ensite NavX system during pregnancy: two case reports and literature review. Medicine (Baltimore). 2016;95:e4487.
Wang Y, Chen GZ, Yao Y, Bai Y, Chu HM, Ma KZ, et al. Ablation of idiopathic ventricular arrhythmia using zero-fluoroscopy approach with equivalent efficacy and less fatigue: a multicenter comparative study. Medicine (Baltimore). 2017;96:e6080.
Marinskis G, Bongiorni MG, Dagres N, Lewalter T, Pison L, 2013 Blomstrom-Lundqvist C; Scientific Initiative Committee, European Heart Rhythm Association. X-ray exposure hazards for physicians performing ablation procedures and device implantation: results of the European Heart Rhythm Association survey. Europace.15:444–6.
Świętoniowska-Mścisz A, Zagrodzka M, Chrabąszcz M, Śledź J, Biernikiewicz W, Stec S. Diagnosis of persistent left superior vena cava during zero-fluoroscopy catheter ablation of three substrates of supraventricular arrhythmia. Kardiol Pol. 2019;77:236.
Morka A, Śledź J, Deutsch K, Ludwik B, Zagrodzka M, Szydłowski L, Stec S. Feasibility and performance of catheter ablation with zero-fluoroscopy approach for regular supraventricular tachycardia in patients with structural and/or congenital heart disease. Medicine (Baltimore). 2019;98:e17333.
Shimamoto K, Yamagata K, Wakamiya A, Ueda N, Kamakura T, Wada M, Inoue-Yamada Y, Miyamoto K, Nagase S, Kusano KF. Zero-fluoroscopy ablation in patients with cardiac electronic implantable devices. J Cardiovasc Electrophysiol. 2022;33:423–9.
Douglas L, Packer MD. Three-dimensional mapping in interventional electrophysiology: techniques and technology. J Cardiovasc Electrophysiol. 2005;16:1110–6.
Earley MJ, Showkathali R, Alzetani M, Kistler PM, Gupta D, Abrams DJ, et al. Radiofrequency ablation of arrhythmias guided by non-fluoroscopic catheter location: a prospective randomized trial. Eur Heart J. 2006;27:1223–9.
Borlich M, Iden L, Kuhnhardt K, Paetsch I, Hindricks G, Sommer P. 3D Mapping for PVI- geometry, image integration and incorporation of contact force into work flow. J Atr Fibrillation. 2018;10:1795.
Gist K, Tigges C, Smith G, Clark J. Learning curve for zero-fluoroscopy catheter ablation of AVNRT: early versus late experience. Pacing Clin Electrophysiol. 2011;34:264–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients provided their written informed consent prior to the procedure, and the study was approved by the Bioethics Committee in accordance with the Declaration of Helsinki. The RARE-A-CARE-REGISTRY of rare cardiac arrhythmias and their current or unusual treatment was approved by the Bioethics Committee of the University of Rzeszów (Act No 5/4/2017).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Świętoniowska-Mścisz, A., Stec, P., Stec, S. et al. Efficacy and safety of zero-fluoroscopy approach for ablation of atrioventricular nodal reentry tachycardia: experience from more than 1000 cases. J Interv Card Electrophysiol 66, 1231–1242 (2023). https://doi.org/10.1007/s10840-022-01419-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-022-01419-2