Abstract
Background
Implantable cardioverter defibrillators (ICD) are widely accepted therapy in children and adolescents who are survivors of cardiac arrest or for high-risk patients with inheritable channelopathies, cardiomyopathies, or congenital heart disease. Initial experience with subcutaneous ICD (S-ICD) systems has shown a high efficacy in adults. However, the use of S-ICD in children and adolescents implies some specific considerations, as the safety for these patients is unknown and recommendations among physicians may vary widely.
Methods
We reviewed the data and studied the indications for S-ICD in children and adolescents and discuss the preliminary clinical experience.
Results
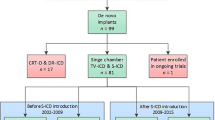
From a cohort of 297 patients enrolled in the S-ICD “Monaldi care” registry that encompass all the patients implanted in the Monaldi Hospital of Naples, we considered 21 consecutive children and adolescents (mean age 13.9 years, range 8-18 years, mean body weight 59.3 kg, range 38-100 kg) who underwent S-ICD implant from April 2014 to June 2020. Mean follow-up was 41.9±21.9 months. Only one patient presented, 6 weeks after implantation, skin erosion at the inferior parasternal incision that resolved after antibiotic therapy, without the necessity of any system revision. Two patients experienced appropriate shocks and four inappropriate shocks, due to T wave oversensing or atrial arrhythmia. Only one patient, with arrhythmogenic right ventricular dysplasia, required a system revision after 36 months of the first implantation and then a reintervention with a replacement of the S-ICD by a conventional ICD system.
Conclusions
Our experience suggests that the S-ICD device can be used in some children over the age of 8 as well as adults, with a similar rate of unwanted side effects, and early evidence of apparent efficacy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Implantable cardioverter defibrillators (ICD) are widely accepted therapy in children and adolescents who are survivors of cardiac arrest in the absence of reversible causes or for high-risk patients with inheritable channelopathies, cardiomyopathies, or congenital heart disease [1,2,3,4,5]. However, placement of traditional transvenous ICD system, especially in children and adolescents, may be problematic because of the elevated rate of lead malfunction and failure, children’s growth, various anatomic constraints, or lack of vascular access [6,7,8].
Although the subcutaneous implantable cardioverter defibrillator (S-ICD) has been advocated to overcome many of these limitations and seems to be ideally suited especially for young patients with a life expectancy of more than 10 years [1, 9, 10], there is little clinical experience with this technology particularly for youngest patients, while it has showed a high efficacy in adults [11,12,13]. The present study from the S-ICD “Monaldi Care” registry, that encompasses all the patients implanted in the Monaldi Hospital of Naples, examines the preliminary clinical experience with the S-ICD in children and adolescents.
2 Methods
This is an observational, retrospective study on S-ICD implantation and follow-up in children and adolescents. Data were collected prospectively in the S-ICD “Monaldi care” registry and analyzed retrospectively. In 2013, we started our own Monaldi Hospital registry, named S-ICD “Monaldi Care” registry, which was later incorporated into the S-ICD Rhythm Detect Registry [14]. We prospectively entered data from all patients who underwent S-ICD implantation in our Hospital. The S-ICD “Monaldi Care” registry was developed under the agreement of different EP teams working in the hospital to perform epidemiological analyses and publish their results for the population of patients with implanted S-ICD. The registry was approved by the local ethics committee, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments.
Informed consent for data storage and analysis was obtained from the patient’s guardians.
2.1 Selection of the patients
All the patients aged less than 18 years, who underwent S-ICD implantation between April 2014 and June 2020, were included in the study, as they were already enrolled in the “Monaldi Care” registry including a total of 297 patients.
2.1.1 Inclusion criteria (specific indications for S-ICD implantation)
International guidelines were followed for ICD implantation [1, 2].
In particular, the indication for S-ICD was considered for children or adolescents who had no favorable venous access (occluded veins, congenital anomalies), patients with intracardiac shunts or with a history of endocarditis, and when pacing therapy for bradycardia support, cardiac resynchronization, or anti-tachycardia pacing was not needed.
2.2 S-ICD screening
All the enrolled patients were eligible for S-ICD suitability as they had at least one surface ECG lead (sensing vector) considered acceptable for all postures tested (i.e., supine and standing position) and during exercise test. In patients already having pacemakers appropriate S-ICD sensing was also verified during atrial and/or ventricular pacing.
2.3 Implantation procedure
All procedures were performed in the electrophysiology/cardiac pacing laboratory by a single team of five electrophysiologists of the Adult Congenital Heart Disease (ACHD) Unit with the support of the manufacturer’s technicians. Implantations were performed under general anesthesia or, only in the procedures performed in 2019 and 2020, through ultrasound-guided serratus anterior plane block [15]. At the onset of the experience a complete subcutaneous approach was performed, thereafter, the inter-muscular approach was preferred. Antibiotic prophylaxis was given to every patient. In the 2014-2015 cases, S-ICDs (model Emblem A209, Boston Scientific, Natick, NA, USA) were implanted via a standard three-incision approach. Subsequently, S-ICDs (models Emblem A219, Boston Scientific, Natick, NA, USA) were implanted applying a three- or two-incision technique [16].
Acute efficacy of the system was tested by single shock and was defined as successful conversion of induced ventricular tachycardia/ventricular fibrillation by an intraoperative defibrillation test (DT) to sinus rhythm. If the test was unsuccessful, the shock vector polarity was reversed.
For patients in which the intra-operative DT repeated twice showed a lack of inducibility, 10 J shocks were delivered synchronously in sinus rhythm. An impedance of <90 Ω was considered indicative of device system integrity and appropriate system position achieved [17]. All patients received individualized dual-zone programming (conditional shock zone and shock zone).
2.4 Data collection
Following data was collected:
-
(1).
Patient demographics
-
(2).
-Preimplant clinical characteristics (congenital diagnosis and details regarding surgical repair/palliation; a copy of the preimplant 12-lead ECG; results of S-ICD eligibility screening both at baseline in supine and standing positions, as well as with exercise testing, if suitable for the patient, existing cardiac implantable electronic device details; drug therapy; ICD indication and reasons for use of the S-ICD)
-
(3).
Implant characteristics (implant techniques, results of defibrillation testing, initial S-ICD programming, a copy of the post-procedural chest x-ray, procedural complications, and post-procedural length of stay)
Acute complications were defined as those occurring before discharge from the hospital or within 30 days of implant.
Therapies were classified as appropriate if delivered for ventricular tachycardia/ventricular fibrillation (VT/VF); otherwise, they were considered inappropriate (IAS = inappropriate shock).
2.5 Follow-up
Patients were regularly followed between February 2015 and October 2020 at the Pediatric and Adult CHD Unit in accordance with the following protocol: patients underwent clinical evaluation, ECG, and device interrogation 1 month after the implant and every 3–4 months thereafter.
Trans-thoracic echocardiography, Holter monitoring, and, when possible, exercise testing were performed every 12 months for periodic functional evaluation of the disease.
The outcomes analyzed included patients’ characteristics, long-term complications, all post-operative VF episodes, time to the first appropriate shock, first inappropriate shock, and all appropriate and inappropriate shocks during follow-up.
2.6 Data analysis
Data are presented as mean ± standard deviation or median (interquartile range) for continuous variables as appropriate and as frequencies and percentages for dichotomous variables. The study is descriptive, with no inferential statistics performed.
3 Results
All the patient’s data are reported in Tables 1 and 2.
3.1 Baseline patient characteristics
From a large cohort of 297 patients enrolled in the S-ICD “Monaldi care” registry, 21 consecutive children and adolescents (mean age 13.9 years; range 8-18 years, 7 females) who underwent S-ICD implant from February 2015 to June 2020 were included in the study.
Mean weight was 59.3kg (range 38-100 kg), height 158.1cm (range 135-182 cm), body mass index 23 (range 16.8-36.73), and body surface area 1.6 (range 1.14-2.1). Notably, five patients (patient #2, #4 Fig. 1, #8, #19 Fig. 2, #21 Fig. 3) underwent cardiac surgery early in the life. Only one patient (#19, Fig. 2) with a repaired tetralogy of Fallot had a previously pacemaker device at the time of S-ICD implantation. Primary prevention was the indication in 7 (33% of the patients). Two patients (patient #9, #17) with dilated cardiomyopathy following myocarditis and two patients with complex CHD (patient #4, Fig. 1, patient #19, Fig. 2) showed persistent reduced ejection fraction (<35%) despite maximal therapy and entered the criteria for S-ICD implantation. One patient with hypertrophic cardiomyopathy (HCM) (patient #1) presented 3 major pediatric risk factors for sudden cardiac death (SCD): severe ventricular hypertrophy with Z score ≥6, non-sustained VT, and family history of SCD [18]. The patient with arrhythmogenic right ventricular dysplasia (ARVD) (patient #14) presented 2 major risk factors: non-sustained VT and moderate right ventricle dysfunction [19]. The patient with Brugada syndrome (BrS) (patient #16) had a spontaneous diagnostic type I ECG pattern, history of syncope, and induced VF during programmed ventricular stimulation [1].
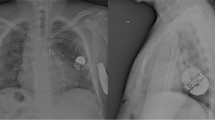
Post-implant chest x-rays (AP/LL). Patient #21 (14 years old/M, 53kg, 1.48m2 BSA): Aortic arch interruption +ventricular septal defect + atrial septal defect s/p Damus-Kaye-Stansel modified procedure s/p bidirectional Glenn shunt s/p Yasui operation s/p ventricular septal defect device closure s/p melody valve implantation
Notably, in the period between April 2014 and June 2020, no patients who entered the inclusion criteria (specific indications for S-ICD implantation) were rejected for the S-ICD implant, as all passed the S-ICD screening.
3.2 Procedural data
Eighteen patients passed the S-ICD eligibility test with the electrode in the right parasternal position and three in a left parasternal position. Three patients presented 1 sensing vector acceptable for all postures tested, 8 patients showed 2 sensing vectors acceptable, and 10 patients presented all the 3 sensing vectors. The standard three-incision approach was adopted in the first 4 patients, and the two-incision technique was used in the following 17. The generator was positioned in an inter-muscular pocket in all but 3 patients. In 5 patients, a defibrillation test was not performed: in one patient, the test was not performed due to low ejection fraction, and in four patients, a test repeated twice intraoperatively showed a lack of inducibility. For these patients, 10 J shocks were delivered synchronously in sinus rhythm. An impedance ranging between 10 and 55Ω was found in all, and it was considered highly indicative of device system integrity and appropriate system position. In the last implanted patients (pt. #18, 19, 20, 21) in which was available AP/LL post-procedural chest X-ray, PRAETORIAN scores [20], adopted since 2019, documented 30 to 60 points representing a low risk of conversion failure. All patients had dual-zone programming. The conditional shock zone was programmed between 200 and 220 bpm, and the shock zone was programmed for all at 250bpm. Seven patients received the “Latitude system” for remote automatic, in-home monitoring, most of them implanted in the period 2018-2020.
No complications were reported during the procedures.
3.3 Follow-up
The post-operative course was uneventful, and all the patients were discharged between 2 and 3 days after the procedure. Mean follow-up was 41.9±21.9 months (range 4-78 months). No acute (within 30 days of implant) complications (infections or skin erosions) were reported. Patient #13 presented, 6 weeks after implantation, skin erosion at the inferior parasternal incision. A culture was positive for staphylococcus epidermidis. After specific antibiotic therapy, the skin erosion had no sequelae and the patient did not need any system revision. Two patients (patient #7 and #14) experienced VF appropriately detected and treated. Four patients (patient #5, #14, #15, and #21) experienced IAS. In patients #5 (Fig. 4), #14, and #21, the IAS was due to T wave oversensing and in patient #15 was due to atrial arrhythmia. Notably, in one patient (#14) affected by arrhythmogenic right ventricular dysplasia, following repeated inappropriate shocks, due to T wave oversensing and progressive reduced amplitude of QRS complexes over the time, a system revision after 36 months, the first implantation was required and then, as the screening vectors were considered unsatisfactory, a reintervention with a replacement of the S-ICD by a conventional ICD system.
4 Discussion
This study on children and adolescents with S-ICD includes the largest population of patients <18 years old analyzed so far. Most reports have been limited to isolated case reports or case series on children and adolescents, some include older patients as well and registry studies evaluating the general S-ICD population. The results of this study suggest that the S-ICD device can be used in some children over the age of 8 as well as adults, with a similar rate of unwanted side effects, and early evidence of apparent efficacy.
4.1 Potential benefits of S-ICD in young patients
The S-ICD system was developed because of its perceived benefits over transvenous ICD systems especially for young patients with a life expectancy of more than 10 years [21]. It is likely that the eliminated need for transvenous lead placement substantially reduces the implant-related complications associated with transvenous lead insertion, especially in young physically active patients. The advantages of the S-ICD are the absence of leads within the heart and the preservation of central venous circulation other than the extremely low risk of systemic infections [22, 23]. Furthermore, mechanically induced pro-arrhythmia from the lead [24] and lead-associated tricuspid regurgitation have also been postulated as possible adverse consequences of transvenous lead use [25, 26]. Difficulties in achieving venous access [27], which can prolong the procedure and occasionally results in failed implantable cardioverter defibrillator implantation, can be avoided. Lead failure, sometimes due to lead fracture remains a major limitation in the use of transvenous ICD systems over the long term [6, 7], as lead failure generates inappropriate shocks or impedes appropriate therapy. The S-ICD with its entirely subcutaneous and more robust lead probably elongates lead longevity because the lead is less subject to mechanical stress. The S-ICD also promises to offer advantages for extraction procedures, which are associated with substantial morbidity and mortality, when required. These advantages might become particularly evident in cases where lead extraction is indicated because of lead fractures or infections, a condition quite frequent in the youngest patients.
An important drawback of the S-ICD system may be the size of the can, especially in children and adolescents with little subcutaneous tissue. The larger generator might increase the risk of skin erosion, patient’s discomfort, and infection as compared with the conventional ICDs. In addition, the heavier weight could cause device dislodgement and could potentially lead to a change of the shock configuration, with unpredictable consequences on algorithm detection properties and defibrillation threshold. In our series, the implantation procedure was absolutely successful and free from complications. In all the tested patients, S-ICD was effective in terminating induced ventricular fibrillation. In our study, the post-operative course was uneventful. All the patients were mobilized shortly after the implantation and were discharged between 2 and 3 days after the procedure. Nevertheless, no acute complications, infections, or skin erosions were observed. Notably, only one patient (patient #13) presented, 6 weeks after implantation, skin erosion at the inferior parasternal incision. After specific antibiotic therapy, as a culture was positive for staphylococcus epidermidis, the skin erosion had no sequelae and the patient did not need any system revision. This patient was the only one in which the horizontal incision at the xiphoid process was closed by means of absorbable sutures, differently from all the others. It is not clear if it can exist in any association between this type of suture and the occurrence of the observed late complication. More observations would clarify this aspect. Although pocket infections can occur with the subcutaneous implantable cardioverter defibrillator (5-10%, similar to transvenous devices), infection resolves with antibiotics in the majority of cases. Explantation is rarely necessary; however, explanting a subcutaneous implantable cardioverter defibrillator is much simpler and safer than endovascular lead extraction. The lack of acute complications, and the presence of a single late complication, differently from other studies interesting similar populations [9, 10, 22, 23], could be related in our series to the smaller size of the can used (Emblem), that has a 20% reduction in device profile compared to the previous model, and the prevalent use of inter-muscular approach and two incision techniques. Our experience with S-ICD shows a high efficacy. Only three patients showed episodes of IAS due to T-wave oversensing, with double-counting (in one case the IAS was position-dependent sitting on the floor in the squatting position) and only one patient showed IAS due to atrial arrhythmias. All the IAS disappeared with better strategic programming over the time and increased operator experience. Changing of the sensing vector, activation of the SMART PASS filter, improved detection algorithms other than extended use of “latitude system” for remote monitoring, and adequate antiarrhythmic therapy can reduce unwanted inappropriate shocks. Remote monitoring has been already shown to have an important role in the timely diagnosis of atrial tachyarrhythmias, device-related complications, and inappropriate therapies. If these events could be detected earlier, appropriate measures could be undertaken to reduce the number of shocks and spare the device battery [28]. Only one patient required a system revision after 36 months of the first implantation and then, as the screen vectors were still considered unsatisfactory, a reintervention with a replacement of the S-ICD by a conventional ICD system. The patient was affected by familial ARVD, with a progressive reduction of QRS complexes amplitude over the time. Explantation of the S-ICD was uncomplicated. This case highlights the need for a strict follow-up and probably a periodic defibrillation threshold testing during growth in young children or particularly in the subset of patients in which a modification of QRS complexes can be supposed. Furthermore, this case underlines some peculiar aspects of the ARVD patients that should be taken into account in the assessment of S-ICD eligibility. Perhaps, those patients that typically exhibit reduced QRS voltages amplitude [17, 29, 30] and large negative T-waves and/or right atrial enlargement (peaked P waves) may be more prone to double-counting and IAS over the time [23, 31]. Consequently, probably for young patients with ARVD, it may be desirable to have at least two surface ECG lead (sensing vector at screening) considered acceptable for all postures tested (i.e., supine and standing position) and during a treadmill exercise test.
4.2 Potential benefits of S-ICD in primary prevention
In our series, primary prevention was the S-ICD indication in seven patients (33% of all, aged 14.5±3 years, 1.7±0.3 BSA). For these young patients, with a supposed long-life expectancy might be preferable for the implantation of a fully S-ICD device as young patients may expect many ICD changes during their life-time and therefore are most likely to undergo multiple procedures for lead revision or extractions, with a high risk of infections. In our study, there were no issues regarding growth and size. The youngest patient implanted was 8 years old (patient #5, LQTS, height 138cm, weight 38kg), and the smallest patient had a BMI of 16.8 kg/m2 (patient #19, Fig. 2 repaired tetralogy of Fallot). Moreover, the two-incision inter-muscular technique [32] used for the majority of the patients might be safe and useful in reducing potentially pocket-related complications, providing a better cosmetic outcome (Fig. 5), condition really important, especially in thin young individuals.
4.3 Limitations
The main limitations of this study are the small sample size, the low event rate, the retrospective design of the analysis, and the relatively limited follow-up period.
5 Conclusions
Our experience suggests that S-ICD device can be used in some children over the age of 8 as well as adults, with a similar rate of unwanted side effects and early evidence of apparent efficacy, especially in high-risk patients with inheritable channelopathies, cardiomyopathies or CHD, complex anatomy, and limited venous access to the heart, albeit conditional to the screening test being positive. In this population, the use of S-ICD promises to offer advantages and lesser risks than the classic ICD transvenous system [7, 28, 33]. The S-ICD is no real option when there is clearly pace-terminable arrhythmia history, or possibility of resynchronisation via an additional LV epicardial lead (CRT-ICDs), or pacemaker requirement, but could certainly be of value in primary prophylaxis patients. Large prospective comparative trials will be needed to fully gauge S-ICD potential compared with classical transvenous ICD system in young high-risk patients. Ongoing and future studies will help guide our decisions.
Abbreviations
- ICD:
-
Implantable cardioverter defibrillator
- S-ICD:
-
Subcutaneous implantable cardioverter defibrillator
- CHD:
-
Congenital heart disease
- VF:
-
Ventricular fibrillation
- VT:
-
Ventricular tachycardia
- IAS:
-
Inappropriate shock
- SCD:
-
Sudden cardiac death
- HCM:
-
Hypertrophic cardiomyopathy
- ARVD:
-
Arrhythmogenic right ventricular dysplasia
- BrS:
-
Brugada syndrome
References
Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867.
Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e73–e189.
Baumgartner H, Bonhoeffer P, De Groot NM, et al. Task force on the management of grown-up congenital heart disease of the European Society of Cardiology (ESC); Association for European Paediatric Cardiology (AEPC); ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J. 2010;31(23):2915–57.
Baumgartner H, De Backer J, Babu-Narayan SV, et al. ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2020;ehaa554.
Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmia in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11(10):e102–65.
Sherrid MV, Daubert JP. Risks and challenges of implantable cardioverter-defibrillators in young adults. Prog Cardiovasc Dis. 2008;51:237–63.
Maisel WH, Kramer DB. Implantable cardioverter-defibrillator lead performance. Circulation. 2008;117:2721–3.
Khairy P, Landzberg MJ, Gatzoulis MA, Mercier LA, Fernandes SM, Côté JM, et al. Epicardial versus endocardial pacing and thromboembolic events investigators. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: a multicenter study. Circulation. 2006;113(20):2391–7.
Silvetti MS, Pazzano V, Verticelli L, Battipaglia I, Saputo FA, Albanese S, et al. Subcutaneous implantable cardioverter-defibrillator: is it ready for use in children and young adults?. A single-centre study. Europace. 2018;20(12):1966–73.
Bettin M, Larbig R, Rath B, et al. Long-term experience with the subcutaneous implantable cardioverter-defibrillator in teenagers and young adults. J Am Coll Cardiol EP. 2017;3:1499–506.
McLeod CJ, Boersma L, Okamura H, et al. The subcutaneous implantable cardioverter defibrillator: state-of-the-art review. Eur Heart J. 2017;38:247–57.
Lambiase PD, Barr C, Theuns DAMJ, Knops R, Neuzil P, Johansen JB, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–65.
Burke MC, Gold MR, Knight BP, Barr CS, Theuns DAMJ, Boersma LVA, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–15.
D’Onofrio A, Pieragnoli P, Biffi M, et al. Subcutaneous implantable cardioverter defibrillator implantation: an analysis of Italian practice and its evolution. Int. J. Cardiol. 2018;272:162–7.
Droghetti A, Basso RE, Scimia P, et al. Ultrasound-guided serratus anterior plane block combined with the two-incision technique for subcutaneous ICD implantation. Pacing Clin Electrophysiol. 2018 May;41(5):517–23.
Knops RE, Olde Nordkamp LR, de Groot JR, et al. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2013;10:1240–3.
Amin AK, Gold MR, Burke MC, Knight BP, Rajjoub MR, Duffy E, et al. Factors associated with high-voltage impedance and subcutaneous implantable defibrillator ventricular fibrillation conversion success. Circ Arrhythm Electrophysiol. 2019;12(4):e006665.
Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy. Eur Heart J. 2014;35:2733–9.
Corrado D, Wichter T, Link MS, Hauer RNW, Marchlinski FE, Anastasakis A, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy dysplasia: an international task force consensus statement. Circulation. 2015;132:441–53.
Quast A-FBE, Baalman SWE, Brouwer TF, Smeding L, Wilde AAM, Burke MC, et al. A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable defibrillator: the PRAETORIAN score. Heart Rhythm. 2019;16:403–10.
Poole JE, Gold MR. Who should receive the subcutaneous implanted defibrillator?: the subcutaneous implantable cardioverter defibrillator (ICD) should be considered in all ICD patients who do not require pacing. Circ Arrhythm Electrophysiol. 2013;6(6):1236–44.
Moore JP, Mondesert B, Lloyd MS, et al. Clinical experience with the subcutaneous implantable cardioverter-defibrillator in adults with congenital heart disease. Circ- Arrhythm Electrophysiol. 2016;9:e004338.
Willy K, Reinke F, Bogeholz N, et al. The entirely subcutaneous ICD system in patients with congenital heart disease: experience from a large single-centre analysis. Europace. 2019;21:1537–42.
Li W, Sarubbi B, Somerville J. Iatrogenic ventricular tachycardia from endocardial pacemaker late after repair of tetralogy of Fallot. PACE. 2000;23:2131–4.
Lee JC, Epstein LM, Huffer LL, Stevenson WG, Koplan BA, Tedrow UB. ICD lead pro-arrhythmia cured by lead extraction. Heart Rhythm. 2009;6(5):613–8.
Lin G, Nishimura RA, Connolly HM, et al. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45(10):1672–5.
Van OJ, Geskes G, Debie L. A completely subcutaneous implantable cardioverter defibrillator system functioning simultaneously with an endocardial implantable cardioverter defibrillator programmed as pacemaker. Europace. 2011;13:141–2.
Coutinho Cruz M, Viveiros Monteiro A, Portugal G, et al. Long-term follow-up of adult patients with congenital heart disease and an implantable cardioverter defibrillator. Congenit Heart Dis. 2019;1–9.
Corrado D, Leoni L, Link MS, Bella PD, Gaita F, Curnis A, et al. Implantable cardioverter defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–91.
Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, et al. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144–52.
Migliore F, Viani S, Bongiorni MG, et al. Subcutaneous implantable cardioverter defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy: results from an Italian multicenter registry. In J Cardiol. 2019;280:74–9.
Migliore F, Allocca G, Calzolari V, et al. Intermuscular two-incision technique for subcutaneous implantable cardioverter defibrillator implantation: results from a multicenter registry. Pacing Clin Electrophysiol. 2017;40(3):278–85.
Olde Nordkamp LRA, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AAM, et al. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13(2):443–54.
Acknowledgments
Special thanks to the Adult Congenital Heart Disease Unit nursing staff and specially to the head nurse Mrs. Assunta Carandente for their essential contribution and support in maintaining high-quality standard of care for our complex patients. We thank furthermore Dr. Gabriella Piccolo and Dr. Nadia Puzone, data manager, for data collecting and analysis, and Dr. Cecilia Spinelli Barrile for her professional support in reviewing the English language and style of the manuscript and reviewing the data.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarubbi, B., Colonna, D., Correra, A. et al. Subcutaneous implantable cardioverter defibrillator in children and adolescents: results from the S-ICD “Monaldi care” registry. J Interv Card Electrophysiol 63, 283–293 (2022). https://doi.org/10.1007/s10840-021-00966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10840-021-00966-4