Abstract
Background
Subcutaneous implantable cardioverter defibrillators (S-ICD) are widely accepted therapy in congenital heart disease (CHD) patients at risk of life-threatening ventricular arrhythmias or sudden cardiac death (SCD) when pacing is not required.
Occasionally, pacemaker (PM)-dependent CHD patients will subsequently develop an indication for a cardioverter defibrillator. The use of S-ICD in complex CHD patients who have had already PM devices implanted implies some specific considerations, as the safety for these patients in unknown and recommendations among physicians may vary widely.
Methods
We review the data and studied the indications for S-ICD in complex CHD with previous PM and discuss its usefulness in clinical practice.
Results
From a large cohort of 345 patients enrolled in the S-ICD Monaldi care registry, which encompass all the patients implanted in the Monaldi Hospital of Naples, we considered 11 consecutive complex CHD patients (10M/1F aged 40.4 ±18.4 years) who underwent S-ICD implant after a previous PM implant, from February 2015 to October 2022. Mean follow-up was 25.5 ± 22 months. All the patients showed a good compliance to the device system with no complications (infections or skin erosions).
Conclusions
In complex CHD with already implanted PM devices, S-ICD implant appears to be a safe alternative to PM upgrading to transvenous ICD system, avoiding abandoned leads or life-threatening lead extraction. However, there are important issues with regard to testing and programming that need to be addressed at the time of implantation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Implantable cardioverter defibrillators (ICD) are widely accepted therapy in congenital heart disease (CHD) patients at risk of life-threatening ventricular arrhythmias or sudden cardiac death (SCD) [1,2,3,4,5]. Occasionally, pacemaker (PM)-dependent CHD patients will subsequently develop an indication for an ICD. In such a scenario, common options for upgrade include implantation of additional transvenous ICD lead with or without extracting the existing pacing lead. Sometimes such an approach may not be possible or desirable due to central venous obstruction, various anatomic constraints, technical difficulties, high-risk procedures, or patient preferences.
The addition of a subcutaneous implantable cardioverter defibrillator (S-ICD) to an existing transvenous or epicardial pacing device may be another option, instead of implantation of an ICD lead. Although the S-ICD has been advocated to be ideally suited to the adult congenital heart disease (ACHD) population [6,7,8,9,10,11,12], there is a very limited clinical experience with this technology in PM recipients with complex CHD.
2 Methods
This is a study on S-ICD implantation and follow-up in complex CHD patients who have had already a PM device implanted. Data were collected prospectively in the “Monaldi Care” Registry, and analysed retrospectively. In 2013, we started our own Monaldi Hospital registry, named S-ICD Monaldi Care registry which was later incorporated into the S-ICD Rhythm Detect Registry [13]. We prospectively entered data from all patients who underwent S-ICD implantation in our hospital. The S-ICD Monaldi Care registry was developed under the agreement of different EP teams working in the hospital to perform epidemiological analyses and publish their results for the population of patients with implanted S-ICD. The registry was approved by the local ethics committee, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and its later amendments.
Informed consent was obtained from the patients or their guardians, respectively.
2.1 Selection of the patients
All the patients affected by complex CHD, who underwent S-ICD implantation between February 2015 and November 2022 and have already had a previous PM device implanted, were included in the study, as they were already enrolled in the Monaldi Care registry.
A complex CHD was defined as moderate or severe CHD complexity according to the latest ESC/ACC Guidelines [1, 5].
2.1.1 Inclusion criteria (specific indications for S-ICD implantation)
International guidelines were followed for ICD implantation [1,2,3,4,5].
The indication for S-ICD was considered for patients with complex CHD and previous PM device implanted who had no favourable venous access (occluded veins, congenital anomalies) or hypothesized venous occlusion following further intracavitary lead positioning or history of endocarditis or at high infective risk who presented the following conditions:
-
Survivors of an aborted cardiac arrest, after the exclusion of any reversible causes
-
Symptomatic sustained VT after haemodynamic and electrophysiological evaluation that excluded any reversible causes
-
Systemic left ventricular ejection fraction (LVEF) < 35%, biventricular physiology, symptomatic heart failure (HF) despite optimal medical treatment and NYHA functional class II or III
-
Syncope of unknown origin in the presence of either advanced ventricular dysfunction or inducible sustained VT or VF on programmed ventricular stimulation (PVS)
-
Tetralogy of Fallot (TOF) and multiple risk factors for SCD, including left ventricle dysfunction, non-sustained VT, QRS duration > 180 ms or inducible sustained VT on PVS
-
Advanced single or systemic right ventricle dysfunction in the presence of other risk factors such as non-sustained VT, NYHA functional class II or III or severe systemic AV valve regurgitation.
2.1.2 Exclusion criteria:
-
All the patients with a simple heart defect (isolated defect, defects repaired or unrepaired without any haemodynamic impairment) or with a mild CHD complexity according the latest ESC/ACC Guidelines [1, 5]
-
An inherited arrhythmia (long QT syndrome, Brugada syndrome, catecholaminergic polymorphic ventricular tachycardia), cardiomyopathies (dilated, hypertrophic, restrictive, non-compaction, arrhythmogenic right ventricular), myocarditis
-
Patients with CRT/D indications according the latest ESC Guidelines [1]
2.2 S-ICD screening
All the enrolled patients were already eligible for S-ICD suitability as they had at least one surface ECG lead (sensing vector) considered acceptable for all postures tested (i.e. supine and standing position) and, if suitable for the patient, during exercise test, either during intrinsic QRS, either during atrial and/or ventricular pacing. PM devices were temporarily programmed to VVI mode with a lower rate 10–20 bpm faster than the patient’s intrinsic rhythm. Using the Boston Scientific screening templates, a patient was classified as a “screen-in” if no portion of the electrocardiogram exceeded the template in both positions (supine and upright) at any gain. Only ventricularly paced complexes that did not have any fusion with intrinsic rhythm were analyzed. Evaluation of QRS morphologies and pacing stimuli at both clinical voltage and maximal voltage parameters to replicate the possibility of a power-on-reset phenomenon was performed. The tracings were evaluated by at least two reviewers.
2.3 Implantation procedure
All procedures were performed in the electrophysiology/cardiac pacing laboratory, by a single team composed of six electrophysiologists of the ACHD Unit with the support of the manufacturer’s technicians. Implantations were performed under general anaesthesia or only in the procedures performed after 2019, through ultrasound-guided serratus anterior plane block [14]. At the onset of the experience, a complete subcutaneous approach was performed; thereafter, the inter-muscular approach was preferred. Antibiotic prophylaxis was given to all patients. In the 2015 cases, S-ICDs (model Emblem A209, Boston Scientific, Natick, NA, USA) were implanted via a standard three-incision approach. Subsequently, S-ICDs (models Emblem A219, Boston Scientific, Natick, NA, USA) were implanted applying a three- or two-incision technique [15]. During S-ICD lead insertion, fluoroscopy was used in those patients with a prior sternotomy to ensure positioning away from the nearest sternal wire out of concern for noise from sensing chatter.
Acute efficacy of the system was not tested due to low ejection fraction, the unstable haemodynamic conditions of the enrolled patients and high risk of complications [16]. For all the patients, 10 J shocks were delivered synchronously in sinus rhythm. An impedance of < 90 Ω was considered highly predictive of defibrillation testing (DT) success [17].
All patients received individualized dual-zone programming (conditional therapy zone between 180 and 220 beats/min and a shock zone of 230–250 beats/min). If feasible, the pacing upper rate was programmed at ≤ 50% of the S-ICD tachycardia zone to reduce the risk that double counting would not cause an inappropriate shock.
2.4 Data collection
The following data were collected:
-
Patient demographics
-
Preimplant clinical characteristics (congenital diagnosis and details regarding surgical repair/palliation and existing PM device details; results of the most recent catheterization and non-invasive imaging; results of latest PM device test screening, a copy of the preimplant 12-lead ECG; results of S-ICD eligibility screening in sinus rhythm and during atrial and/or ventricular pacing both at baseline in supine and standing positions, as well as with exercise testing, if suitable for the patient, drug therapy, ICD indication, and motivation for use of the S-ICD)
-
Implant characteristics (implant techniques, results of defibrillation testing, initial S-ICD programming, a copy of the postprocedural chest x ray, procedural complications, and postprocedural length of stay)
Acute complications were defined as those occurring before discharge from the hospital or within 30 days of implant. Therapies were classified as appropriate if delivered for VT/VF; otherwise, they were considered inappropriate (IAS = inappropriate shock).
2.5 Follow-up
Patients were regularly followed between February 2015 and February 2023 at ACHD Unit in accordance with the following protocol: patients underwent clinical evaluation, ECG and device interrogation (PM and S-ICD) 1 month after the S-ICD implant and every 3–4 months thereafter. Trans-thoracic echocardiography and Holter monitoring were performed every 12 months, unless clinical symptoms, for periodic functional evaluation of the disease. The outcomes analysed included patients’ characteristics, long-term complications, all post-operative arrhythmias monitored by the devices, any VF episodes, time to the first appropriate shock, first inappropriate shock and all appropriate and inappropriate shocks during follow-up.
2.6 Data analysis
Data are presented as mean ± standard deviation or median (interquartile range) for continuous variables as appropriate and as frequencies and percentages for dichotomous variables. The study is descriptive, with no inferential statistics performed.
3 Results
All the patient’s data are reported in Tables 1 and 2.
3.1 Baseline patient characteristics
From a large cohort of 345 patients enrolled in the S-ICD Monaldi care registry, included in the study were 11 consecutive complex CHD patients (10M/1F aged 40.4 ± 18.4 years, range 13–73 years) who underwent S-ICD implant after a previous PM implant, from February 2015 to October 2022. Mean follow-up was 25.5 ± 22 months. Mean weight was 73.9 ± 17.7 kg, height 172.9 ±10.6 cm, body mass index 24.9 ±4.9 and body surface area 1.9 ±0.2. Notably, all but one (patient no. 9) had a cardiac surgery operation early in the life.
Seven had endocardial-lead PM (2 DDD PM, 3 VVI PM, 2 VDD PM), three had epicardial-lead PM (2 DDD PM, 1 CRT-P device) and one a leadless PM at the time of SICD implant. Between them, 5/11 were chronically paced (> 40% of the time; 4 endocardial PM and 1 epicardial PM). The endocardial lead was positioned along the right ventricle (RV) septum in 2 patients, along the RV apex in two patients, along the sub-pulmonary, morphologically left ventricle, free wall, septum or inferior wall in the other three patients.
The epicardial lead was positioned along the free wall in one patient with a single ventricle morphology and along RV free wall in the other two patients.
In the patient with a leadless PM, the device was positioned in the RV apex.
Primary prevention was the indication for S-ICD implantation in 9/11 (81.8% of the patients).
3.2 Procedural data
All the patients but two passed the S-ICD eligibility test with the electrode in a left parasternal position. In details, one patient presented one sensing vector acceptable for all postures tested, seven patients showed two sensing vectors acceptable, and three patients presented all the three sensing vectors. The standard three-incision approach was adopted only in the first patient of the series, and the two-incision technique was used in the following 10. The generator was positioned for all in an inter-muscular pocket in the left lateral thoracic region. As the defibrillation test was not performed, for all the patients, 10-J shocks were delivered synchronously in sinus rhythm. An impedance ranging between 10 and 55Ω was found in all, and it was considered highly predictive of device system integrity and appropriate system position. Furthermore, in 10/11 patients (all except patient no. 1, already implanted in 2015) in which was available AP/LL postprocedural chest X- ray, PRAETORIAN scores [18], adopted since 2019, documented 30 to 60 points representing a low risk of conversion failure.
All patients had dual-zone programming. The conditional shock zone was programmed between 180 and 220 bpm, and the shock zone was programmed for all at 250 bpm.
Nine patients received the “Latitude system” for remote automatic, in-home monitoring.
No complications were reported during the procedures.
3.3 Follow-up
The post-operative course was uneventful, and all the patients were discharged between 2 and 3 days after the procedure. Mean follow-up was 25.5 ± 22 months. No acute or late complications (infections or skin erosions) were reported. Only one patient (patient no. 5) experienced IAS due to double counting due to T wave oversensing; for him, the conditional shock zone was reprogrammed from 180 to 230 bpm; after re-programming, no other IAS occurred.
No patients experienced appropriate shocks during follow-up.
Seven patients presented at device interrogation or home-monitoring evaluation arrhythmias not requiring electrical therapies (three atrial fibrillation, one atrial tachycardia, three non-sustained VT).
One patient underwent heart transplantation (HTX).
4 Discussion
S-ICD has become a widely accepted therapy in CHD patients who are deemed high risk for ventricular arrhythmias [6, 8,9,10,11,12]. For those patients who have already a pacing device and need an ICD treatment but in whom standard transvenous approaches are not feasible or desirable, the combination of a PM device and an S-ICD might therefore be a useful strategy, alternative to PM upgrading to transvenous ICD system, avoiding abandoned leads or life-threatening lead extraction. Special groups of complex CHD patients could particularly benefit of such approach.
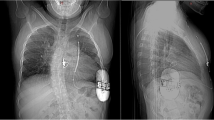
The presence of severe tricuspid and/or pulmonary valve regurgitation is quite common in complex CHD, especially in the patients already implanted with an endocardial PM lead. In these high-risk patients, a further abandoned lead without extraction could be supposed to progress the valve incompetence leading to worse haemodynamic condition and further RV volume overload, facilitating ventricular arrhythmia occurrence. In these settings, an increase in lead-related tricuspid incompetence [19, 20] can be easily avoided with a S-ICD implantation. In our series, the presence of a moderate to severe tricuspid incompetence was quite frequent (patients no. 1, no. 2, no. 4 Fig. 1, no. 6, no. 7, no. 9, no. 11). This condition gave more reasons for selecting the S-ICD device.
In patients with univentricular circulation with an intracardiac shunt due to a huge atrial and/or ventricular septal defect (patient no. 5 — Fig. 2), the implantation of fully S-ICD devices is absolutely mandatory, so in patients with a tricuspid valve prosthesis (patient no. 8) that may develop valvular degeneration necessitating a need for a new valve in the tricuspid position in due time.
In patients with transposition of the great arteries treated by an atrial switch procedure (Mustard or Senning) (patients no. 3 Fig. 3, no. 10), ICD lead placement can be technically difficult, other than sometimes contraindicated due to the possibility of pathway obstruction or baffle damage. Furthermore, in this condition, the S-ICD promises to offer advantages for potentially extraction procedures, when required, for lead fractures or infections, as an abandoned malfunctioned lead can even increase the risk of intra-cardiac obstruction.
Epicardial options, with necessity of a thoracotomy, should be alternatively considered in these cases if vector testing fails.
In our series, no complications, infections or skin erosions were observed. The lack of complications, differently from other studies involving CHD patients [9, 10, 21, 22], could be related in our series to the smaller size of the generator used (Emblem), which has a 20% reduction in device profile compared to the previous model, and the prevalent use of intermuscular approach and two incisions technique. Rates of appropriate and inappropriate shocks in S-ICD system are usually similar to those occurring with the transvenous ICD. Our experience with S-ICD shows a high efficacy. Only one patient (patient no. 5 — Fig. 2) showed episodes of inappropriate shocks, due to T-wave oversensing, with double-counting, conditions eliminated with improved device programming (reprogramming shock zone, changing of the sensing vector and later activation of the SMART PASS filter). Probably, the small number of inappropriate shock rates in our series, moreover reduced consistently during the follow-up, is related to better strategic programming over time and increased operator experience. Improved detection algorithms other than extended use of “latitude system” for remote monitoring and adequate antiarrhythmic therapy can reduce unwanted inappropriate shocks. Remote monitoring has been already shown to have an important role in the timely diagnosis of atrial tachyarrhythmias, device-related complications and inappropriate therapies. If these events are detected earlier, appropriate measures could be undertaken to reduce the number of shocks and increase the longevity of the battery.
4.1 Suitability of S-ICD implant in patients already with a permanent PM device
Overall experience with simultaneous use of the S-ICD and a permanent pacemaker prior to device implantation is limited [23,24,25,26,27,28,29,30,31,32] and mostly referred to single-case reports.
S-ICD implantation in the setting of unipolar pacing has been relatively contraindicated. The primary concern is due to the indwelling pacemaker under-sensing ventricular fibrillation and providing inappropriate pacing. Artifact from unipolar pacing could interfere with appropriate detection of ventricular arrhythmias by the S-ICD and hence withhold vital intervention [26].
Exclusion criteria for the FDA mandated US Investigational Device Exemption (IDE) Registry and the Evaluation oF FactORs ImpacTing CLinical Outcome and Cost EffectiveneSS of the S-ICD (EFFORTLESS S-ICD) registry included patients with unipolar pacemakers, or implanted devices that revert to unipolar pacing, based on concerns of potential ventricular oversensing and inappropriate shocks [33, 34].
Reversion to a unipolar pacing mode is an inherent risk during a power-on-reset phenomenon, a rare occurrence seen most commonly in older devices. While unipolar pacing coupled with an S-ICD may be safe in some circumstances, the risk of the S-ICD undersensing true VF due to inappropriate pacing or providing an inappropriate shock from double counting remains. In general, pacemakers that can enter “safety core” mode after a shock with unipolar pacing should be avoided. In our patients, the previous implanted pacemaker did not go to unipolar pacing with a power-on-reset, and appropriate sensing by the S-ICD was confirmed during ventricular pacing with maximal output to address these concerns as much as possible. Furthermore, for the patient with a leadless PM (patient no. 11), which implements fixed bipolar pacing, compatibility was theoretically guaranteed.
For the patients completely pacemaker-dependent, even in the worst scenario of fixed double counting of QRS complexes, conservatively programming the pacemaker at VVI 60 bpm would have maintained the S-ICD sensed rate abundantly below the therapy window (200 bpm).
Our series provide additional evidences that the S-ICD can be used safely with permanent PM devices (with endocardial, epicardial lead or leadless systems).
To minimize risks of cross talk between the two devices, as already indicated, it is important that during implantation, S-ICD screening of paced and native bizarre morphologies should be done to assess best sensing vector and avoid oversensing. Moreover, the upper tracking rate limit of the PM should be programmed below the S-ICD shock zone rate detection, and consideration can be given to programming the pacing upper rate to ≤ 50% the conditional shock zone rate. With these settings, even if there is double counting of the pacing spike, it will still be below the conditional shock zone. The sensing vector, which is least likely to have pacemaker artifact, should be used.
While exercise testing was not performed in all our cases, due to the severely impaired clinical condition, it can be an additional option that may reduce the risk of inappropriate shocks in these particularly cases. Interference between the devices should be always evaluated. Pacing spikes could be counted independently from the R waves by the S-ICD. Post-shock pacing from the S-ICD could inhibit pacing from the pacemaker and should be turned off.
The non-inferiority of defibrillation testing (DT) omission at the time of implantation was already demonstrated in transvenous ICD [35]. In our series, S-ICD defibrillation test was omitted, as it already happens in clinical practice, especially in very sick patients with worse systolic function [16].
No dysfunction of the pacemaker devices after delivery of S-ICD shocks was found in our series, neither for endocardial or for epicardial leads (that are quite common in CHD patients due to impossibility to perform an endocardial pacing implant or decision during surgical procedures). Moreover, no misinterpretation of pacing artifacts was perceived by the S-ICD. On the other hand, untreated self-limiting VT episodes were all correctly detected in our series.
In our series, S-ICD therapy was shown to be technically feasible in patients with a single-chamber pacemaker as well as those who have more complex pulse generators, with either endocardial or epicardial leads or leadless devices. Furthermore, endocardial or epicardial lead position (septum, apex, free wall or inferior wall) in RV, LV or single ventricle did not influence S-ICD eligibility or system efficacy.
Conversely, some patients that have an existing S-ICD may develop a pacing indication that was not present during their initial implant. In such a scenario, it would be important to know whether addition of a pacemaker is potentially feasible since ventricular pacing may lead to different QRS amplitude.
4.2 Limitations
The main limitations of this study are the small sample size, the low event rate, the retrospective design of the analysis and the relatively limited follow-up period.
5 Conclusions
To the best of our knowledge, only a very small number of cases of S-ICD implantation in patients already paced with complex CHD have been reported in the literature. This study on S-ICD in complex CHD with previous PM devices includes the largest population of patients analysed so far.
Our series, which exhibit several unique and challenging elements, demonstrate that S-ICD treatment combined with an endocardial, epicardial or leadless pacemaker devices might be a safe and effective approach providing pacing and S-ICD functions avoiding PM upgrading to transvenous ICD system, abandoned leads or life-threatening lead extraction.
S-ICDs could be safely and effectively used in patients with pre-existing PM devices, albeit conditional to the screening test being positive. Anyway, it is important for the evaluation of QRS morphologies and pacing stimuli at both clinical voltage and maximal voltage parameters to replicate the possibility of a power-on-reset phenomenon. The successful combination of the S-ICD with a PM that has either a transvenous PM electrode or an epicardial electrode is technically feasible and offers both cardiac stimulation and arrhythmia protection even in complex CHD patients.
However, there are important issues with regard to testing and programming that need to be addressed at the time of implantation.
The S-ICD is no real option when there is clearly pace-terminable arrhythmia history, or possibility of resynchronisation via an additional LV epicardial lead (CRT-ICDs), but could certainly be of value in primary prophylaxis patients. Large prospective comparative trials will be needed to fully gauge S-ICD potential compared with classical transvenous ICD system in high-risk patients with complex CHD and previous PM implant.
Ongoing and future studies will help guide our decisions.
Data Availability
All patient's data are available upon request.
References
Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, Lung B, Kluin J, Lang IM, Meijboo F, Moons P, Mulder BJM, Oechslin E, Roos-Hesselink JW, Schwerzmann M, Sondergaard L, Zeppenfeld K, ESC Scientific Document Group. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021;42(6):563–645.
Khairy P, Van Hare GF, Balaji S, et al. PACES/HRS expert consensus statement on the recognition and management of arrhythmia in adult congenital heart disease: developed in partnership between the Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS). Endorsed by the governing bodies of PACES, HRS, the American College of Cardiology (ACC), the American Heart Association (AHA), the European Heart Rhythm Association (EHRA), the Canadian Heart Rhythm Society (CHRS), and the International Society for Adult Congenital Heart Disease (ISACHD). Heart Rhythm. 2014;11(10):e102–65.
Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15(10):e73–e189.
Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. ESC Scientific Document Group. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36(41):2793–867.
Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guideline for the Management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):1494–563.
Peters B, Will A, Berger F, Butter C. Implantation of a fully subcutaneous ICD in a patient with single ventricle morphology and Eisenmenger physiology. Acta Cardiol. 2012;67:473–5.
Zeb M, Curzen N, Veldtman G, et al. Potential eligibility of congenital heart disease patients for subcutaneous implantable cardioverter-defibrillator based on surface electrocardiogram mapping. Europace. 2015;17:1059–67.
Vehmeijer JT, Brouwer TF, Limpens J, et al. Implantable cardioverter-defibrillators in adults with congenital heart disease: a systematic review and meta-analysis. Eur Heart J. 2016;37:1439–48.
Moore JP, Mondesert B, Lloyd MS, et al. Clinical experience with the subcutaneous implantable cardioverter-defibrillator in adults with congenital heart disease. Circ Arrhythm Electrophysiol. 2016;9:e004338.
Silvetti MS, Pazzano V, Verticelli L, et al. Subcutaneous implantable cardioverter-defibrillator: is it ready for use in children and young adults ?. A single-centre study. Europace. 2018;20(12):1966–73.
Sarubbi B, Correra A, Colonna D, et al. Subcutaneous implantable cardioverter defibrillator in complex adult congenital heart disease. Results from the S-ICD “Monaldi Care” registry. Int Journal of Cardiol CHD. 2021;3:100091. https://doi.org/10.1016/j.ijcchd.2021.100091.
Sarubbi B, Colonna D, Correra A, et al. Subcutaneous implantable cardioverter defibrillator in children and adolescents: results from the S-ICD “Monaldi care” registry. J Interv Card Electrophysiol. 2022;63(2):283–93. https://doi.org/10.1007/s10840-021-00966-4.
D’Onofrio A, Pieragnoli P, Biffi M, et al. Subcutaneous implantable cardioverter defibrillator implantation: an analysis of Italian practice and its evolution. Int J Cardiol. 2018;272:162–7.
Droghetti A, Basso RE, Scimia P, Harizai F, Marini M. Ultrasound-guided serratus anterior plane block combined with the two-incision technique for subcutaneous ICD implantation. Pacing Clin Electrophysiol. 2018;41(5):517–23.
Knops RE, Olde Nordkamp LR, de Groot JR, Wilde AA. Two-incision technique for implantation of the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm. 2013;10:1240–3.
Bianchi V, Bisignani G, Migliore F, et al. “S-ICD rhythm detect” investigators. Safety of omitting defibrillation efficacy testing with subcutaneous defibrillators: a propensity-matched case-control study. Circ Arrhythm Electrophysiol. 2021;14(12):e010381. https://doi.org/10.1161/CIRCEP.121.010381.
Payne JE, Badertscher P, Field ME, Sturdivant JL, Gold MR. Relationship of shock energy to impedance during subcutaneous implantable cardioverter-defibrillator testing. Circ Arrhythm Electrophysiol. 2020;13:e008631.
Quast A-FBE, Baalman SWE, Brouwer TF, et al. A novel tool to evaluate the implant position and predict defibrillation success of the subcutaneous implantable defibrillator: the PRAETORIAN score. Heart Rhythm. 2019;16:403–10.
Lee JC, Epstein LM, Huffer LL, Stevenson WG, Koplan BA, Tedrow UB. ICD lead pro-arrhythmia cured by lead extraction. Heart Rhythm. 2009;6(5):613–8.
Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45(10):1672–5.
Willy K, Reinke F, Bogeholz N, et al. The entirely subcutaneous ICD system in patients with congenital heart disease: experience from a large single-centre analysis. Europace. 2019;21:1537–42.
Bettin M, Larbig R, Rath B, et al. Long-term experience with the subcutaneous implantable cardioverter-defibrillator in teenagers and young adults. J Am Coll Cardiol EP. 2017;3:1499–506.
Porterfield C, DiMarco JP, Mason PK. Effectiveness of implantation of a subcutaneous implantable cardioverter-defibrillator in a patient with complete heart block and a pacemaker. Am J Cardiol. 2015;115(2):276–8.
Kuschyk J, Stach K, Tülümen E, et al. Subcutaneous implantable cardioverter-defibrillator: First single-center experience with other cardiac implantable electronic devices. Heart Rhythm. 2015;12(11):2230–8.
Steinberg C, Chakrabarti S, Krahn AD, Bashir J. Nothing inside the heart - combining epicardial pacing with the S-ICD. Heart Rhythm Case Rep. 2015;1(6):419–23.
Huang J, Patton KK, Prutkin JM. Concomitant use of the subcutaneous implantable cardioverter defibrillator and a permanent pacemaker. Pacing Clin Electrophysiol. 2016;39(11):1240–5.
Gemein C, Haj M, Schmitt J. Combining a subcutaneous ICD and a pacemaker with abdominal device location and bipolar epicardial left ventricular lead: first-in-man approach. Europace. 2016;18(8):1279.
Tjong FV, Brouwer TF, Smeding L, et al. Combined leadless pacemaker and subcutaneous implantable defibrillator therapy: feasibility, safety, and performance. Europace. 2016;18(11):1740–7.
Ip JE, Wu MS, Kennel PJ, et al. Eligibility of pacemaker patients for subcutaneous implantable cardioverter defibrillators. J Cardiovasc Electrophysiol. 2017;28(5):544–8.
Lüker J, Sultan A, Sreeram N, Brockmeier K, Steven D. Implantation of a subcutaneous implantable cardioverter defibrillator with right parasternal electrode position in a patient with D-transposition of the great arteries and concomitant AAI pacemaker: a case report. Eur Heart J Case Rep. 2018;2(3):yty099.
Baroni M, Colombo G, Testoni A, et al. Combined leadless pacemaker and subcutaneous implantable cardioverter-defibrillator to manage recurrent transvenous system failures. J Electrocardiol. 2019;54:43–6.
Abbott N, Bender A, Henrikson C, et al. Adverse device-device interaction between pacemaker and subcutaneous implantable cardiac defibrillator. Pacing Clin Electrophysiol. 2021;44(11):1944–8.
Lambiase PD, Barr C, Theuns DAMJ, et al. Worldwide experience with a totally subcutaneous implantable defibrillator: early results from the EFFORTLESS S-ICD Registry. Eur Heart J. 2014;35:1657–65.
Burke MC, Gold MR, Knight BP, et al. Safety and efficacy of the totally subcutaneous implantable defibrillator: 2-year results from a pooled analysis of the IDE study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–15.
Healey JS, Hohnloser SH, Glikson M, et al. Shockless IMPLant Evaluation [SIMPLE] investigators. Cardioverter defibrillator implantation without induction of ventricular fibrillation: a single-blind, non- inferiority, randomised controlled trial (SIMPLE). Lancet. 2015;385:785–91.
Acknowledgements
Special thanks to the Adult Congenital Heart Disease Unit nursing staff and specially to the head nurse Mrs. Assunta Carandente for their essential contribution and support in maintaining high-quality standard of care for our complex patients. We thank furthermore Dr. Gabriella Piccolo, Dr. Nadia Puzone, Dr. Cecilia Spinelli Barrile and Dr. Tiziana Varriale, data manager and research assistants, for data collecting and analysis and support in remote control monitoring.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sarubbi, B., Ciriello, G.D., Papaccioli, G. et al. Combined subcutaneous implantable cardioverter defibrillator and pacemaker devices in complex congenital heart disease: a single-center experienced based study. J Interv Card Electrophysiol (2023). https://doi.org/10.1007/s10840-023-01670-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10840-023-01670-1