Abstract
Purpose
To clarify the associations of the maternal age, history of miscarriage, and embryonic/fetal size at miscarriage with the frequencies and profiles of cytogenetic abnormalities detected in spontaneous early miscarriages.
Methods
Miscarriages before 12 weeks of gestation, whose karyotypes were evaluated by G-banding between May 1, 2005, and May 31, 2017, were included in this study. The relationships between their karyotypes and clinical findings were assessed using trend or chi-square/Fisher’s exact tests and multivariate logistic analyses.
Results
Three hundred of 364 miscarriage specimens (82.4%) had abnormal karyotypes. An older maternal age was significantly associated with the frequency of abnormal karyotype (ptrend < 0.001), particularly autosomal non-viable and viable trisomies (ptrend 0.001 and 0.025, respectively). Women with ≥ 2 previous miscarriages had a significantly lower possibility of miscarriages with abnormal karyotype than women with < 2 previous miscarriages (adjusted odds ratio [aOR], 0.48; 95% confidence interval [95% CI], 0.27–0.85). Although viable trisomy was observed more frequently in proportion to the increase in embryonic/fetal size at miscarriage (ptrend < 0.001), non-viable trisomy was observed more frequently in miscarriages with an embryonic/fetal size < 10 mm (aOR, 2.41; 95% CI, 1.27–4.58), but less frequently in miscarriages with an embryonic/fetal size ≥ 20 mm (aOR, 0.01; 95% CI, 0.00–0.07) than in anembryonic miscarriages.
Conclusions
The maternal age, history of miscarriage, and embryonic/fetal size at miscarriage may be independently associated with the frequencies or profiles of cytogenetic abnormalities in early miscarriages.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The cytogenetic status is an essential factor determining the viability of a conceptus. Most chromosomal abnormalities occurring in a conceptus are likely to lead to arrest of pregnancy, resulting in spontaneous miscarriage or stillbirth. The chromosomal analysis of products of conception (POC) is not routine practice for women who have miscarried; however, the cytogenetic identification of POC may be beneficial not only for the management of subsequent pregnancies in women with recurrent miscarriage (RM) [1] but also for the elucidation of genetic associations in the early development of pregnancy, as the viability of a conceptus is expected to differ among chromosomal abnormalities [2]. Apart from inheritable structural abnormalities, chromosomal abnormalities of POC are incidentally generated during gametogenesis, fertilization, or early embryogenesis; however, this does not necessarily occur in all women expecting pregnancy in the same way. For instance, both maternal and fetal clinical factors, such as the maternal age, history of miscarriage, and gestational age, can greatly affect the cytogenetic results of miscarriages [3].
The most convincing clinical factor is the maternal age at miscarriage. The frequency of autosomal trisomy, the most frequent cause of early miscarriages, remarkably increases with advancing maternal age at pregnancy because of age-related meiotic errors in oogenesis [3,4,5,6,7]. It is also reported that the profile of detected trisomy can vary by the maternal age [6,7,8]. Another possible factor is maternal history of RM. The results concerning the relationship between maternal history of RM and the frequency of chromosomal abnormalities are conflicting; several studies have indicated a lower frequency of chromosomal abnormalities in women with RM [9,10,11,12,13], whereas others have shown a similar or higher frequency [7, 8, 14,15,16,17,18,19]. The composition of chromosomal abnormalities can be also different in miscarriages from women with and without RM [7, 18]. In addition, several studies have revealed ultrasonographic findings, such as the presence of a fetal pole or fetal cardiac activity, as possible factors related to the frequency or composition of chromosomal abnormalities in early miscarriages [8, 12, 20,21,22,23,24]; however, a comparison by only the presence or absence of a fetal pole or fetal cardiac activity may be insufficient to specify the contribution of each cytogenetic abnormality to miscarriage. Few studies have investigated the frequencies/profiles of chromosomal abnormalities at more than two developmental stages of pregnancy [18, 25,26,27]. Furthermore, few studies on the associations between those clinical factors and the cytogenetic results of miscarriages have been so far conducted regarding each confounding effect [5, 7, 13, 28]. Therefore, the currently available data are insufficient to draw conclusions on the relationships of chromosomal abnormalities with clinical maternal factors or fetal findings in early miscarriage.
In this study, we comprehensively investigated the associations between chromosomal abnormalities of early miscarriages and clinical factors, including the maternal age, history of miscarriage, and embryonic/fetal size at miscarriage, in order to clarify the relevance of these clinical factors themselves on the frequencies or profiles of chromosomal abnormalities in POC that can cause early miscarriages.
Materials and methods
The cytogenetic findings of 394 miscarriages, managed in our perinatal center between May 1, 2005, and May 31, 2017, were retrospectively reviewed. Our hospital is a tertiary perinatal care center and has a special clinic for women with infertility or RM. All pregnancies were clinically confirmed by the presence of an intrauterine gestational sac. Spontaneous miscarriage was diagnosed by ultrasonographic findings, such as a persistent anembryonic (empty) gestational sac or arrest of fetal cardiac activity. When an embryo or fetus was recognized in the gestational sac, the maximal length of the embryo/fetus, or crown-rump length (CRL) if possible, was measured just before miscarriage and used for the analysis. To focus on early pregnancy, only miscarriages with an empty sac or CRL < 45 mm or biparietal diameter (BPD) < 20 mm, approximately corresponding to < 12 weeks of gestation, were included in this study, as early miscarriage is defined as miscarriage before 12 weeks of gestation in Japan. Cases of biochemical pregnancy, ectopic pregnancy, and (vanishing) twin pregnancy were excluded from this study. POC specimens were collected mostly by medical procedures, namely dilation and curettage (n = 332) and the induction of labor by the transvaginal administration of gemeprost (n = 10). When the expulsion of POC spontaneously occurred, villous tissue was carefully separated from the discharged specimens and used for the analysis (n = 52).
The cytogenetic analysis of POC was performed after obtaining informed consent from women who miscarried. After removing the maternal tissue/blood clot from the villous tissue and washing with saline, the specimen was collected in a sterile container of 10 mL of RPMI-1640 with fetal bovine serum and penicillin-streptomycin/glutamine or gentamicin and GlutaMAX™ (Gibco/Life Technologies, Grand Island, NY, USA). A cytogenetic analysis was performed by the G-banding method at Integrated Genetics, USA. Trained cytogenetic technologists dissected and selected the placental chorionic villi, which were enzymatically digested with Trypsin-EDTA (Gibco/Life Technologies) and collagenase Type 2 (Worthington Biochemical, Lakewood, NJ, USA) and cultured in alpha-MEM complete medium (Gibco/Life Technologies) at 37 °C in a 5% CO2 incubator. All procedures, including cell harvesting, slide preparation, and staining, were conducted following standard protocols [8, 29]. At least 20 metaphases were analyzed by the G-banding method in each case. Cases of abnormal karyotypes were classified into the following categories in this study: autosomal non-viable trisomy, viable trisomy (trisomies 13, 18, 21), triploidy, tetraploidy, monosomy X, structural abnormalities, 47,XXY, and complicated abnormalities defined by having two distinct abnormalities, such as trisomy and monosomy, or aneuploidy and structural abnormality, including mosaicism.
Clinical information on miscarriages was retrospectively collected from medical records. Our primary clinical factors of interest were the maternal age, history of early miscarriage before 12 weeks of gestation, and embryonic/fetal size at miscarriage. Maternal age was calculated by days from the date of birth to the date of miscarriage and classified into the following three groups: < 35, 35 to < 40, and ≥ 40 years old. The number of previous miscarriages before 12 weeks of gestation was divided into the following two groups: < 2 and ≥ 2. The embryonic/fetal size was classified into the following four categories based on the ultrasonographic findings at miscarriage: empty (gestational sac was seen, but embryo/fetus not), < 10 mm, 10 to < 20 mm, and ≥ 20 mm of embryonic/fetal size, or CRL if possible.
This study was approved by the institutional ethical committee to publicize the clinical findings of women and cytogenetic results of their miscarriages, in August 2015 (No. 991).
Statistical analyses
First, the success rate of G-banding analysis was compared by the chi-square test between different collecting methods. Second, we analyzed the associations between each abnormal karyotype and clinical factors using a trend test for the maternal age and embryonic/fetal size, and the chi-square test or Fisher’s exact test for the number of previous miscarriages. Third, to eliminate confounding effects, we used a multivariate logistic analysis including the maternal age, history of miscarriage, and embryonic/fetal size. As non-viable and viable trisomies are the most major components of abnormal karyotypes detected in miscarriages, only the frequency of abnormal karyotype or non-viable/viable trisomy was assessed in the multivariate association analysis. Finally, we evaluated the details of autosomal trisomy among the categories to identify which trisomies contributed to a significant difference. All statistical analyses were conducted using the statistical software package Stata SE 13 (STATA Corp, College Station, TX), and p < 0.05 was considered statistically significant.
Results
The G-banding analysis was successful in 364 of 394 spontaneous miscarriages (92.4%), and the success rate was clearly different between the specimens obtained by medical procedures and those derived from spontaneous discharge: 97.1% (332/342) and 61.5% (32/52), respectively (p < 0.001). A further analysis was therefore conducted based on 364 miscarriage cases. The clinical background characteristics of these miscarriages are shown in Table 1.
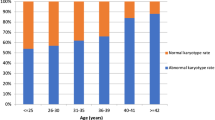
Abnormal karyotypes were found in 82.4% (300/364) of successfully analyzed cases, and the associations between each abnormal karyotype and clinical factors, such as the maternal age, number of previous miscarriages, and embryonic/fetal size at miscarriage, are shown in Table 2. Overall, the most frequently detected abnormality was autosomal non-viable trisomy (48.9%, n = 178), followed by autosomal viable trisomy (16.8%, n = 61), triploidy (4.4%, n = 16), and monosomy X (3.6%, n = 13). The ratio of normal female/male karyotype was 1.37 (37/27). The frequency of abnormal karyotype significantly increased with advancing maternal age (ptrend < 0.001), and the frequency of both autosomal non-viable and viable trisomies significantly increased as well (ptrend 0.001 and 0.025, respectively). In contrast, triploidy and structural abnormality had a significant negative association with the maternal age (ptrend 0.016 and 0.017, respectively). The frequencies of other abnormalities did not significantly differ by the maternal age.
The frequency of abnormal karyotype was significantly lower in women with ≥ 2 previous miscarriages compared with women with < 2 previous miscarriages (p = 0.023). Regarding the individual comparisons, only autosomal viable trisomy was significantly related to the number of previous miscarriages (p = 0.004). By embryonic/fetal size, although the frequency of abnormal karyotype did not show a significant trend, autosomal non-viable and viable trisomies showed opposite significant trends in frequencies, with a significant negative trend noted for non-viable trisomy (ptrend < 0.001) and a significant positive trend noted for viable trisomy (ptrend < 0.001). Tetraploidy had a significant negative trend (ptrend 0.006) and was only detected in miscarriages with an empty sac or embryonic/fetal size < 10 mm, whereas monosomy X had a significant positive trend (ptrend 0.002) and was only detected in miscarriages with an embryonic/fetal size ≥ 10 mm. The details of structural abnormalities and complicated abnormalities are shown in Supplemental Table 1. Three parental balanced translocations were found in cases with structural abnormalities.
Associations between the three clinical factors and the frequency of abnormal karyotype or non-viable/viable trisomy in crude and multivariate logistic analyses are shown in Table 3. Compared with women < 35 years old, the frequency of abnormal karyotype was higher among women aged 35 to < 40 (adjusted odds ratio [aOR], 2.73; 95% confidence interval [95% CI], 1.45–5.15) and women ≥ 40 years old (aOR, 5.15; 95% CI, 2.35–11.3). For both non-viable and viable trisomies, a similar significant association was found. Women with ≥ 2 previous miscarriages were less likely to have abnormal karyotypes than women with < 2 previous miscarriages (aOR, 0.48; 95% CI, 0.27–0.85). In contrast, the frequencies of non-viable/viable trisomies in women with ≥ 2 previous miscarriages were not markedly different from those in women with < 2 previous miscarriages by a multivariate analysis, although the frequency of viable trisomy was significantly lower according to a crude analysis. In addition, the frequency of abnormal karyotype was significantly lower in miscarriages with an embryonic/fetal size ≥ 20 mm than in those with an empty sac (aOR, 0.41; 95% CI, 0.18–0.95). Compared with miscarriages with an empty sac, the frequency of non-viable trisomy was significantly higher in miscarriages with an embryonic size < 10 mm (aOR, 2.41; 95% CI, 1.27–4.58) but lower in those with an embryonic/fetal size ≥ 20 mm (aOR, 0.01; 95% CI, 0.00–0.07). A significant increase in frequency of viable trisomy was noted in miscarriages both with embryonic/fetal size 10 to < 20 mm (aOR, 8.49; 95% CI, 1.90–37.9) and ≥ 20 mm (aOR, 36.8; 95% CI, 8.26–164), compared with those with an empty sac.
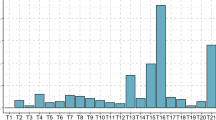
The profiles of autosomal non-viable and viable trisomies are described according to the maternal age, history of miscarriages, and embryonic/fetal size at miscarriage in Table 4. Overall, the most frequent trisomy was trisomy 22, followed by trisomies 21, 15, and 16. Trisomies 1 and 19 were not detected in early miscarriages enrolled in this study. With advancing maternal age, the proportions of trisomies 15 and 21 and plural trisomy became high, whereas trisomy 16 became less common. The proportions of trisomies 16 and 21 were higher in women with < 2 previous miscarriages, whereas the proportions of trisomy 22 and plural trisomy were higher in women with ≥ 2 previous miscarriages. In addition, a wide variety of trisomies was detected in miscarriages with an empty sac or embryonic/fetal size < 10 mm, but the detected trisomies were limited mainly to trisomies 15, 21, and 22 in miscarriages with embryonic/fetal size 10 to < 20 mm, and most trisomies detected in miscarriages with embryonic/fetal size ≥ 20 mm were trisomies 18 and 21. The gestational ages when common non-viable trisomies (trisomy 15, 16, and 22) were frequently detected were different, as follows: trisomy 15 in miscarriages with embryonic/fetal size 10 to < 20 mm, trisomy 16 in miscarriages with an empty or embryonic/fetal size < 10 mm, and trisomy 22 in miscarriages with embryonic/fetal size < 20 mm. Plural trisomy was frequently detected in miscarriages with an empty sac or embryonic/fetal size < 10 mm as trisomy 16.
Discussion
In the present study, we showed that the maternal age, history of miscarriage, and embryonic/fetal size at miscarriage may be independently associated with the frequencies or profiles of cytogenetic abnormalities in early miscarriages. The frequency of abnormal karyotype was significantly higher in women with an older age and lower in women with ≥ 2 previous early miscarriages and miscarriages with an embryonic/fetal size ≥ 20 mm. The maternal age or embryonic/fetal size at miscarriage was independently associated with the occurrence of non-viable/viable autosomal trisomy. This may be the first study to assess the detailed associations between these clinical factors and the frequency of abnormal karyotype or trisomy in early miscarriages by a multivariate logistic analysis in order to avoid confounding effects.
In accordance with previous studies [4,5,6,7], the frequency of abnormal karyotype remarkably increased with the maternal age in this study. This trend is likely due to both non-viable and viable trisomies, which showed a significantly increased trend with the maternal age. In addition, we found the age-related associations of both types of trisomy were not attenuated by other clinical factors, namely the number of previous miscarriages and embryonic/fetal size at miscarriage, suggesting the independent association of these factors. Although trisomies 15 and 21 and plural trisomy showed increases in frequency, the rate of trisomy 16 decreased with the maternal age, in line with previous studies [6, 8]. This peculiar tendency of trisomy 16 might result from the difference in the effect of maternal age on meiosis of each chromosome [30, 31]. The frequencies of triploidy and structural abnormalities decreased with the maternal age, as the previous results demonstrated [4, 6, 7], whereas the frequency of monosomy X did not change with the maternal age, in disagreement with previous studies claiming a decreased frequency in women with advanced age [4, 6, 8]. As monosomy X is more likely to be derived from meiotic error of the father rather than the mother [8, 32], the frequency of monosomy X is not likely to be associated with the maternal age.
Regarding the association of the number of previous miscarriages with the cytogenetic results of miscarriages, some studies claimed that there was a lower frequency of abnormal karyotypes in RM patients [9,10,11,12,13], suggesting an undetermined maternal cause for RM, whereas others reported that the frequency did not change or even increased in miscarriages from RM patients [7, 8, 14,15,16,17,18,19]. RM patients may have some genetic vulnerability leading to non-disjunction or superfertility, leading to the conception of embryos with severe chromosomal abnormalities [33, 34], but these hypotheses remain to be investigated. In this study, the frequency of abnormal karyotype was significantly lower in women with ≥ 2 previous miscarriages than in women with < 2 previous miscarriages, and viable trisomy seemed to be the main contributor to this trend. A previous study showed that a decrease in viable trisomies and an increase in non-viable trisomies were recognized in miscarriages from RM women ≥ 35 years of age, which might be related to the increased incidence of preembryonic miscarriages [7]. In this study, however, a multivariate analysis showed that the frequency of non-viable/viable trisomy was not markedly different between in women with < 2 and ≥ 2 previous miscarriages, whereas women with ≥ 2 previous miscarriages had a significantly lower possibility to miscarry due to fetal chromosome abnormality even after adjusting for the maternal age and embryonic/fetal size, suggesting the possibility of maternal causes for RM.
Miscarriages before 12 weeks’ gestation were classified into four gestational stages in the present study according to embryonic/fetal size as determined by ultrasonography. The gestational age is generally calculated by the date of the last menstrual period, but an estimation based on ultrasonographic findings can be more reliable because there is a possibility of delayed ovulation or a delayed diagnosis of miscarriage. In addition, a detailed assessment of cytogenetic results based on classification into four stages of early miscarriages has not yet been performed. Our analysis showed that the frequency of abnormal karyotype was significantly lower in miscarriages with an embryonic/fetal size ≥ 20 mm than in those with an empty sac, and the frequencies and profiles of cytogenetic abnormalities differed considerably according to embryonic/fetal size. We found a negative trend in non-viable trisomies with embryonic/fetal size, but the association analysis showed a significant aOR in the opposite direction between miscarriages with an embryonic/fetal size < 10 mm and miscarriages with an embryonic/fetal size ≥ 20 mm compared with those with an empty sac. These inconsistent tendencies may have been caused by differences in the peak frequencies of major non-viable trisomies. Previous studies have also shown the frequencies of these trisomies to differ according to the presence of a fetal pole or fetal cardiac activity and trisomy 15 or 22 is more frequently detected at a later stage of miscarriages than trisomy 16 [8, 22, 25]. In contrast, a positive trend in viable trisomy with embryonic/fetal size was noted, which was in agreement with previous studies comparing miscarriages with two different gestational ages [8, 12, 13, 20, 22,23,24]; the aOR for viable trisomy became significant in miscarriages with an embryonic/fetal size ≥ 10 mm, and markedly increased in miscarriages with an embryonic/fetal size ≥ 20 mm. These results concerning autosomal non-viable/viable trisomy strongly suggest that each chromosome plays a different genetic role in the early embryonic/fetal development and contributes to miscarriage at different gestational stages. The frequency of triploidy did not change with gestational age in this study, although some studies have reported a higher frequency in later miscarriages [6, 12, 13, 24]. This might be due to the inclusion of near-triploidy (triploidy including aneuploidy), leading to a shorter life, into the category “triploidy” in this study. In contrast, this study showed that tetraploidy carried a significantly higher risk of miscarriage at the early stage of pregnancy. This means that polyploidy should be divided into triploidy and tetraploidy when assessing the cytogenetic results of miscarriages. Monosomy X cases were relatively long-lived (similar to trisomies 21 and 18), which was in agreement with the findings of previous reports [6, 12, 13, 22,23,24]. Structural abnormalities did not change with gestational age in this study, although some studies have reported a higher frequency in miscarriages without a fetal pole or with CRL < 15 mm [13, 21]. This discrepancy may be caused by differences in the severity of structural abnormalities detected in miscarriages.
The present study has several limitations. First, the maternal age was higher in this study than in previous studies [10,11,12,13, 18, 23,24,25], which may lead to a biased interpretation concerning the association of the frequencies of abnormal karyotypes and maternal history of miscarriage or embryonic/fetal size. To reduce this possibility, we performed a multivariate analysis for the frequency of abnormal karyotype or non-viable/viable trisomy, in which the association of maternal history of miscarriage or embryonic/fetal size and the frequency of abnormal karyotype or embryonic/fetal size and the frequency of non-viable/viable trisomy was confirmed to have independent significance. Second, we did not evaluate the size of gestational sac, although some studies have suggested that a smaller embryo/fetus with/without smaller gestational sac than expected by gestational age is suggested to be associated with chromosomal abnormalities [35, 36]. The size or appearance of the gestational sac might change depending on the timing of the diagnosis of miscarriage. Third, other potential clinical factors, including the mode of conception, maternal obesity, and complications such as polycystic ovary syndrome [37, 38], were not evaluated in this study because of lack of sufficient information on these points. In addition, the management of RM was inconsistent due to the relatively long period of the study, which may have limited our evaluation of the associations regarding the number of previous miscarriages. Finally, the results of this study might be affected by the drawbacks of the G-banding method, such as the need for culture, maternal contamination, or low resolution; indeed, the success rate of POC derived from spontaneous discharge was quite low, and 46,XX was predominant in normal karyotypes. DNA-based analyses, such as single nucleotide polymorphism (SNP) microarray, have been recently performed in the cytogenetic analysis of miscarriages in order to overcome these drawbacks [39,40,41,42]; however, a portion of tetraploidy and balanced structural abnormalities cannot be detected by this method [43]. At present, the most suitable method depends on the situation, and an additional analysis by another method should be considered when necessary in order to obtain correct cytogenetic results for miscarriages [43].
In conclusion, the frequencies and profiles of cytogenetic abnormalities in early miscarriages are strongly associated with clinical factors, such as the maternal age, history of miscarriage, and embryonic/fetal size at miscarriage. Clinicians should be aware of these facts when assessing the cytogenetic results of miscarriages. Further detailed investigations of these associations are awaited in order to elucidate the genetic contributions of chromosomal abnormalities to fetal and placental development as well as to early miscarriage and RM.
References
Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod. 2002;17(2):446–51.
Sorokin Y, Johnson MP, Uhlmann WR, Zador IE, Drugan A, Koppitch FC 3rd, et al. Postmortem chorionic villus sampling: correlation of cytogenetic and ultrasound findings. Am J Med Genet. 1991;39(3):314–6.
Page JM, Silver RM. Genetic causes of recurrent pregnancy loss. Clin Obstet Gynecol. 2016;59(3):498–508.
Eiben B, Bartels I, Bahr-Porsch S, Borgmann S, Gatz G, Gellert G, et al. Cytogenetic analysis of 750 spontaneous abortions with the direct-preparation method of chorionic villi and its implications for studying genetic causes of pregnancy wastage. Am J Hum Genet. 1990;47(4):656–63.
Cowchock FS, Gibas Z, Jackson LG. Chromosome errors as a cause of spontaneous abortion: the relative importance of maternal age and obstetric history. Fertil Steril. 1993;59(5):1011–4.
Hardy K, Hardy PJ, Jacobs PA, Lewallen K, Hassold TJ. Temporal changes in chromosome abnormalities in human spontaneous abortions: results of 40 years of analysis. Am J Med Genet A. 2016;170(10):2671–80.
Grande M, Borrell A, Garcia-Posada R, Borobio V, Munoz M, Creus M, et al. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod. 2012;27(10):3109–17.
Segawa T, Kuroda T, Kato K, Kuroda M, Omi K, Miyauchi O, et al. Cytogenetic analysis of the retained products of conception after missed abortion following blastocyst transfer: a retrospective, large-scale, single-centre study. Reprod BioMed Online. 2017;34(2):203–10.
Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73(2):300–4.
Carp H, Toder V, Aviram A, Daniely M, Mashiach S, Barkai G. Karyotype of the abortus in recurrent miscarriage. Fertil Steril. 2001;75(4):678–82.
Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104(4):784–8.
Liu Y, Liu Y, Chen H, Du T, Tan J, Zhang J. The frequencies of the presence of embryonic pole and cardiac activity in early miscarriages with abnormal karyotypes. Clin Exp Obstet Gynecol. 2015;42(4):490–4.
Liu Y, Liu Y, Chen H, Li Q, Meng L, Chen L, et al. Relationship of karyotype to embryo crown-rump length and maternal serum human chorionic gonadotropin level in early miscarriage. Am J Perinatol. 2015;32(1):15–22.
Stern JJ, Dorfmann AD, Gutierrez-Najar AJ, Cerrillo M, Coulam CB. Frequency of abnormal karyotypes among abortuses from women with and without a history of recurrent spontaneous abortion. Fertil Steril. 1996;65(2):250–3.
Coulam CB, Stephenson M, Stern JJ, Clark DA. Immunotherapy for recurrent pregnancy loss: analysis of results from clinical trials. Am J Reprod Immunol. 1996;35(4):352–9.
Morikawa M, Yamada H, Kato EH, Shimada S, Yamada T, Minakami H. Embryo loss pattern is predominant in miscarriages with normal chromosome karyotype among women with repeated miscarriage. Hum Reprod. 2004;19(11):2644–7.
Marquard K, Westphal LM, Milki AA, Lathi RB. Etiology of recurrent pregnancy loss in women over the age of 35 years. Fertil Steril. 2010;94(4):1473–7.
Choi TY, Lee HM, Park WK, Jeong SY, Moon HS. Spontaneous abortion and recurrent miscarriage: a comparison of cytogenetic diagnosis in 250 cases. Obstet Gynecol Sci. 2014;57(6):518–25.
Goldstein M, Svirsky R, Reches A, Yaron Y. Does the number of previous miscarriages influence the incidence of chromosomal aberrations in spontaneous pregnancy loss? J Matern Fetal Neonatal Med. 2017;30(24):2956–60.
Schmidt-Sarosi C, Schwartz LB, Lublin J, Kaplan-Grazi D, Sarosi P, Perle MA. Chromosomal analysis of early fetal losses in relation to transvaginal ultrasonographic detection of fetal heart motion after infertility. Fertil Steril. 1998;69(2):274–7.
Lathi RB, Mark SD, Westphal LM, Milki AA. Cytogenetic testing of anembryonic pregnancies compared to embryonic missed abortions. J Assist Reprod Genet. 2007;24(11):521–4.
Munoz M, Arigita M, Bennasar M, Soler A, Sanchez A, Borrell A. Chromosomal anomaly spectrum in early pregnancy loss in relation to presence or absence of an embryonic pole. Fertil Steril. 2010;94(7):2564–8.
Cheng HH, Ou CY, Tsai CC, Chang SD, Hsiao PY, Lan KC, et al. Chromosome distribution of early miscarriages with present or absent embryos: female predominance. J Assist Reprod Genet. 2014;31(8):1059–64.
Liu Y, Liu Y, Zhang S, Chen H, Liu M, Zhang J. Etiology of spontaneous abortion before and after the demonstration of embryonic cardiac activity in women with recurrent spontaneous abortion. Int J Gynaecol Obstet. 2015;129(2):128–32.
Ouyang Y, Tan Y, Yi Y, Gong F, Lin G, Li X, et al. Correlation between chromosomal distribution and embryonic findings on ultrasound in early pregnancy loss after IVF-embryo transfer. Hum Reprod. 2016;31(10):2212–8.
Azmanov DN, Milachich TV, Zaharieva BM, Michailova GI, Dimitrova VG, Karagiozova ZH, et al. Profile of chromosomal aberrations in different gestational age spontaneous abortions detected by comparative genomic hybridization. Eur J Obstet Gynecol Reprod Biol. 2007;131(2):127–31.
Romero ST, Geiersbach KB, Paxton CN, Rose NC, Schisterman EF, Branch DW, et al. Differentiation of genetic abnormalities in early pregnancy loss. Ultrasound Obstet Gynecol. 2015;45(1):89–94.
Ljunger E, Stavreus-Evers A, Cnattingius S, Ekbom A, Lundin C, Anneren G, et al. Ultrasonographic findings in spontaneous miscarriage: relation to euploidy and aneuploidy. Fertil Steril. 2011;95(1):221–4.
Simoni G, Brambati B, Danesino C, Rossella F, Terzoli GL, Ferrari M, et al. Efficient direct chromosome analyses and enzyme determinations from chorionic villi samples in the first trimester of pregnancy. Hum Genet. 1983;63(4):349–57.
Warburton D, Kinney A. Chromosomal differences in susceptibility to meiotic aneuploidy. Environ Mol Mutagen. 1996;28(3):237–47.
Hussin J, Roy-Gagnon MH, Gendron R, Andelfinger G, Awadalla P. Age-dependent recombination rates in human pedigrees. PLoS Genet. 2011;7(9):e1002251.
Hassold T, Benham F, Leppert M. Cytogenetic and molecular analysis of sex-chromosome monosomy. Am J Hum Genet. 1988;42(4):534–41.
Quenby S, Vince G, Farquharson R, Aplin J. Recurrent miscarriage: a defect in nature’s quality control? Hum Reprod. 2002;17(8):1959–63.
Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27(8):2297–303.
Angiolucci M, Murru R, Melis G, Carcassi C, Mais V. Association between different morphological types and abnormal karyotypes in early pregnancy loss. Ultrasound Obstet Gynecol. 2011;37(2):219–25.
Li X, Ouyang Y, Yi Y, Tan Y, Lu G. Correlation analysis between ultrasound findings and abnormal karyotypes in the embryos from early pregnancy loss after in vitro fertilization-embryo transfer. J Assist Reprod Genet. 2017;34(1):43–50.
Kroon B, Harrison K, Martin N, Wong B, Yazdani A. Miscarriage karyotype and its relationship with maternal body mass index, age, and mode of conception. Fertil Steril. 2011;95(5):1827–9.
Wang Q, Luo L, Lei Q, Lin MM, Huang X, Chen MH, et al. Low aneuploidy rate in early pregnancy loss abortuses from patients with polycystic ovary syndrome. Reprod BioMed Online. 2016;33(1):85–92.
van den Berg MM, van Maarle MC, van Wely M, Goddijn M. Genetics of early miscarriage. Biochim Biophys Acta. 2012;1822(12):1951–9.
Lathi RB, Massie JA, Loring M, Demko ZP, Johnson D, Sigurjonsson S, et al. Informatics enhanced SNP microarray analysis of 30 miscarriage samples compared to routine cytogenetics. PLoS One. 2012;7(3):e31282.
Lathi RB, Gustin SL, Keller J, Maisenbacher MK, Sigurjonsson S, Tao R, et al. Reliability of 46,XX results on miscarriage specimens: a review of 1,222 first-trimester miscarriage specimens. Fertil Steril. 2014;101(1):178–82.
Ozawa N, Sago H, Matsuoka K, Maruyama T, Migita O, Aizu Y, et al. Cytogenetic analysis of spontaneously discharged products of conception by array-based comparative genomic hybridization. Springerplus. 2016;5(1):874.
Shah MS, Cinnioglu C, Maisenbacher M, Comstock I, Kort J, Lathi RB. Comparison of cytogenetics and molecular karyotyping for chromosome testing of miscarriage specimens. Fertil Steril. 2017;107(4):1028–33.
Acknowledgements
We wish to thank Brian Quinn, editor-in-chief, Japan Medical Communication, for editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the institutional ethical committee to publicize the clinical findings of women and cytogenetic results of their miscarriages, in August 2015 (No. 991).
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 12.1 kb)
Rights and permissions
About this article
Cite this article
Ozawa, N., Ogawa, K., Sasaki, A. et al. Maternal age, history of miscarriage, and embryonic/fetal size are associated with cytogenetic results of spontaneous early miscarriages. J Assist Reprod Genet 36, 749–757 (2019). https://doi.org/10.1007/s10815-019-01415-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01415-y